Key Points
Question
What are the clinical characteristics and outcomes associated with antibiotic treatment of hospitalized patients with asymptomatic bacteriuria?
Findings
In this cohort study of 2733 hospitalized adults with asymptomatic bacteriuria, 82.7% received inappropriate antibiotic treatment; patients who were older, had altered mental status, or had abnormal urinalysis results were more likely to receive antibiotics. Antibiotic treatment was associated with a 37% longer duration of hospitalization after urine testing without improved clinical outcomes.
Meaning
Antibiotic treatment of asymptomatic bacteriuria in hospitalized patients appears to be common, may not be associated with improved clinical outcomes, and may be associated with longer duration of hospitalization after urine testing.
Abstract
Importance
Treatment of asymptomatic bacteriuria (ASB) with antibiotics is a common factor in inappropriate antibiotic use, but risk factors and outcomes associated with treatment of ASB in hospitalized patients are not well defined.
Objective
To evaluate factors associated with treatment of ASB among hospitalized patients and the possible association between treatment and clinical outcomes.
Design, Setting, and Participants
A retrospective cohort study was conducted from January 1, 2016, through February 1, 2018, at 46 hospitals participating in the Michigan Hospital Medicine Safety Consortium. A total of 2733 hospitalized medical patients with ASB, defined as a positive urine culture without any documented signs or symptoms attributable to urinary tract infection, were included in the analysis.
Exposures
One or more antibiotic dose for treatment of ASB.
Main Outcomes and Measures
Estimators of antibiotic treatment of ASB. Secondary outcomes included 30-day mortality, 30-day hospital readmission, 30-day emergency department visit, discharge to post–acute care settings, Clostridioides difficile infection (formerly known as Clostridium difficile) at 30 days, and duration of hospitalization after urine testing.
Results
Of 2733 patients with ASB, 2138 were women (78.2%); median age was 77 years (interquartile range [IQR], 66-86 years). A total of 2259 patients (82.7%) were treated with antibiotics for a median of 7 days (IQR, 4-9 days). Factors associated with ASB treatment included older age (odds ratio [OR], 1.10 per 10-year increase; 95% CI, 1.02-1.18), dementia (OR, 1.57; 95% CI, 1.15-2.13), acutely altered mental status (OR, 1.93; 95% CI, 1.23-3.04), urinary incontinence (OR, 1.81; 95% CI, 1.36-2.41), leukocytosis (white blood cell count >10 000/μL) (OR, 1.55; 95% CI, 1.21-2.00), positive urinalysis (presence of leukocyte esterase or nitrite, or >5 white blood cells per high-power field) (OR, 2.83; 95% CI, 2.05-3.93), and urine culture with a bacterial colony count greater than 100 000 colony-forming units per high-power field (OR, 2.30; 95% CI, 1.83-2.91). Treatment of ASB was associated with longer duration of hospitalization after urine testing (4 vs 3 days; relative risk, 1.37; 95% CI, 1.28-1.47). No other differences in secondary outcomes were identified after propensity weighting.
Conclusions and Relevance
Hospitalized patients with ASB commonly receive inappropriate antibiotic therapy. Antibiotic treatment did not appear to be associated with improved outcomes; rather, treatment may be associated with longer duration of hospitalization after urine testing. To possibly reduce inappropriate antibiotic use, stewardship efforts should focus on improving urine testing practices and management strategies for elderly patients with altered mental status.
This cohort study examines the outcomes of treatment of asymptomatic bacteriuria in hospitalized patients.
Introduction
Urinary tract infections (UTIs) are the second most common infection among hospitalized patients.1 Accurate diagnosis requires a combination of relevant signs and symptoms, generally supported by a positive urine culture (UC). Bacterial growth in a UC without accompanying symptoms is known as asymptomatic bacteriuria (ASB).2,3 Asymptomatic bacteriuria is commonly misdiagnosed as UTI.4 However, unlike UTI, treatment of ASB with antibiotics does not improve outcomes in outpatient and long-term care settings except in pregnancy or patients undergoing invasive urologic procedures.2,3,5 Despite national guidelines recommending against antibiotic therapy for ASB, high treatment rates persist.6,7,8,9
Inappropriate use of antibiotics can result in adverse events,10 increased antibiotic resistance, and Clostridioides difficile infection (CDI; formerly known as Clostridium difficile), prompting hospitals and antimicrobial stewardship programs to adopt strategies to reduce inappropriate antibiotic use, in particular, ASB treatment. However, data to guide interventions are limited; few studies have assessed factors and outcomes associated with antibiotic treatment for ASB in hospitalized populations.6,7,8,9,11,12 Surveys assessing clinician knowledge regarding ASB in inpatients found that deficits are prevalent. Even when clinicians are knowledgeable, guideline-discordant practices persist owing to concern for adverse outcomes if ASB is not treated.13,14,15 Therefore, a large, multihospital registry inclusive of a diverse patient population was used to identify factors possibly associated with inappropriate treatment of ASB and evaluate the clinical outcomes of antibiotic treatment.
Methods
Study Setting
The Michigan Hospital Medicine Safety (HMS) Consortium comprises 46 hospitals with shared goals of improving the quality of care and decreasing adverse events among hospitalized medical patients.16,17,18 Participation in the HMS Consortium is voluntary, representing half (46 of 92) of Michigan hospitals and including a mix of academic and community, small (<200 bed) and large (≥200 bed) hospitals.
Because the purpose of the HMS Consortium is to measure and improve the quality of existing care practices, this project received a status designation of not regulated by the University of Michigan Medical School’s Institutional Review Board, which waives the requirement of informed consent.
Data Collection
From January 1, 2016, through February 1, 2018 (10 hospitals in 2016, expanded to 46 in 2017), trained abstractors retrospectively collected data from patients with a positive UC collected during hospitalization (defined as a UC with any bacterial growth and identified as abnormal by the hospital’s microbiology policy). The HMS Consortium procedures for data collection and quality assurance have been previously described.16,17,18 Deidentified data were collected from 90 days before admission until follow-up was terminated by a major complication (eg, death) or 30 days after discharge. Data abstracted from medical records included demographics, signs and symptoms of UTI, laboratory and radiographic findings, vital signs, antibiotic type and duration, and outcomes. Symptoms were collected from clinician and nursing documentation 3 days before and after UC collection. At 30 days after discharge, patients were contacted for additional outcome data by scripted telephone follow-up (up to 3 attempts). A standardized data dictionary and random audits at each hospital by quality coordinators (including S.B.) ensured data integrity.
Patient Sampling and Selection
Abstractors at each hospital consecutively screened patients 30 days after discharge and included the first patient daily with a positive UC.18 Patients were not eligible for inclusion if they met any of the following criteria: (1) age younger than 18 years; (2) pregnant; (3) urinary stent, nephrostomy, altered urinary tract anatomy, or urologic surgery during hospitalization; (4) intensive care unit admission within 3 days before or after UC; (5) entered hospice during hospitalization; (6) left against medical advice; (7) concomitant infection (documentation by physician of an additional bacterial infection during hospitalization except CDI); (8) active treatment and/or prophylaxis for UTI on admission; (9) solid organ or bone marrow transplant recipient; (10) HIV with CD4 count less than 200 cells/mm3; (11) neutropenia (absolute neutrophil count <0.5 cells/μL [to convert to ×109 per liter, multiply by 0.001]) on hospital day 1 or 2; (12) isolated candiduria; or (13) within the 30 days after discharge from index hospitalization already abstracted for that patient.
Patients with bacteriuria were categorized as having ASB if there was no documentation of signs or symptoms meeting diagnostic criteria for UTI per Infectious Diseases Society of America Guidelines and National Healthcare Safety Network definitions.2,3,19 Specifically, patients could not have one of the following documented signs or symptoms: dysuria, urinary frequency/urgency, suprapubic pain, fever (temperature ≥38 °C), costovertebral pain/tenderness, hematuria, and autonomic dysreflexia or increased spasticity in patients with spinal cord injury (eFigure in the Supplement). Patients with acute alterations in mental status (AMS), who often cannot communicate symptoms, were categorized as having ASB if they had none of the aforementioned signs or symptoms and no systemic signs of possible infection (peripheral white blood cell count >10 000 cells/μL [to convert to ×109 per liter, multiply by 0.001], systolic blood pressure <90 mm Hg, or ≥2 criteria for systemic inflammatory response syndrome).3,20,21 Patients were excluded from analysis if relevant data were missing.
Outcomes
The primary outcome was antibiotic treatment, defined as receiving at least 1 dose of an oral or intravenous antibiotic (assessed 3 days before UC through discharge, including discharge prescriptions). Metronidazole and/or oral vancomycin alone was not considered ASB treatment.
Variables included (1) demographics, (2) receipt (in prior 90 days) of antibiotic, (3) urinary catheter presence, (4) nonspecific signs or symptoms not consistent with UTI definition (including fatigue, change in urine color/sediment), (5) severity of illness, and (6) laboratory results (including elevated peripheral white blood cell count, urinalysis, and UC results).
Secondary outcomes assessed included discharge to post–acute care facility, development of CDI within 30 days, duration of hospitalization after urine testing, and 30-day mortality, readmission, and emergency department visit. The CDI events included laboratory diagnosis (positive C difficile polymerase chain reaction and/or glutamate dehydrogenase level with toxin enzyme immunoassay testing)22 occurring 48 hours after UC or new CDI diagnosis documented within 30 days post discharge. Hospital duration was assessed from the day urine testing was performed (urinalysis or UC).
Sensitivity Analyses
Sensitivity analyses were performed to account for potential unmeasured confounders of duration of hospitalization after urine testing. First assessed was the outcome of ASB treatment on hospital duration only for patients with urine testing performed on hospital day 1. This sample limit addressed potential differences among patients with a later change prompting testing, which would be a possible marker for a complicated hospitalization. Additional analysis assessed duration of hospitalization for patients who had testing and antibiotics ordered on day 1.
Statistical Analysis
Factors in antibiotic treatment of ASB were summarized using descriptive analyses. Unadjusted associations of factors with antibiotic treatment were assessed using logistic generalized estimating equation models accounting for hospital level clustering, with results expressed as odds ratios (ORs) for dichotomous variables and relative risks (RRs) for continuous variables with 95% CIs. The final multivariable model was determined by a stepwise selection procedure based on covariate contributions to the model fit as measured by the Schwarz criterion.23 Associations of secondary outcomes with antibiotic treatment of ASB were assessed using logistic generalized estimating equation models, inverse probability of treatment24 weighted by baseline covariates identified to be significant in the bivariate and/or multivariate analysis, and other factors potentially associated with the outcome (eAppendix in the Supplement).25,26,27,28,29,30,31,32,33 To account for variables with missing data, a 10-fold multiple imputation was completed.34 A 2-sided P value <.05 was used to indicate significance. All analyses were performed in SAS, version 9.4 (SAS Institute Inc).
Results
Baseline Demographics
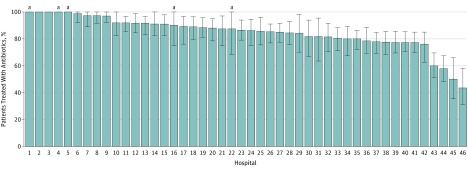
Between January 1, 2016, and February 1, 2018, 7252 patients with a positive UC were identified among 46 hospitals. Of these, 2772 patients (38.2%) lacked documented urinary symptoms and were classified as having ASB (eFigure in the Supplement). Among 2733 patients with ASB and complete treatment data, 2259 individuals (82.7%) received antibiotics for a median of 7 days (interquartile range [IQR], 4-9) (Table 1). Most individuals (1933/2259 [85.6%]) were treated with 3 or more days of antibiotics. The median age was 77 years (IQR, 66-86); 2138 of 2733 patients (78.2%) were women. At the time of UC, 375 of 2733 patients (13.7%) had an indwelling urinary catheter, 1138 patients (41.6%) had chronic kidney disease, 1076 patients (39.4%) had diabetes, and 560 patients (20.5%) had dementia. A total of 2308 patients (84.4%) were admitted to the hospital for reasons other than UTI. Discharge diagnoses more frequently included UTI for patients who received ASB treatment compared with those who did not receive treatment (485/2259 [21.5%] vs 0/474) (eTable 1 in the Supplement). Almost all patients (2499 [94.1%]) had pyuria. The most common urine pathogens isolated included Escherichia coli (50.2%) and Klebsiella species (15.3%). Ceftriaxone (61.6%) was the most common initial antibiotic treatment; fluoroquinolones (33.2%) were the antibiotics most frequently prescribed at discharge (Table 1). The indication for UC was documented in 1565 of the cases (57.3%) (Table 1). Of documented indications, abnormal urinalysis results was the most common (666/1565 [42.6%]). The proportion of patients with ASB treated with antibiotics varied by hospital, with 35 of the 46 facilities (76.1%) treating ASB in 80% or more of the patients (Figure).
Table 1. Characteristics of 2733 Patients With Asymptomatic Bacteriuria.
| Characteristic | No. (%) |
|---|---|
| Age, median (IQR), y | 77 (66-86) |
| Women | 2138 (78.2) |
| Comorbiditiesa | |
| Diabetes | 1076 (39.4) |
| Moderate to severe chronic kidney disease | 1138 (41.6) |
| Hemodialysis | 50 (1.8) |
| Liver disease | 176 (6.4) |
| Congestive heart failure | 804 (29.4) |
| Cerebrovascular disease | 708 (25.9) |
| History of cancer | 582 (21.3) |
| Spinal cord injury | 42 (1.5) |
| Immunosuppressionb | 95 (3.5) |
| Dementia | 560 (20.5) |
| Urinary catheter | |
| Indwelling | 375 (13.7) |
| Other | 46 (1.7) |
| Urinalysis obtained | 2662 (97.4) |
| Urinalysis result | |
| Positive LE and/or >5 WBCs/high-power field | 2499 (91.4) |
| Positive nitrite | 1005 (36.8) |
| Documentation of reason for culture in records | 1565 (57.3) |
| Abnormal urinalysis | 666 (42.6) |
| Altered mental status | 110 (7.0) |
| Fatigue (new or worsening) | 108 (6.9) |
| Abdominal pain (new or worsening) | 105 (6.7) |
| Other | 576 (36.8) |
| Urine pathogens | |
| Escherichia coli | 1373 (50.2) |
| Klebsiella spp | 417 (15.3) |
| Enterococcus spp | 294 (10.8) |
| Proteus spp | 154 (5.6) |
| Pseudomonas aeruginosa | 104 (3.8) |
| Enterobacter spp | 97 (3.5) |
| Citrobacter spp | 71 (2.6) |
| ≥2 Bacteria | 340 (12.4) |
| Treatment | |
| Duration of therapy for those receiving antibiotics on day 1 of treatment, median (IQR) | 7 (4-9) |
| Ceftriaxone | 1392 (61.6) |
| Fluoroquinolonec | 428 (18.9) |
| Cephalexin | 72 (3.2) |
| Antibiotics at discharged | |
| Fluoroquinolonec | 469 (33.2) |
| Cephalosporin (1st or 2nd generation) | 444 (31.5) |
| Trimethoprim with sulfamethoxazole | 132 (9.4) |
| Nitrofurantoin | 97 (6.9) |
Abbreviations: IQR, interquartile range; LE, leukocyte esterase; spp, species; TNF, tumor necrosis factor; WBC, white blood cell.
Comorbidities are not mutually exclusive.
Defined as chemotherapy administered within 30 days, HIV positive with a CD4 count greater than 200 cells/mm3, prednisone dose of 10 mg/d or more for at least 30 days (or equivalent corticosteroid dose), receiving biologic agents (eg, TNF inhibitors or other immunosuppressant agents), or congenital or acquired immunodeficiency.
Fluoroquinolones given were levofloxacin and ciprofloxacin.
Listed if more than 5% of total prescribed.
Figure. Proportion of Patients With Asymptomatic Bacteriuria Who Received Antibiotic Treatment Across 46 Hospitals in Michigan.
Error bars indicate 95% CIs.
aLow-volume hospital was defined as being in the 10th percentile or below for asymptomatic bacteriuria cases across the collaborative.
Variables Associated With ASB Treatment
Variables associated with ASB treatment included patient characteristics, such as older age and acute AMS, and laboratory results, such as positive urinalysis (presence of leukocyte esterase or nitrite, or >5 white blood cells per high-power field). Severity of illness, measured by qSOFA (quick sequential organ failure assessment)35 score (328/2733 [12.0%]) or 2 or more systemic inflammatory response syndrome criteria (909/2733 [33.3%]), was not associated with increased treatment rates (Table 2).
Table 2. Distribution of Patient-Level Variables and Treatment of Asymptomatic Bacteriuria.
| Variable | No. (%) | Odds Ratio (95% CI) | P Valuea | |
|---|---|---|---|---|
| Antibiotics (n = 2259) | No Antibiotics (n = 474) | |||
| Baseline characteristics | ||||
| Age, median (IQR), y | 78 (67-87) | 74 (62-83) | 1.02 (1.01-1.02) | <.001 |
| Women | 1774 (78.6) | 364 (76.8) | 1.09 (0.84-1.42) | .51 |
| White race | 1813 (80.5) | 397 (84.1) | 0.99 (0.73-1.34) | .95 |
| Charlson Comorbidity Index score | ||||
| 0 | 258 (11.4) | 54 (11.4) | 1 [Reference] | .009 |
| 1-2 | 656 (29.0) | 150 (31.7) | 0.88 (0.63-1.24) | |
| 3-4 | 720 (31.9) | 113 (23.8) | 1.35 (0.98-1.88) | |
| ≥5 | 625 (27.7) | 157 (33.1) | 0.87 (0.62-1.22) | |
| Diabetes | 876 (38.8) | 200 (42.2) | 0.84 (0.69-1.02) | .07 |
| Moderate to severe chronic kidney disease | 934 (41.4) | 204 (43.0) | 1.00 (0.80-1.26) | .98 |
| History of cancer | 477 (21.1) | 105 (22.2) | 1.00 (0.80-1.25) | .99 |
| Spinal cord injury | 36 (1.6) | 6 (1.3) | 1.66 (0.69-3.99) | .26 |
| Dementia | 506 (22.4) | 54 (11.4) | 2.10 (1.53-2.89) | <.001 |
| Immunosuppressionb | 73 (3.2) | 22 (4.6) | 0.79 (0.49-1.29) | .35 |
| IV chemotherapy in preceding 30 d | 19 (0.8) | 10 (2.1) | 0.46 (0.23-0.92) | .03 |
| Hemodialysis | 38 (1.7) | 12 (2.5) | 0.60 (0.32-1.12) | .11 |
| Transfer from post–acute care facilityc | 96 (4.3) | 11 (2.3) | 2.02 (0.78-5.20) | .15 |
| Nonambulatory | 355 (15.7) | 44 (9.3) | 1.65 (1.22-2.23) | .001 |
| Hospitalization in past 90 d | 662 (29.3) | 144 (30.4) | 0.91 (0.69-1.21) | .52 |
| Antibiotics in past 90 d | 492 (21.8) | 99 (20.9) | 1.00 (0.76-1.32) | .99 |
| Indwelling catheter | 331 (14.7) | 44 (9.3) | 1.45 (1.10-1.91) | .009 |
| Any catheterd | 369 (16.3) | 48 (10.1) | 1.57 (1.18-2.08) | .002 |
| Signs and symptoms | ||||
| Abdominal pain | 383 (17.0) | 100 (21.1) | 0.81 (0.60-1.08) | .15 |
| Incontinence | 534 (23.6) | 59 (12.4) | 2.31 (1.68-3.19) | <.001 |
| Functional decline | 165 (7.3) | 23 (4.9) | 1.42 (0.94-2.16) | .09 |
| Acutely altered mental status | 425 (18.8) | 45 (9.5) | 1.96 (1.28-3.00) | .002 |
| Fatigue, malaise, or lethargy | 659 (29.2) | 128 (27.0) | 1.12 (0.83-1.51) | .47 |
| Nausea or vomiting | 481 (21.3) | 123 (25.9) | 0.82 (0.64-1.05) | .11 |
| Change in color, sediment, or malodorous urine | 254 (11.2) | 42 (8.9) | 1.63 (1.04-2.57) | .03 |
| Urinary retention or postvoid residual >200 mL | 207 (9.2) | 27 (5.7) | 1.75 (1.19-2.57) | .004 |
| Heart rate >90 beats/min | 1276 (56.5) | 254 (53.6) | 1.20 (0.94-1.54) | .13 |
| Respiratory rate ≥22 breaths/min | 767 (34.0) | 169 (35.7) | 0.98 (0.73-1.33) | .92 |
| Systolic blood pressure <90 mm Hg | 286 (12.7) | 60 (12.7) | 1.09 (0.74-1.59) | .67 |
| Severity of illness | ||||
| qSOFA (≥2 vs <2)e | 273 (12.1) | 55 (11.6) | 1.12 (0.69-1.82) | .64 |
| ≥2 SIRS criteriaf | 760 (33.6) | 149 (31.4) | 1.28 (0.95-1.71) | .10 |
| Laboratory results | ||||
| Leukocytosisg | 816 (36.1) | 151 (31.9) | 1.26 (0.99-1.60) | .06 |
| Positive urinalysish | 2104 (93.1) | 382 (80.6) | 3.36 (2.44-4.62) | <.001 |
| Urine culture with ≥100 000 CFU | 1903 (84.2) | 308 (65.0) | 2.85 (2.26-3.60) | <.001 |
| Pseudomonas aeruginosa | 90 (4.0) | 14 (3.0) | 1.24 (0.69-2.25) | .47 |
| Escherichia coli | 1192 (52.8) | 180 (38.0) | 1.67 (1.35-2.07) | <.001 |
| Resistant E colii | 81 (3.6) | 8 (1.7) | 1.47 (0.90-2.37) | .12 |
Abbreviations: CFU, colony-forming unit; IQR, interquartile range; IV, intravenous; SIRS, systemic inflammatory response syndrome; qSOFA, quick sequential organ failure assessment; TNF, tumor necrosis factor.
P < .05 was considered significant.
Defined in a footnote to Table 1.
Includes transfer from subacute rehabilitation center, skilled nursing home, acute rehabilitation center, assisted living, or other hospital. Also includes whether patient had been admitted or resided in a nursing home, subacute rehabilitation center, or extended care facility in the past 30 days.
Includes Foley catheter, intermittent straight catheterization, and suprapubic catheter present on day of or 1 day before urine culture collection.
Systolic blood pressure of 100 mm Hg or lower = 1, respiratory rate of 22 or more breaths/min = 1; and Glasgow Coma Scale score less than 15 = 1.
Temperature less than 36 °C or greater than 38 °C, heart rate more than 90 beats/min, respiratory rate more than 20 breaths/min, and peripheral white blood cell count less than 4000/μL or greater than 12 000/μL (to convert to ×109 per liter, multiply by 0.001).
Defined as a peripheral white blood cell count greater than 10 000/μL.
Defined as presence of leukocyte esterase or nitrite, or more than 5 white blood cells per high-power field.
Resistant to ceftriaxone or labeled as extended-spectrum β-lactamase E coli in culture result.
On multivariable analysis, patient characteristics of older age (OR, 1.10 per 10-year increase; 95% CI, 1.02-1.18), acute AMS (OR, 1.93; 95% CI, 1.23-3.04), dementia (OR, 1.57; 95% CI, 1.15-2.13), and urinary incontinence (OR, 1.81; 95% CI, 1.36-2.41), as well as laboratory test results including a positive urinalysis (OR, 2.83; 95% CI, 2.05-3.93), E coli bacteriuria (OR, 1.42; 95% CI, 1.12-1.79), high bacteriuria colony counts (>100 000 colony-forming units per high-power field) (OR, 2.30; 95% CI, 1.83-2.91), and peripheral leukocytosis (white blood cell count >10 000 cells/μL) (OR, 1.55; 95% CI, 1.21-2.00), were associated with ASB treatment (Table 3).
Table 3. Multivariable Model of Patient Factors Associated With Treatment of Asymptomatic Bacteriuria (N = 2773).
| Variable | No. | Odds Ratio (95% CI)a | P Valueb |
|---|---|---|---|
| Patient characteristics | |||
| Age (per 10-y increase) | 1.10 (1.02-1.18) | .01 | |
| Dementia | 560 | 1.57 (1.15-2.13) | .004 |
| Incontinence | 593 | 1.81 (1.36-2.41) | <.001 |
| Acutely altered mental status | 470 | 1.93 (1.23-3.04) | .004 |
| Laboratory tests | |||
| Urine culture with Escherichia coli | 1372 | 1.42 (1.12-1.79) | .003 |
| Peripheral leukocytosisc | 967 | 1.55 (1.21-2.00) | <.001 |
| Bacteriuria ≥100 000 CFU | 2211 | 2.30 (1.83-2.91) | <.001 |
| Positive urinalysisd | 2486 | 2.83 (2.05-3.93) | <.001 |
Abbreviation: CFU, colony-forming unit.
Odds ratios greater than 1 indicate factors associated with treatment of asymptomatic bacteriuria.
P < .05 was considered significant.
Defined as white blood cell count greater than 10 000/μL (to convert to ×109 per liter, multiply by 0.001).
Defined as presence of leukocyte esterase or nitrite, or more than 5 white blood cells per high-power field.
Patient Outcomes
Within 30 days of discharge, 2375 of 2733 patients (86.9%) had follow-up terminated by a major complication or were assessed by record review and/or telephone follow-up. According to data presented in Table 4 after adjustment, no differences were found in mortality, readmission, emergency department visit, or CDI within 30 days post discharge or discharge to post–acute care facility between patients with ASB who received vs did not receive antibiotics. Most urine testing (2068/2733 [75.7%]) was performed on day 1 of hospitalization. Duration of hospitalization after urine testing was 37% longer (median, 4 vs 3 days; RR, 1.37; 95% CI, 1.28-1.47) in patients with ASB who received vs did not receive antibiotic treatment.
Table 4. Outcomes for Treatment vs No Treatment for Asymptomatic Bacteriuria (N = 2733).
| Outcomea | No. (%) | Unadjusted Odds Ratio (95% CI) | Unadjusted P Value | Adjusted Odds Ratio (95% CI) | Adjusted P Value | |
|---|---|---|---|---|---|---|
| Antibiotics (n = 2259) | No Antibiotics (n = 474) | |||||
| 30-d Postdischarge mortalityb | 63 (2.8) | 11 (2.3) | 1.22 (0.66-2.26) | .53 | 1.34 (0.72-2.49) | .35 |
| 30-d Postdischarge readmissionb | 362 (16.0) | 66 (13.9) | 1.16 (0.87-1.56) | .31 | 1.29 (0.92-1.81) | .14 |
| 30-d Postdischarge ED visitb | 272 (12.0) | 62 (13.1) | 0.91 (0.70-1.18) | .48 | 0.90 (0.66-1.24) | .52 |
| Discharge to post–acute care facilityb,c | 811 (35.9) | 102 (21.5) | 1.98 (1.58-2.48) | <.001 | 1.19 (0.90-1.57) | .22 |
| Clostridioides difficile infectiond | 14 (0.6) | 2 (0.4) | 1.39 (0.41-4.68) | .59 | 0.88 (0.20-3.86) | .86 |
| Duration of hospitalization, median (IQR), de | 4 (3-6) | 3 (2-5) | 1.37 (1.28-1.47)f | <.001 | 1.37 (1.28-1.47)f | <.001 |
Abbreviations: ED, emergency department; IQR, interquartile range.
Outcomes were adjusted for patient variables found to be significant (P < .05) and associated with treatment in the bivariate and multivariate analysis.
Mortality, readmissions, ED visits, and discharge to post–acute care facility were adjusted for age, Charlson Comorbidity Index score, hospitalization in 90 days preceding current admission, admission from nursing home, and insurance type.
Long-term acute care hospital, skilled nursing facility, inpatient rehabilitation, and subacute rehabilitation.
Infection occurring within 30 days of discharge was adjusted for age, history of antibiotic use and number of antibiotics in previous 90 days, admission from skilled nursing facility, prior hospitalization, proton-pump inhibitor use, immunosuppression, and Charlson Comorbidity Index score.
From date of urine testing (either urine culture or urinalysis, whichever was performed first). Adjusted for age, sex, Charlson Comorbidity Index score, prior hospitalization, admission from nursing home, and insurance type.
Relative risk given because because duration of hospitalization is a continuous variable.
Sensitivity Analyses
Treatment of ASB remained associated with duration of hospitalization on sensitivity analysis. When including only patients who had urine testing performed on hospital day 1 (n = 2068), treatment of ASB remained associated with longer hospitalization (median, 5 vs 3 days without treatment; RR, 1.33; 95% CI, 1.22-1.46) (eTable 2 in the Supplement). When only patients who had urine testing performed and antibiotics started on hospital day 1 were included (n = 1810), ASB treatment remained associated with longer hospitalization (median, 4 vs 3 days without treatment; RR, 1.27; 95% CI, 1.15-1.40).
Discussion
In this study of 2733 hospitalized patients with ASB across 46 hospitals, 80% or more received antibiotic treatment. Both patient and laboratory characteristics were associated with ASB treatment, including older age, acute AMS, dementia, and abnormal urinalysis results. In addition, treatment of ASB was not associated with differences in most outcomes but was associated with longer duration of hospitalization after urine testing. These findings support guideline recommendations against ASB treatment among hospitalized patients and suggest potential harm associated with treatment.
We found high treatment rates of ASB in hospitalized patients and identified patient characteristics associated with antibiotic treatment of ASB. Similar to findings in prior studies, older age and AMS were risk factors for ASB treatment.9,36 In addition, our study identified new characteristics associated with treatment: dementia and urinary incontinence. These factors, although independently associated with treatment, are more common with older age, placing elderly patients—who have the highest morbidity and mortality due to antibiotic-associated adverse events—at the highest risk of receiving inappropriate antibiotic therapy. Acute AMS and dementia are particularly challenging because patients with these conditions are often not able to reliably communicate symptoms, causing clinician hesitation in withholding treatment.12,37,38 From reports of experience in the long-term care setting, patients with bacteriuria and with acute AMS and no localizing UTI symptoms or signs of systemic infection may be observed without antibiotics for 24 to 48 hours while other potential sources of AMS (eg, dehydration, medication adverse effects) are addressed.37,38,39 Prior studies describing lower treatment rates (47%-62%) in hospitalized patients with ASB have differing or unspecified ASB definitions.7,40,41 Higher treatment rates (72%-77%) of ASB were described among institutionalized patients with dementia or studies excluding AMS as a UTI criterion.8,9,42,43 Our inclusion of this population (ASB with AMS) likely contributed to our observed higher treatment rates and enabled us to identify an association of dementia and AMS with increased ASB treatment. Our approach in patients with AMS is supported by the recently updated Infectious Diseases Society of America ASB guidelines, which addressed the impaired elderly patient with bacteriuria and delirium, recommending assessment for other causes and careful observation instead of antibiotics.3 This population would likely benefit from targeted stewardship interventions.
We also found ASB treatment to be more common in patients with certain laboratory test results. Despite common misperceptions, positive urinalysis or UC results do not define a UTI or necessitate antibiotic therapy.2,44,45,46 A negative urinalysis result makes a UTI unlikely, but a positive urinalysis does not diagnose infection given its poor positive predictive value.2,46,47 However, abnormal urinalysis was the top indication documented for UC and the strongest factor associated with treatment. These findings are consistent with prior studies that identified pyuria, abnormal urinalysis, gram-negative bacteriuria, and higher bacterial colony counts to be associated with ASB treatment.6,9,11 Furthermore, the rate of pyuria in this cohort was 91.4%, which is similar to the rate in a prior study in an elderly institutionalized population.45 Given the high prevalence of both pyuria and ASB in this population, reflex urine culturing (UC performed after abnormal urinalysis) may potentially further contribute to inappropriate antibiotic use in hospitalized elderly patients, as clinicians may be more likely to misinterpret an abnormal urinalysis linked to a positive UC.47 To decrease inappropriate testing and treatment of ASB, diagnostic stewardship interventions and education should emphasize the poor positive predictive value of abnormal urinalysis results and emphasize symptoms as the correct prompt for urine testing and UTI diagnosis.
In addition, we found no difference in mortality, readmission, emergency department visits, or CDI within 30 days in patients with ASB treated with antibiotics compared with those who received no treatment. Multiple randomized clinical trials demonstrated no improvement in outcomes with ASB treatment for certain populations (patients who are elderly, diabetic, or institutionalized), and these data have been extrapolated to the hospitalized patient.2 Data on clinical outcomes in the hospitalized population with ASB are limited.12,48,49 In addition to our study finding no clinical benefit, antibiotic treatment of ASB was associated with a 37% increase in duration of hospitalization after urine testing, suggesting potential harm and additional cost. Similarly, a recent study found that hospitalized patients with a UC on admission, irrespective of their admitting diagnosis, had longer hospital stays compared with patients with no UC.50 The association of antibiotic treatment with longer duration of hospitalization was robust in sensitivity analyses attempting to account for differences in patients with testing performed later during hospitalization. It is possible that ASB treatment may be associated with longer hospitalization because clinicians may delay discharge in stable patients while awaiting antibiotic susceptibility test results. Given that UTI is one of the most commonly treated and misdiagnosed conditions in US hospitals, a 1-day difference in length of stay would have a substantial effect on hospitalization-related costs.
Limitations and Strengths
Our study has limitations. First, because this was an observational retrospective study, we cannot prove causation. Second, ascertaining urinary symptoms is limited by the accuracy of documentation, so ASB frequency may have been overestimated. Third, by excluding concomitant infections, we may not have captured the full scope of ASB treatment in hospitalized patients. Fourth, adverse outcomes were infrequent, limiting our power to detect differences. However, given the study size, if a difference in outcomes existed, it would be small. Fifth, although we adjusted for potential confounding variables and completed sensitivity analyses for duration of hospitalization, residual confounding may still exist. Sixth, one-third of the patients had 2 or more systemic inflammatory response syndrome criteria (by definition, none had AMS or fever), and 328 of 2733 patients (12.0%) met qSOFA criteria. Patients with sepsis syndrome may warrant empirical use of antibiotics, but systemic inflammatory response syndrome criteria are neither sensitive nor specific for infection, and indiscriminate early antibiotic use may cause harm without proven benefit in less sick populations.51,52,53,54 However, the qSOFA score should prompt consideration of possible infection in patients with nonlocalizing symptoms; thus, some proportion of early antibiotic use for this population may be appropriate.
Despite these limitations, our study has multiple strengths. Trained abstractors collected data uniformly for accuracy. We also included a large, heterogeneous cohort of hospitalized patients with ASB managed at diverse hospitals for generalizability. We found significant differences in ASB treatment between hospitals, suggesting that certain stewardship strategies may help to reduce ASB treatment and warrant future study. Furthermore, outcomes were assessed by both medical record review and telephone follow-up, increasing our ability to identify adverse events. Attempts were made to minimize potential bias by inverse probability of treatment weighting by propensity scores. Because a randomized clinical trial of hospitalized patients with ASB may be unethical given the preponderance of available outpatient data arguing against treatment, this multicenter retrospective cohort may provide the best data available to address this population.
This study has important implications for stewardship policy and intervention design. It suggests that inappropriate treatment of ASB is common in hospitals. Treatment was associated with both patient symptoms (AMS, incontinence) and test results (particularly urinalysis), likely representing a misinterpretation of the UTI diagnostic criteria. No benefit associated with ASB treatment in the hospitalized patient was identified, but potential harm includes an association with longer duration of hospitalization. Stewardship efforts should prioritize ASB, targeting older patients with dementia. Inappropriate antibiotic use has been associated with harm; these findings add to the growing body of literature supporting a less-is-more approach, especially in elderly patients who are at higher risk of experiencing adverse drug events. Furthermore, to reduce ASB treatment, programs should consider diagnostic stewardship interventions addressing decreasing urine testing in asymptomatic patients (eg, through computerized decision support and education).
Conclusions
Inappropriate antibiotic treatment of ASB is common among hospitalized patients. Treatment was associated with certain patient characteristics (elderly, dementia, and AMS) and laboratory test findings (particularly abnormal urinalysis results), which were possibly related to a misunderstanding of the diagnostic criteria for UTI. Treatment did not improve clinical outcomes; rather, it may have been associated with an increase in duration of hospitalization after urine testing. Our findings suggest that, to reduce inappropriate antibiotic use, stewardship efforts should focus on improving urine testing practices and management strategies for stable elderly patients with AMS.
eAppendix. Outcome Adjustment Variables
eReferences.
eFigure. Flow Diagram of Study Population
eTable 1. Top Ten Primary Admitting and Discharge Diagnoses Among Treated vs Not Treated Asymptomatic Bacteriuria Patients, N = 2733
eTable 2. Sensitivity Analyses for Duration of Hospital Stay after Urine Testing for Treatment vs No Treatment of Asymptomatic Bacteriuria, N = 2733
References
- 1.Magill SS, Edwards JR, Beldavs ZG, et al. ; Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. doi: 10.1001/jama.2014.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM; Infectious Diseases Society of America; American Society of Nephrology; American Geriatric Society . Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40(5):643-654. doi: 10.1086/427507 [DOI] [PubMed] [Google Scholar]
- 3.Nicolle LE, Gupta K, Bradley SF, et al. . Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of America. Clin Infect Dis. 2019;68(10):1611-1615. doi: 10.1093/cid/ciy1121 [DOI] [PubMed] [Google Scholar]
- 4.Fridkin S, Baggs J, Fagan R, et al. ; Centers for Disease Control and Prevention (CDC) . Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200. [PMC free article] [PubMed] [Google Scholar]
- 5.Köves B, Cai T, Veeratterapillay R, et al. . Benefits and harms of treatment of asymptomatic bacteriuria: a systematic review and meta-analysis by the European Association of Urology Urological Infection Guidelines Panel. Eur Urol. 2017;72(6):865-868. doi: 10.1016/j.eururo.2017.07.014 [DOI] [PubMed] [Google Scholar]
- 6.Flokas ME, Andreatos N, Alevizakos M, Kalbasi A, Onur P, Mylonakis E. Inappropriate management of asymptomatic patients with positive urine cultures: a systematic review and meta-analysis. Open Forum Infect Dis. 2017;4(4):ofx207. doi: 10.1093/ofid/ofx207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury F, Sarkar K, Branche A, et al. . Preventing the inappropriate treatment of asymptomatic bacteriuria at a community teaching hospital. J Community Hosp Intern Med Perspect. 2012;2(2). doi: 10.3402/jchimp.v2i2.17814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartley S, Valley S, Kuhn L, et al. . Overtreatment of asymptomatic bacteriuria: identifying targets for improvement. Infect Control Hosp Epidemiol. 2015;36(4):470-473. doi: 10.1017/ice.2014.73 [DOI] [PubMed] [Google Scholar]
- 9.Spivak ES, Burk M, Zhang R, et al. ; Management of Urinary Tract Infections Medication Use Evaluation Group . Management of bacteriuria in Veterans Affairs hospitals. Clin Infect Dis. 2017;65(6):910-917. doi: 10.1093/cid/cix474 [DOI] [PubMed] [Google Scholar]
- 10.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308-1315. doi: 10.1001/jamainternmed.2017.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin E, Bhusal Y, Horwitz D, Shelburne SA III, Trautner BW. Overtreatment of enterococcal bacteriuria. Arch Intern Med. 2012;172(1):33-38. doi: 10.1001/archinternmed.2011.565 [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta M, Brymer C, Elsayed S. Treatment of asymptomatic UTI in older delirious medical in-patients: a prospective cohort study. Arch Gerontol Geriatr. 2017;72:127-134. doi: 10.1016/j.archger.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 13.Drekonja DM, Abbo LM, Kuskowski MA, Gnadt C, Shukla B, Johnson JR. A survey of resident physicians’ knowledge regarding urine testing and subsequent antimicrobial treatment. Am J Infect Control. 2013;41(10):892-896. doi: 10.1016/j.ajic.2013.01.020 [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Kim M, Kim NH, et al. . Why is asymptomatic bacteriuria overtreated? a tertiary care institutional survey of resident physicians. BMC Infect Dis. 2015;15:289. doi: 10.1186/s12879-015-1044-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trautner BW, Petersen NJ, Hysong SJ, Horwitz D, Kelly PA, Naik AD. Overtreatment of asymptomatic bacteriuria: identifying provider barriers to evidence-based care. Am J Infect Control. 2014;42(6):653-658. doi: 10.1016/j.ajic.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 16.Flanders SA, Greene MT, Grant P, et al. . Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. doi: 10.1001/jamainternmed.2014.3384 [DOI] [PubMed] [Google Scholar]
- 17.Greene MT, Flanders SA, Woller SC, Bernstein SJ, Chopra V. The association between PICC use and venous thromboembolism in upper and lower extremities. Am J Med. 2015;128(9):986-993.e1. doi: 10.1016/j.amjmed.2015.03.028 [DOI] [PubMed] [Google Scholar]
- 18.Vaughn VMGT, Gandhi T, Conlon A, Chopra V, Malani AN, Flanders SA. The association of antibiotic stewardship with fluoroquinolone prescribing in Michigan hospitals: a multi-hospital cohort study [published online February 13, 2019]. Clin Infect Dis. doi: 10.1093/cid/ciy1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Healthcare Safety Network (NHSN) Long-term Care Facility Component: Tracking Infections in Long-term Care Facilities. Atlanta, GA: Division of Healthcare Quality Promotion, National Center for Emerging and Zoonotic Infectious Diseases; May 2018. https://www.cdc.gov/nhsn/pdfs/ltc/ltcf-manual-508.pdf. Accessed July 25, 2019. [Google Scholar]
- 20.Dellinger RP, Levy MM, Rhodes A, et al. ; Surviving Sepsis Campaign Guidelines Committee Including the Pediatric Subgroup . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580-637. doi: 10.1097/CCM.0b013e31827e83af [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Fink MP, Marshall JC, et al. ; SCCM/ESICM/ACCP/ATS/SIS . 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250-1256. doi: 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 22.McDonald LC, Gerding DN, Johnson S, et al. . Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48. doi: 10.1093/cid/cix1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buckland ST, Burnham KP, Augustin NH. Model selection: an integral part of inference. Biometrics. 1997;53(2):603-618. doi: 10.2307/2533961 [DOI] [Google Scholar]
- 24.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Billings J, Dixon J, Mijanovich T, Wennberg D. Case finding for patients at risk of readmission to hospital: development of algorithm to identify high risk patients. BMJ. 2006;333(7563):327. doi: 10.1136/bmj.38870.657917.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comette P, D’Hoore W, Malhomme B, Van Pee D, Meert P, Swine C. Differential risk factors for early and later hospital readmission of older patients. Aging Clin Exp Res. 2005;17(4):322-328. doi: 10.1007/BF03324617 [DOI] [PubMed] [Google Scholar]
- 27.Allaudeen N, Vidyarthi A, Maselli J, Auerbach A. Redefining readmission risk factors for general medicine patients. J Hosp Med. 2011;6(2):54-60. doi: 10.1002/jhm.805 [DOI] [PubMed] [Google Scholar]
- 28.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: Atherosclerosis Risk in Communities cohort (1987 to 2004). Circ Heart Fail. 2011;4(3):308-316. doi: 10.1161/CIRCHEARTFAILURE.110.959031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vassallo A, Tran MC, Goldstein EJ. Clostridium difficile: improving the prevention paradigm in healthcare settings. Expert Rev Anti Infect Ther. 2014;12(9):1087-1102. doi: 10.1586/14787210.2014.942284 [DOI] [PubMed] [Google Scholar]
- 30.Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med. 2016;176(12):1801-1808. doi: 10.1001/jamainternmed.2016.6193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCollum DL, Rodriguez JM. Detection, treatment, and prevention of Clostridium difficile infection. Clin Gastroenterol Hepatol. 2012;10(6):581-592. doi: 10.1016/j.cgh.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 32.Shayne M, Culakova E, Poniewierski MS, et al. . Risk factors for in-hospital mortality and prolonged length of stay in older patients with solid tumor malignancies. J Geriatr Oncol. 2013;4(4):310-318. doi: 10.1016/j.jgo.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 33.Damrauer SM, Gaffey AC, DeBord Smith A, Fairman RM, Nguyen LL. Comparison of risk factors for length of stay and readmission following lower extremity bypass surgery. J Vasc Surg. 2015;62(5):1192-200.e1. doi: 10.1016/j.jvs.2015.06.213 [DOI] [PubMed] [Google Scholar]
- 34.Rubin DB. Multiple imputations in sample surveys—a phenomenological bayesian approach to nonresponse. Proceedings of the Survey Research Methods Section of the American Statistical Association; 1978. http://www.asasrms.org/Proceedings/papers/1978_004.pdf. Accessed July 23, 2019. [Google Scholar]
- 35.Angus DC, Seymour CW, Coopersmith CM, et al. . A framework for the development and interpretation of different sepsis definitions and clinical criteria. Crit Care Med. 2016;44(3):e113-e121. doi: 10.1097/CCM.0000000000001730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trautner BW, Bhimani RD, Amspoker AB, et al. . Development and validation of an algorithm to recalibrate mental models and reduce diagnostic errors associated with catheter-associated bacteriuria. BMC Med Inform Decis Mak. 2013;13:48. doi: 10.1186/1472-6947-13-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie R, Stewart MT, Bellantoni MF, Finucane TE. Bacteriuria in individuals who become delirious. Am J Med. 2014;127(4):255-257. doi: 10.1016/j.amjmed.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 38.Mody L, Juthani-Mehta M. Urinary tract infections in older women: a clinical review. JAMA. 2014;311(8):844-854. doi: 10.1001/jama.2014.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nace DA, Drinka PJ, Crnich CJ. Clinical uncertainties in the approach to long term care residents with possible urinary tract infection. J Am Med Dir Assoc. 2014;15(2):133-139. doi: 10.1016/j.jamda.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 40.Gau JT, Shibeshi MR, Lu IJ, et al. . Interexpert agreement on diagnosis of bacteriuria and urinary tract infection in hospitalized older adults. J Am Osteopath Assoc. 2009;109(4):220-226. [PubMed] [Google Scholar]
- 41.Kelley D, Aaronson P, Poon E, McCarter YS, Bato B, Jankowski CA. Evaluation of an antimicrobial stewardship approach to minimize overuse of antibiotics in patients with asymptomatic bacteriuria. Infect Control Hosp Epidemiol. 2014;35(2):193-195. doi: 10.1086/674848 [DOI] [PubMed] [Google Scholar]
- 42.Dufour AB, Shaffer ML, D’Agata EM, Habtemariam D, Mitchell SL. Survival after suspected urinary tract infection in individuals with advanced dementia. J Am Geriatr Soc. 2015;63(12):2472-2477. doi: 10.1111/jgs.13833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Agata E, Loeb MB, Mitchell SL. Challenges in assessing nursing home residents with advanced dementia for suspected urinary tract infections. J Am Geriatr Soc. 2013;61(1):62-66. doi: 10.1111/jgs.12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nicolle LE. Consequences of asymptomatic bacteriuria in the elderly. Int J Antimicrob Agents. 1994;4(2):107-111. doi: 10.1016/0924-8579(94)90042-6 [DOI] [PubMed] [Google Scholar]
- 45.Rodgers K, Nicolle L, McIntyre M, Harding G, Hoban D, Murray D. Pyuria in institutionalized elderly subjects. Can J Infect Dis. 1991;2(4):142-146. doi: 10.1155/1991/139202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta K, Hooton TM, Naber KG, et al. ; Infectious Diseases Society of America; European Society for Microbiology and Infectious Diseases . International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52(5):e103-e120. doi: 10.1093/cid/ciq257 [DOI] [PubMed] [Google Scholar]
- 47.Humphries RM, Dien Bard J. Reflex cultures reduce laboratory workload and improve antimicrobial stewardship in patients suspected of having urinary tract infections. J Clin Microbiol. 2016;54(2):254-258. doi: 10.1128/JCM.03021-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leis JA, Rebick GW, Daneman N, et al. . Reducing antimicrobial therapy for asymptomatic bacteriuria among noncatheterized inpatients: a proof-of-concept study. Clin Infect Dis. 2014;58(7):980-983. doi: 10.1093/cid/ciu010 [DOI] [PubMed] [Google Scholar]
- 49.Collins CD, Kabara JJ, Michienzi SM, Malani AN. Impact of an antimicrobial stewardship care bundle to improve the management of patients with suspected or confirmed urinary tract infection. Infect Control Hosp Epidemiol. 2016;37(12):1499-1501. doi: 10.1017/ice.2016.199 [DOI] [PubMed] [Google Scholar]
- 50.Horstman MJ, Spiegelman AM, Naik AD, Trautner BW. Urine culture on admission impacts antibiotic use and length of stay: a retrospective cohort study. Infect Control Hosp Epidemiol. 2018;39(5):547-554. doi: 10.1017/ice.2018.55 [DOI] [PubMed] [Google Scholar]
- 51.Klompas M, Calandra T, Singer M. Antibiotics for sepsis—finding the equilibrium. JAMA. 2018;320(14):1433-1434. doi: 10.1001/jama.2018.12179 [DOI] [PubMed] [Google Scholar]
- 52.Marik PE, Farkas JD, Spiegel R, Weingart S. POINT: should the Surviving Sepsis Campaign guidelines be retired? yes. Chest. 2019;155(1):12-14. doi: 10.1016/j.chest.2018.10.008 [DOI] [PubMed] [Google Scholar]
- 53.Prescott HC, Iwashyna TJ. Improving sepsis treatment by embracing diagnostic uncertainty. Ann Am Thorac Soc. 2019;16(4):426-429. doi: 10.1513/AnnalsATS.201809-646PS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singer M, Deutschman CS, Seymour CW, et al. . The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Outcome Adjustment Variables
eReferences.
eFigure. Flow Diagram of Study Population
eTable 1. Top Ten Primary Admitting and Discharge Diagnoses Among Treated vs Not Treated Asymptomatic Bacteriuria Patients, N = 2733
eTable 2. Sensitivity Analyses for Duration of Hospital Stay after Urine Testing for Treatment vs No Treatment of Asymptomatic Bacteriuria, N = 2733