SUMMARY
Protection against Mesocestoides corti, a cestode that invades vital organs, is dependent on the production of IL-4, as IL-4−/− mice were found to have higher parasite burdens when compared with wild-type mice. The goal of this study was to investigate the role of IL-4 in immunity to M. corti, focusing on the immunological profile and on potential mediators of pathology. IL-4−/− mice infected with M. corti showed 100% mortality by 32 days, whereas wild-type mice survived for approximately 1 year. Parasite burdens were significantly increased in the liver, peritoneal, and thoracic cavities of IL-4−/− mice, associated with impaired recruitment of inflammatory cells and a reduction in monocytes and macrophages. IL-5 production by splenocytes and expression in liver tissue was decreased in infected IL-4−/− mice compared with wild-type mice. In contrast, IL-4−/− mice produced increased amounts of IFNγ and TNFα. Alternatively activated macrophages were a major feature of liver granulomas in wild-type mice evidenced by Arginase I expression, while livers from infected IL-4−/− mice showed impaired alternative macrophage activation without increased classical macrophage activation. Thus, lethality during M. corti infection of IL-4−/− mice is associated with decreased Th2 cytokines, increased Th1 cytokines and impairment of alternatively activated macrophages.
Keywords: alternatively activated macrophages, classically activated macrophages, interleukin-4, Mesocestoides corti
INTRODUCTION
Mesocestoides corti is a tapeworm parasite that invades the host liver, causing hepatocyte destruction as the parasites reproduce via budding (1). Fibrosis develops around the parasite, after which a regenerative phase of hepatocyte proliferation occurs that may control excessive damage caused by invading parasites (2). Mesocestoides corti infected IL-4−/− mice produce increased amounts of Th1-associated immunoglobulins and decreased production of Th2-associated immunoglobulins (3). Indeed, IL-4−/− mice were found to have higher parasite burdens when compared with wild-type mice.
Immune responses during parasitic infection can be generalized into two groups: T helper (Th) type 1 vs. 2. Th1 responses are important for control of many intracellular parasite infections, such as Leishmania species, and are characterized by the production of high levels of IL-12, IFNγ, and TNFα (4). On the other hand, Th2 responses are associated with immune control of most extracellular helminths and are characterized by high levels of IL-4, IL-5, and IL-13 production (5). IL-4 has been shown to promote host protection in a number of Th2-associated helminth infections. Studies in IL-4−/− mice support the conclusion that IL-4 is important for control of infection with the nematodes Litomosoides sigmodontis (6) and Onchocerca volvulus (7), as well as the trematode Schistosoma mansoni (8).
Th2 derived cytokines such as IL-4 and IL-13 can promote the development of alternatively activated macrophages (AAMφ). These macrophages have been shown to regulate T helper cell activation and function to promote the release of IL-4, IL-13, and IL-5 (9). AAMφ are characterized by an increased expression of Arginase-1, FIZZ-1, and Ym-1 mRNA, increased surface expression of mannose receptor, and decreased levels of nitric oxide synthase 2 (iNOS), which is in stark contrast to classically activated macrophages (CAMφ) induced by cytokines released during Th1 responses (10–14). The development of AAMφ has been associated with both trematode and nematode infections, where it is coincident with development of Th2 cytokine responses and IL-4 production (10,12,15–17).
The goal of the present study to investigate the role of IL-4 in host-immunity to M. corti, specifically focusing on the immunological profile and on potential mediators of pathology Mesocestoides corti infected IL-4−/− mice succumb to infection within the first month, while wild-type mice live for over a year. IL-4−/− mice infected with M. corti were evaluated for the development of both T helper cell responses and macrophage subpopulations. While wild-type mice infected with M. corti mount an effective Th2 response featuring AAMφ development, infected IL-4−/− mice develop a Th1 response and lack both CAMφ and AAMφ in parasitized tissue.
MATERIALS AND METHODS
Mice and parasites
C57BL/6 and IL-4−/− mice on a B6 background (B6·129P2-Il4tm1Cgn/J) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). All mice were between 5 and 10 weeks of age at the time of infection and controls were matched by sex and weight within the experiments. Mesocestoides corti tetrathyridia were maintained by injecting 100 μL of packed tetrathyridia (approximately 600 parasites) into the peritoneal cavity of wild-type C57BL/6 mice (18). Parasites were collected by PBS lavage of the peritoneal cavity of infected mice followed by washing with PBS.
Experimental infection
Wild-type C57BL/6 and IL-4−/− mice were infected intraperitoneally with approximately 600 tetrathyridia. At various time points from 0 to 28 days, mice were anesthetized using isoflurane (Webster veterinary, Sterling, MA, USA) and euthanized by exsanguination. The peritoneal cavity was washed with PBS, and the fluid collected for determination of parasite and cell numbers. Differential cell analyses were performed by spinning cells onto slides using a Cytospin 3 centrifuge (Thermo Shandon, Pittsburgh, PA, USA) and staining with DiffQuik (Baxter Healthcare, Miami, FL, USA). Parasites collected from the peritoneal and thoracic cavities were counted under a dissecting microscope. Portions of the mouse liver, removed for determination of parasite load, were weighed and incubated overnight at 37°C in 2·5% trypsin in PBS. The following day, the tissue was dissected and parasite numbers were determined by counting under a dissection microscope.
Histology
Portions of the liver and lung tissue were removed following the experiment, fixed in formalin, and sectioned. Sections were then stained using hematoxylin and eosin or Van Gieson’s stain, which stains collagen red (19). Stained sections were examined in a blinded manner, and a grade was assigned based on the degree of fibrosis present in the tissue. The degree of fibrosis was graded based on an estimate of the percentage of the liver parenchyma that was replaced by collagen, ignoring the volume of parasites present. Grade 1: 5%, grade 2: 10–12%, grade 3: 20–25%, grade 4: 30–35%, and grade 5: 40%.
Immunohistochemistry
Three to five micron tissue sections of liver from M. corti-infected mice were de-paraffinized and re-hydrated using standard techniques. Blocking was performed with the M.O.M. immunodetection kit (Vector laboratories, Burlingame, CA, USA) or 3% Goat serum and incubated with anti-mouse Arginase I mAb (BD Signal Transduction laboratories, San Diego, CA, USA) and rat-anti mouse F4/80 (eBioscience, San Diego, CA, USA) followed by trypsin-based antigen retrieval. Isotype matched control antibodies were used to confirm specificity. Following incubation with primary antibodies at 4°C overnight, slides were washed with 1× PBS and antibodies detected with Cy3-donkey anti-mouse, or Cy3-donkey anti-rat mAb (Jackson ImmunoResearch, West Grove, PA, USA), treated with 4′,6-diamidino-2-phenylindole (DAPI). Images were photographed using a Nikon Eclipse E600 compound microscope fitted with a SPOT™ diagnostics digital camera and software (Nikon Precision Inc., Melville, NY, USA).
Preparation of M. corti specific antigen
Mesocestoides corti somatic antigen (SOM Ag) was prepared by homogenizing 1 mL of packed M. corti tetrathyridia in a homogenizer with 5 mL of sterile PBS. The resulting homogenate was filtered using a sterile 25 mm prefilter (Nalgene, Rochester, NY, USA), followed by a sterile 0·22 μm filter (Nalgene). The protein concentration was determined by micro BCA (Pierce, Rockford, IL, USA).
Spleen cell stimulation
Spleens were harvested from infected and control wild-type and IL-4−/− mice. Single-cell suspensions were prepared and cells were resuspended at 5 × 106 cells/mL in complete T-cell medium [Dulbecco’s modified Eagle’s minimal essential medium containing 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine (all from Mediatech, Herndon, VA, USA), 5 × 10−5 M 2-ME (Sigma-Aldrich, St Louis, MO, USA), and 10% fetal calf serum (HyClone, Logan, UT, USA)] with or without the addition of M. corti SOM Ag, and cultured in 96-well round-bottom plates at 37°C and 5% CO2. Culture supernatants were harvested at 72 h for cytokine analysis.
Cytokine detection
Cytokine ELISAs were performed using paired mAb in combination with recombinant cytokine standards (BD Pharmingen, San Diego, CA, USA), according to the manufacturer’s protocol. Briefly, capture antibody (IL-5 – TRFK5; IFNγ – R4–6A2) was added to 96 well plates, which were then incubated with serial dilutions of standard or samples. Detection antibody (IL-5 – TRFK4; IFNγ – XMG1–2) was then added and absorbance was determined by an ELISA reader.
Quantitative PCR
Sections of liver tissue from experimentally infected mice were removed and placed in 10 volumes of RNALater (Ambion, Foster City, CA, USA) and stored at −80°C until RNA purification was carried out. The total RNA was purified using Total RNA Chemistry (Applied Biosystems, Foster City, CA, USA). Eight μg total RNA was reverse transcribed into cDNA using a High Capacity Reverse Transcription Kit (Applied Biosystems). For quantitative PCR, 40 ng total RNA reverse transcribed to cDNA was added to 12·5 μL of Taqman Fast Universal Master Mix (2x), 1·25 μL of the specified Taqman Gene Expression Assay (Applied Biosystems), and water to a final reaction volume of 25 μL. Reactions were performed on the Applied Biosystems 7500 Fast Real-Time PCR System with the following reaction conditions: 95°C for 20 s followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. All technical replicates were done in triplicate. Genes were normalized via relative quantitation using β actin (Applied Biosystems Gene Expression Assay ID Mm00607939_s1) as the endogenous control. Other Applied Biosystems Gene Expression Assay IDs are as follows: Arginase-1 Mm00475988_m1; IL-5, Mm00439646_m1; IL-13, Mm00434204_m1; TNFα, Mm00443258_m1; IFNγ, Mm00801778_m1; FIZZ-1 (Retnla), Mm00445109_m1; Ym-1 (Chi313), Mm00657889_mH; and iNOS, Mm00440485_m1.
Statistical analyses
For all experiments, MGLH multifactorial anova with Fisher’s least-significant-difference test for post hoc analysis was used to compare results between groups and time points. P values of ≤0·05 were considered significant for all experiments. Data are representative of at least three experiments with similar outcomes and each group contained between 3 and 5 animals.
RESULTS
Parasite burden and host cell populations during M. corti infection in IL-4−/− mice
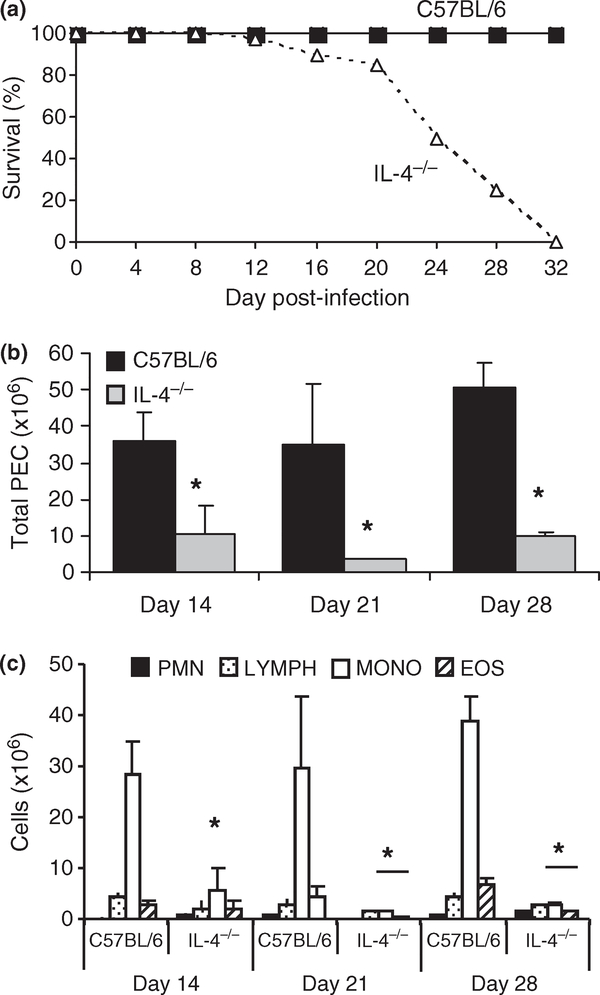
Mesocestoides corti tetrathyridia were injected intraperitoneally into IL-4−/− and wild-type C57BL/6 mice. All of the infected IL-4−/− mice died by day 32 post-infection, while wild-type C57BL/6 mice continued to survive for approximately 1 year (Figure 1a and data not shown). Comparing the numbers of parasites in the peritoneal cavity, liver, and lung with wild-type mice revealed significantly increased parasite burdens in all sites by day 21 (Table 1). The total number of cells in the peritoneal cavity was significantly decreased in infected IL-4−/− mice (Figure 1b). The decrease in the total number of cells in the peritoneal cavity was a reflection of the decrease in recruitment of the monocyte/macrophage population, which at day 14 was only 9 million cells per mL in the IL-4−/− mice compared to 35 million in the wild-type mice (Figure 1c). Similar differences were also seen at days 21 and 28.
Figure 1.
Lethal Mesocestoides corti infection in IL-4−/− mice is associated with decreased numbers of host inflammatory cells. C57BL/6 and IL-4−/− mice were infected intraperitoneally with approximately 600 M. corti tetrathyridia and followed for 32 days (a). The number of peritoneal exudate cells was determined (b). The total number of neutrophils, lymphocytes, monocytes/macrophages and eosinophils within the peritoneal exudate cell population was determined. (c) Data are a compilation of four experiments with equal numbers of wild-type and knock out mice. Asterisks indicate statistical difference from C57BL/6 mice as determined by anova (P < 0·05).
Table 1.
IL-4 −/− mice have higher Mesocestoides corti parasite loads than C57BL/6 mice in liver, peritoneal cavity, and thoracic cavity following infection
| Days post-infection |
Mesocestoides corti parasite counta |
|||||
|---|---|---|---|---|---|---|
| Liver (tetrathyridia/gram) |
Peritoneal cavity |
Thoracic cavity |
||||
| C57BL/6 | IL-4−/− | C57BL/6 | IL-4−/− | C57BL/6 | IL-4−/− | |
| 14 | 174 ± 93 | 536 ± 261 | 1212 ± 250 | 1838 ± 142 | 14 ± 5 | 38 ± 25 |
| 21 | 1186 ± 426 | 6183 ± 1925b | 2134 ± 793 | 4119 ± 797b | 39 ± 10 | 162 ± 65b |
| 28 | 1573 ± 513 | 8854 ± 2142b | 2644 ± 620 | 10561 ± 1290b | 80 ± 9 | 782 ± 80b |
Parasite counts are displayed as mean ± SD of at least four mice per group, and are representative of four separate experiments
Significantly different from parasite count for infected C57BL/6 mice (P ≤0·05) as determined by anova.
Cytokine profile of spleen and liver from M. corti infected IL-4−/− mice
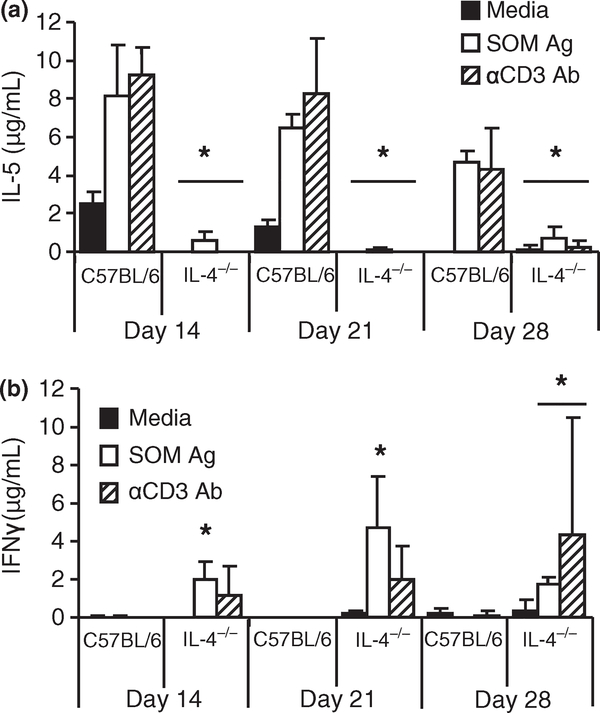
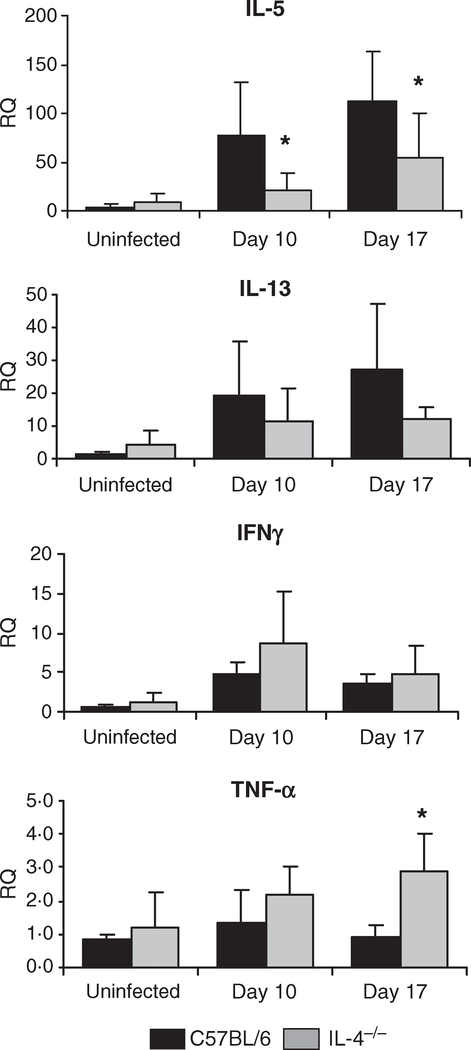
Spleen cell cytokine responses were measured to determine the type of T cell response induced by the parasite in wild-type and IL-4−/− mice. Th1 and Th2 cytokine production by spleen cells from infected mice was measured after stimulation with M. corti SOM Ag or anti-CD3 mAb. Stimulated lymphocytes from infected wild-type C57BL/6 mice produced greater amounts of IL-5 than those from IL-4−/− mice, while IL-4−/− mice produced significantly higher levels of IFNγ than wild-type mice (Figure 2). Quantitative PCR was used to measure cytokine mRNA in the liver to monitor the immune response in the infected organ. Relative expression of IL-5 mRNA was lower in IL-4−/− mice than in wild-type mice, replicating the results seen with the spleen cell stimulation. Neither IL-13 nor IFNγ expression was significantly different in IL-4−/− mice compared to wild-type mice. Conversely, TNFα mRNA levels were significantly increased in the livers of IL-4−/− mice on day 17 post-infection, but not at the earlier time points examined (Figure 3). These results indicate that there was a reduction in the Th2 response with a concomitant increase in the Th1 response in IL-4−/− mice infected with M. corti.
Figure 2.
Stimulation of spleen cells from Mesocestoides corti infected C57BL/6 and IL-4−/− mice. Production of IL-5 (a) and IFNγ (b) following spleen cell stimulation with media, M. corti somatic antigen, or anti-CD3 antibody was measured by ELISA. The data displayed as mean ± SD and are representative of two experiments with each group containing 3–5 samples. A bar across a set indicates all groups below bar were statistically different from wild-type mice. Asterisks indicate statistical difference between IL-4−/− mice and C57BL/6 mice as determined by anova (P < 0·05).
Figure 3.
Expression of liver cytokine mRNA from Mesocestoides corti infected C57BL/6 and IL-4−/− mice. Liver mRNA from M. corti infected C57BL/6 and IL-4 KO mice was isolated and reverse transcribed into cDNA, then analyzed by quantitative PCR. Shown are the relative quantities of IL-5, IL-13, TNFα, and IFNα gene expression after normalization to β-actin and standardization of the relative amount against a day 0 C57BL/6 sample. Statistical differences between IL-4−/− and C57BL/6 mice at a given time point are indicated by asterisks (P < 0·05 as determined by anova).
Histologic changes in the liver during M. corti infection in IL-4−/− mice
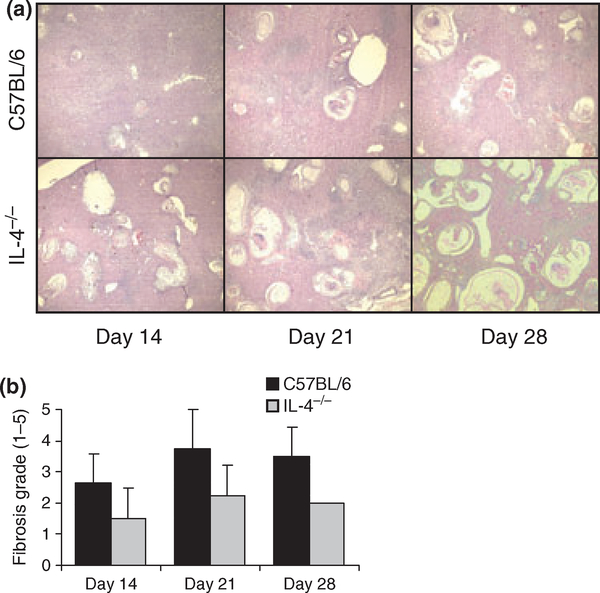
To further investigate the interaction between M. corti and host cells in IL-4−/− mice, histologic analyses were performed on liver tissues. Liver sections from IL-4−/− mice had significantly increased numbers of parasites when compared with wild-type C57BL/6 mice at all time points examined (Figure 4a). Liver sections stained with Van Gieson’s stain to identify collagen were graded in a blinded manner. No significant differences in fibrosis in the liver were apparent between IL-4−/− and C57BL/6 mice at any time point (Figure 4b).
Figure 4.
Histology of livers from C57BL/6 and IL-4−/− mice following infection with Mesocestoides corti. Liver samples from 14, 21, and 28 days post-infection in C57BL/6 wild-type and IL-4KO mice, displayed at low magnification (40×), demonstrate levels of parasite infiltration (a). Sections selected are representative of other sections prepared from the same mouse strain and time point. Levels of fibrosis in each liver sample were graded in a blinded fashion (b). The displayed results are the averages of five mice for each time point, and grading was done on liver regions which were representative of a median section of parasite infiltration. Data are displayed as mean ± SD.
Expression of macrophage markers in the liver during M. corti infection in IL-4−/− mice
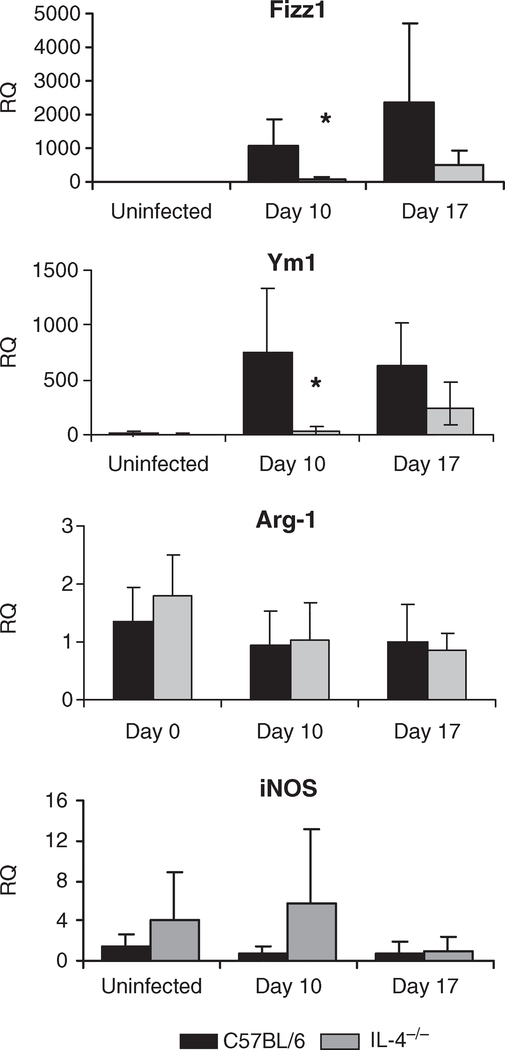
The expression of AAMφ markers in the liver during M. corti infection was assessed using quantitative PCR. Wild-type C57BL/6 mice had elevated levels of Ym-1 and Fizz-1 mRNA, while expression was significantly lower in the infected IL-4−/− mice (Figure 5). There was no difference in the expression of Arg-1 between the two mouse strains, and very low copy numbers were detected in both mice groups. iNOS was also expressed at a relatively low frequency in both IL-4−/− and wild-type mice, and the level of expression was not statistically different.
Figure 5.
Expression of mRNA for macrophage markers in the livers from C57BL/6 and IL-4−/− mice infected with Mesocestoides corti. Liver mRNA from M. corti infected C57BL/6 and IL-4−/− mice was isolated and reverse transcribed into cDNA, then analyzed by quantitative PCR. Shown are the relative quantities of Fizz-1 (Retnla), Ym-1 (Chi3l3), Arginase 1 (Arg 1) and iNOS (NOS2) gene expression after normalization to b actin and standardization of the relative amount against a day 0 C57BL/6 sample. Statistical differences between IL-4−/− and C57BL/6 mice at a given time point are indicated by asterisks (P < 0·05 as determined by anova).
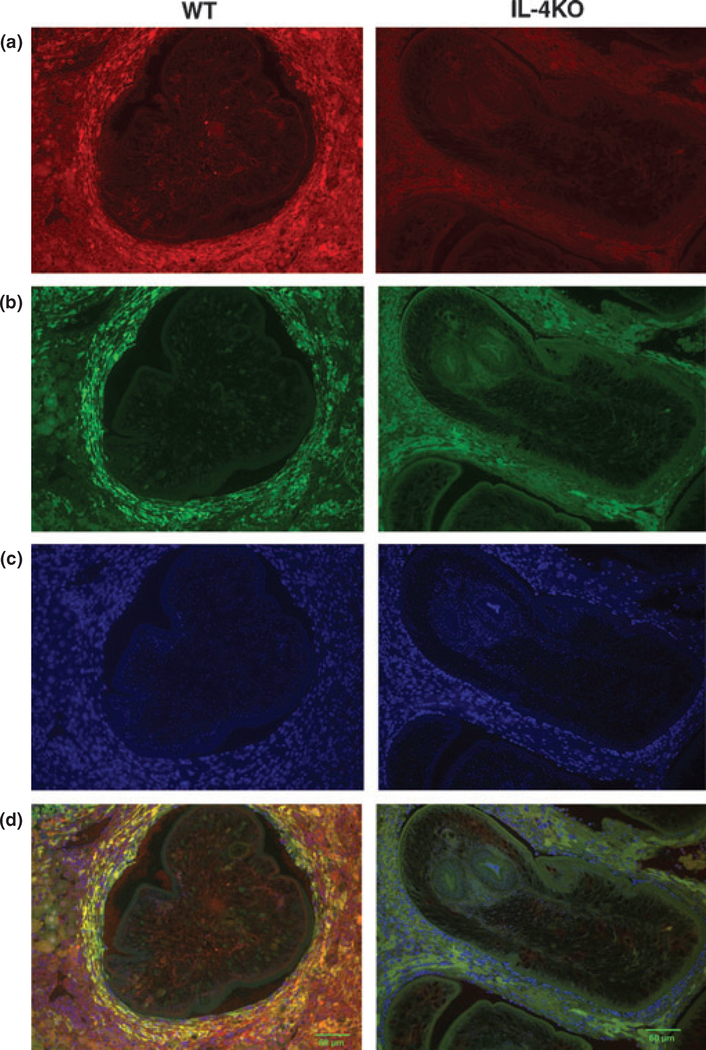
Immunohistochemistry was employed to specifically assess the quantity and phenotype of macrophage populations within the liver granulomas of infected mice. Consistent with observations in the peritoneal cavity, the total number of macrophages in IL-4−/− mice was markedly reduced compared to wild-type controls (Figure 6a). Conversely, there was a marked accumulation of Arginase I positive cells in liver granulomas of wild-type vs. IL-4−/− mice (Figure 6b), which co-localized with the F4/80 staining pattern (Figure 6d). Levels of iNOS, however, were produced at low levels in both the wild-type and IL-4−/− mice, with no significant differences observed between strains.
Figure 6.
IL-4-dependent alternatively activated macrophages surround Mesocestoides corti in liver. Immunohistochemical detection of F4/80 ± macrophages (a) (shown in red) surrounding M. corti larvae in liver at day 14 post-inoculation in C57BL/6 and IL-4−/− mice. Arginase-1 (b) was used as a marker for alternatively activated macrophages (shown in green) and cell nuclei (c) were stained with DAPI (shown in blue). Merged image (d) demonstrates Arginase-1 co-localization in macrophages in C57BL/6, but not in IL-4−/− mice. Representative photos of 50 granulomas examined per strain.
DISCUSSION
This study demonstrates that IL-4 is critical for host protection of mice infected with the parasitic cestode M. corti. IL-4−/− mice showed 100% mortality by 32 days following M. corti infection, with a highly significant increase of tetrathyridia in liver, peritoneal, and thoracic cavity, compared to wild-type mice. This is consistent with a previous study where IL-4−/− mice infected with M. corti developed elevated infection levels, although that study did not show increased mortality. (3). Differences in IL-4−/− mouse survival in these two studies may be explained by differences in initial infection levels and/or experiment duration. Long term infection with M. corti in wild-type C57BL/6 mice leads to extremely high parasite loads. After 3 months of infection in wild-type mice, the liver parasite load was approximately 4000 tetrathryidia per gram of liver (personal observation), yet the mice did not succumb to infection. This observation is consistent with our hypothesis that increased mortality in IL-4−/− mice is because of the host-mediated inflammatory response instead of tissue destruction caused by parasite infection.
IL-4−/− mice infected with M. corti have a shift in the development of antibody isotypes from IgG1 and IgE to IgG2a and IgG2b compared to wild-type mice (3), which indicates a shift from a Th2 to a Th1 response during infection. Indeed, this study demonstrates impaired IL-5 production from splenocytes and decreased IL-5 mRNA levels in hepatic tissue, which indicates a defective Th2 response in the IL-4−/− mice. IFNγ production by splenocytes was significantly increased; however, elevated expression of IFNγ was not clearly demonstrated in the liver. TNFα expression also increased in the liver, in accordance with an increased Th1 cytokine production. The expression of IL-13 by IL-4−/− mice was not different than wild-type mice, which correlates with the observation of no differences in the liver fibrosis levels between mouse strains, as IL-13 levels are known to influence the development of fibrosis (20). Thus, M. corti infected IL-4−/− mice generate an immune response that is shifted from a Th2 to a Th1 response as determined by analysis of spleen and hepatic tissue.
In particular, there was a marked reduction of macrophages, and in particular AAMφ, surrounding parasites within liver granulomas due to the absence of IL-4. Whereas, macrophages from infected wild-type mice expressed high levels of Arginase I that was in close apposition to tetrathyridia, this was not observed in similarly infected IL-4−/− mice. IL-4−/− mice did not show an increase of iNOS, as determined by quantitative PCR and immunohistochemistry, thus, we conclude that neither AAMφ nor CAMφ were expressed in the absence of IL-4. This may suggest a generalized impairment of macrophage recruitment or the presence of an immature macrophage population that lacked F4/80 expression in the absence of IL-4.
Interestingly, parasite numbers correlated with the presence of AAMϕ, as wild-type mice had significantly fewer parasites and IL-4−/− mice had a significantly increased worm burdens. AAMϕ have been demonstrated to kill the muscle stage larvae of the parasitic nematode Heligosomoides polygyrus (17) in an arginase dependent mechanism. Worm-induced AAMϕ have also been shown to downregulate T cell responses and to promote Th2 differentiation (10,21). Combined, this is consistent with a hypothesis that AAMϕ are involved in the IL-4 driven host protective mechanism during M. corti infection.
Although the requirement for IL-4 has been demonstrated in a wide variety of helminth infections (6–8,22), M. corti and S. mansoni are the only reported helminth infections that lead to high rates of mortality in IL-4−/− mice (23). IL-4 levels do not affect the level of fibrosis during S. mansoni infection (24), and neither qualitative fibrosis nor levels of IL-13 expression in the liver were changed in IL-4−/− mice infected with M. corti. In IL-4−/− mice infected with S. mansoni, IL-5 production is impaired, while IFNγ and TNFα production increase (8,25–28), as was seen for splenocytes and liver RNA during M. corti infection. However, mortality in S. mansoni-infected IL-4−/− mice was linked to production of nitric oxide, and inducible nitric oxide synthase was upregulated as a result of IFNγ and TNFα production (8,25,26,29). Increases in iNOS production during S. mansoni infection of IL-4−/− mice was complimented by decreases in surface expression of mannose receptor (16,30), a marker for AAMφ. This is divergent from the IL-4−/− mouse response to M. corti, where there was a decrease in molecules associated with AAMφ with no concomitant increase in molecules associated with CAMφ such as iNOS.
In conclusion, it appears that IL-4−/− mice die from infection with M. corti due to an inability to mount a Th2-cytokine response. This defect leads to an immunological imbalance where the Th1 cytokines cause reduced cell recruitment yet increased pathology in the host. IL-4 appears to protect mice from death caused by infection with M. corti not by controlling parasite replication, but rather by regulating the Th1-associated immunopathology.
ACKNOWLEDGEMENTS
The authors wish to thank Jessica Hess, Kevin Redding, and Udaikumar Padigel for their enthusiastic help with experimental design, implementation and analysis.
Footnotes
Disclosures: None
REFERENCES
- 1.Pollacco S, Nicholas WL, Mitchell GF & Stewart AC. T-cell dependent collagenous encapsulating response in the mouse liver to Mesocestoides corti (Cestoda). Int J Parasitol 1978; 8: 457–462. [DOI] [PubMed] [Google Scholar]
- 2.Specht D & Widmer EA. Response of mouse liver to infection with tetrathyridia of Mesocestoides (Cestoda). J Parasitol 1972; 58: 431–437. [PubMed] [Google Scholar]
- 3.Rawat J, Dixon JB, Macintyre AR, McGarry HF & Taylor MJ. IL-4 dependent resistance to the tapeworm Mesocestoides corti (Cestoda) in mice. Parasite Immunol 2003; 25: 553–557. [DOI] [PubMed] [Google Scholar]
- 4.Reed SG & Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol 1993; 5: 524–531. [DOI] [PubMed] [Google Scholar]
- 5.Urban JF Jr, Madden KB, Svetic A, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev 1992; 127: 205–220. [DOI] [PubMed] [Google Scholar]
- 6.Volkmann L, Saeftel M, Bain O, Fischer K, Fleischer B & Hoerauf A. Interleukin-4 is essential for the control of microfilariae in murine infection with the filaria Litomosoides sigmodontis. Infect Immun 2001; 69: 2950–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson EH, Schynder-Candrian S, Rajan TV, Nelson FK, Lustigman S & Abraham D. Immune responses to third stage larvae of Onchocerca volvulus in interferon-gamma and interleukin-4 knockout mice. Parasite Immunol 1998; 20: 319–324. [DOI] [PubMed] [Google Scholar]
- 8.La Flamme AC, Patton EA, Bauman B & Pearce EJ. IL-4 plays a crucial role in regulating oxidative damage in the liver during schistosomiasis. J Immunol 2001; 166: 1903–1911. [DOI] [PubMed] [Google Scholar]
- 9.Gordon S Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35. [DOI] [PubMed] [Google Scholar]
- 10.Loke P, MacDonald AS, Robb A, Maizels RM & Allen JE. Alternatively activated macrophages induced by nematode infection inhibit proliferation via cell-to-cell contact. Eur J Immunol 2000; 30: 2669–2678. [DOI] [PubMed] [Google Scholar]
- 11.Nair MG, Cochrane DW & Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett 2003; 85: 173–180. [DOI] [PubMed] [Google Scholar]
- 12.Nair MG, Gallagher IJ, Taylor MD, et al. Chitinase and Fizz family members are a generalized feature of nematode infection with selective upregulation of Ym1 and Fizz1 by antigen-presenting cells. Infect Immun 2005; 73: 385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F & Hassanzadeh Gh G. Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J Leukoc Biol 2002; 71: 597–602. [PubMed] [Google Scholar]
- 14.Stein M, Keshav S, Harris N & Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med 1992; 176: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donnelly S, O’Neill SM, Sekiya M, Mulcahy G & Dalton JP Thioredoxin peroxidase secreted by Fasciola hepatica induces the alternative activation of macrophages. Infect Immun 2005; 73: 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 2004; 20: 623–635. [DOI] [PubMed] [Google Scholar]
- 17.Anthony RM, Urban JF Jr, Alem F, et al. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 2006; 12: 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins P, Dixon JB, Rakha NK & Carter SD. Regulation of macrophage-mediated larvicidal activity in Echinococcus granulosus and Mesocestoides corti (Cestoda) infection in mice. Parasitology. 1990; 100(Pt 2): 309–315. [DOI] [PubMed] [Google Scholar]
- 19.Sheehan DC & Hrapchak BB. Chapter 10, Connective Tissue and Muscle Stains Theory and Practice of Histotechnology, 2nd edn. Battelle Press, Columbus, OH, USA, 1980, p. 189. [Google Scholar]
- 20.Kaviratne M, Hesse M, Leusink M, et al. IL-13 activates a mechanism of tissue fibrosis that is completely TGF-b independent. J Immunol 2004; 173: 4020–4029. [DOI] [PubMed] [Google Scholar]
- 21.Loke P, MacDonald AS & Allen JE. Antigen-presenting cells recruited by Brugia malayi induce Th2 differentiation of naive CD4(+) T cells. Eur J Immunol 2000; 30: 1127–1135. [DOI] [PubMed] [Google Scholar]
- 22.Rotman HL, Schnyder-Candrian S, Scott P, Nolan TJ, Schad GA & Abraham D. IL-12 eliminates the Th-2 dependent protective immune response of mice to larval Strongyloides stercoralis. Parasite Immunol 1997; 19: 29–39. [DOI] [PubMed] [Google Scholar]
- 23.Brunet LR, Finkelman FD, Cheever AW, Kopf MA & Pearce EJ. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J Immunol 1997; 159: 777–785. [PubMed] [Google Scholar]
- 24.Metwali A, Elliott D, Blum AM, et al. The granulomatous response in murine Schistosomiasis mansoni does not switch to Th1 in IL-4-deficient C57BL/6 mice. J Immunol 1996; 157: 4546–4553. [PubMed] [Google Scholar]
- 25.Patton EA, La Flamme AC, Pedras-Vasoncelos JA & Pearce EJ. Central role for interleukin-4 in regulating nitric oxide-mediated inhibition of T-cell proliferation and gamma interferon production in schistosomiasis. Infect Immun 2002; 70: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Flamme AC, Patton EA & Pearce EJ. Role of gamma interferon in the pathogenesis of severe schistosomiasis in interleukin-4-deficient mice. Infect Immun 2001; 69: 7445–7452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patton EA, Brunet LR, La Flamme AC, Pedras-Vasconcelos J, Kopf M & Pearce EJ. Severe schistosomiasis in the absence of interleukin-4 (IL-4) is IL-12 independent. Infect Immun 2001; 69: 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pedras-Vasconcelos JA, Brunet LR & Pearce EJ. Profound effect of the absence of IL-4 on T cell responses during infection with Schistosoma mansoni. J Leukoc Biol 2001; 70: 737–744. [PubMed] [Google Scholar]
- 29.Pearce EJ, Cheever A, Leonard S, et al. Schistosoma mansoni in IL-4-deficient mice. Int Immunol 1996; 8: 435–444. [DOI] [PubMed] [Google Scholar]
- 30.Hesse M, Modolell M, La Flamme AC, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol 2001; 167: 6533–6544. [DOI] [PubMed] [Google Scholar]