Abstract
The diagnosis of Prader-Willi syndrome (PWS) is based on clinical findings that change with age. Hypotonia is prominent in infancy. Obesity, mild mental retardation or learning disability, and behavior problems, especially in association with food and eating, result in a debilitating physical and developmental disability in adolescence and adulthood. No consistent biological marker is yet available for PWS in spite of recent research activity in cytogenetics and molecular genetics. Diagnostic criteria for PWS were developed by consensus of seven clinicians experienced with the syndrome in consultation with national and international experts. Two scoring systems are provided: one for children aged 0 to 36 months and another one for children aged 3 years to adults. These criteria will aid in recognition of the syndrome in hypotonic infants and in obese, mildly retarded, behaviorally disturbed adolescents and adults. They will also ensure uniform diagnosis for future clinical and laboratory research in PWS.
Prader-Willi syndrome (PWS) was first described in the medical literature in 1956.1 Subsequently, several case reports appeared in the English-language literature,2–4 but it was not until 1968 that major review articles emerged.5,6 An evaluation of prevalence of symptoms was published in 1972.7 Diagnostic criteria were first proposed 10 years ago based on parental report of symptom prevalence in approximately 100 patients.8 Prader-Willi syndrome remains difficult to diagnose because many features are subtle or nonspecific and others change with age. Misdiagnoses still occur. Underdiagnosis in younger children and overdiagnosis in obese retarded adolescents and adults are both common.
Approximately 70% of clinically typical patients with PWS have a characteristic deletion of the proximal part of the long arm of chromosome 15 [del15(q11q13)].9,10 The remaining 30% cannot be diagnosed using this criterion alone. Recently a majority of nondeletion patients have been shown to have PWS on the basis of maternal disomy (two maternal copies of 15q and no paternal copy) for chromosome 15.11,12 However, the molecular genetic testing required to recognize this disomy is currently not widely available and requires DNA from both parents, which is not always possible. Further complicating the laboratory diagnosis of PWS is the presence of the same chromosomal deletion in patients with Angelman syndrome, a disorder with entirely different symptomatology. Angelman syndrome results from a deletion in the maternally derived chromosome 15, whereas PWS occurs from a deletion in the paternally derived chromosome 15.13–15 Variability in the technical skill of performing the high-resolution chromosome analysis required to identify the interstitial microdeletion of chromosome 15 in PWS adds a final point of confusion. Sometimes a deletion is recognized cytogenetically but not confirmed with molecular genetic analysis. At other times a deletion is detected molecularly but not visible cytogenetically.
Criteria for clinical diagnosis of PWS will serve several purposes. Practicing clinicians, awaiting definite and accessible laboratory testing, can use the criteria to confirm or rule out the diagnosis. Diagnostic criteria can serve as a tool to alert clinicians unfamiliar with the syndrome. They can also serve to identify patients with PWS for whom molecular genetic studies should be undertaken. Finally, they could ensure greater purity of patient populations for research studies.
In recent years, diagnostic criteria have been developed by consensus of clinical experts for several genetic disorders in which diagnosis is largely clinical.16–18 Five physicians, a nurse, and a social worker, each of whom had many years of clinical experience with PWS, held a dedicated workshop in January 1991 to develop such consensus diagnostic criteria for PWS. The criteria were tested by five of the participating clinicians (V.A.H., S.B.C., M.G.B., J.M.H., L.R.G.) on 113 patients. This study compared the accuracy of the proposed criteria with experienced clinical judgment and determined the prevalence of symptoms obtained in these 113 patients.19 Minor alterations in the criteria were made following input from national and international experts at several scientific meetings. The final criteria are presented in this communication. Criteria clarification is provided with pertinent references to aid in the interpretation of the new PWS criteria.
DIAGNOSTIC CRITERIA
Three categories of diagnostic criteria for Prader-Willi syndrome have evolved: major, minor, and supportive (Table). These are scored on a weighted point system. Major criteria are valued at one point and minor at one half point. Supportive criteria are not included in the point system. They serve to increase the confidence of the results determined by the other criteria and to educate the user about nonspecific and less frequent characteristics of the syndrome.
TABLE.
Diagnostic Criteria for Prader-Willi Syndrome*
| Major criteria | |
| 1. | Neonatal and infantile central hypotonia with poor suck, gradually improving with age |
| 2. | Feeding problems in infancy with need for special feeding techniques and poor weight gain/failure to thrive |
| 3. | Excessive or rapid weight gain on weight-for-length chart (ex-cessive is defined as crossing two centile channels) after 12 months but before 6 years of age; central obesity in the absence of intervention |
| 4. | Characteristic facial features with dolichocephaly in infancy, narrow face or bifrontal diameter, almond-shaped eyes, small-appearing mouth with thin upper lip, down-turned corners of the mouth (3 or more required) |
| 5. | Hypogonadism—with any of the following, depending on age: |
| a. Genital hypoplasia (male: scrotal hypoplasia, cryptorchid ism, small penis and/or testes for age [<5th percentile]; female: absence or severe hypoplasia of labia minora and/or clitoris | |
| b. Delayed or incomplete gonadal maturation with delayed pubertal signs in the absence of intervention after 16 years of age (male: small gonads, decreased facial and body hair, lack of voice change; female: amenorrhea/oligomenorrhea after age 16) | |
| 6. | Global developmental delay in a child younger than 6 years of age; mild to moderate mental retardation or learning problems in older children |
| 7. | Hyperphagia/food foraging/obsession with food |
| 8. | Deletion 5q11–13 on high resolution (>650 bands) or other ctyogenetic/molecular abnormality of the Prader-Willi chro mosome region, including maternal disomy |
| Minor criteria | |
| 1. | Decreased fetal movement or infantile lethargy or weak cry in infancy, improving with age |
| 2. | Characteristic behavior problems—temper tantrums, violent outbursts and obsessive/compulsive behavior; tendency to be argumentative, oppositional, rigid, manipulative, possessive, and subborn; perseverating, stealing, and lying (5 or more of these symptoms required) |
| 3. | Sleep disturbance or sleep apnea |
| 4. | Short stature for genetic background by age 15 (in the absence of growth hormone intervention) |
| 5. | Hypopigmentation—fair skin and hair compared to family |
| 6. | Small hands (<25th percentile) and/or feet (<10th percentile) for height age |
| 7. | Narrow hands with straight ulnar border |
| 8. | Eye abnormalities (esotropia, myopia) |
| 9. | Thick viscous saliva with crusting at corners of the mouth |
| 10. | Speech articulation defects |
| 11. | Skin picking |
| Supportive findings (increase the certainty of diagnosis but are not scored) | |
| 1. | High pain threshold |
| 2. | Decreased vomiting |
| 3. | Temperature instability in infancy or altered temperature sensitivity in older children and adults |
| 4. | Scoliosis and/or kyphosis |
| 5. | Early adrenarche |
| 6. | Osteoporosis |
| 7. | Unusual skill with jigsaw puzzles |
| 8. | Normal neuromuscular studies |
Scoring: Major criteria are weighted at one point each. Minor criteria are weighed at one half point. Children 3 years of age or younger: Five points are required for diagnosis, four of which should come from the major group. Children 3 years of age to adulthood: Total score of eight is necessary for the diagnosis. Major criteria must comprise five or more points of the total score.
The symptoms of PWS change dramatically with age.20 Infants and young children demonstrate fewer symptoms than older children and adults. Therefore, the scoring system varies, depending on age. In children 3 years of age and younger, only five points are required for diagnosis; four of those must come from the major group (Table). A total score of eight is necessary for the diagnosis in the 3 year to adulthood group. In this age group major criteria items must comprise five or more points of the total score (Table).
CRITERIA CLARIFICATION
Most of the major diagnostic criteria for Prader-Willi syndrome apply to all age groups. Most of the minor criteria are present only in older children and adults.
Hypotonia in Infancy With Normal Neuromuscular Studies.
Prader-Willi Syndrome should be in the differential diagnosis of neonatal hypotonia and strongly suspected if nonspecific laboratory neuromuscular studies (a supportive finding) result in a diagnosis of “benign, congenital hypotonia.”21,22 It might be difficult to confirm the presence of neonatal hypotonia in an older person in whom PWS is suggested. If known, delayed motor landmarks (eg, the average age of independent sitting is 13 months, walking 28 months) might support the presence of this symptom.8
Feeding Problems in Infancy.
Most infants with PWS require special feeding techniques, eg, gavage feeding or bottles with preemie nipples. The infant does not appear hungry and weight gain is slow. A history of feeding problems in infancy might be difficult to elicit in older persons.
Obesity.
Excessive weight gain follows the period of failure-to-thrive in early infancy in PWS. Early-onset obesity, ie, the infant who was chubby from birth, does not qualify as a diagnostic criterion. In very young children, crossing of two centile channels, eg, from the 5th to the 25th percentile, on weight-for-length charts23 in a short period of time after 12 months of age is a reliable criterion. Onset of obesity must occur before 6 years of age to count as a criterion in this diagnosis. Late childhood and preadolescent onset of obesity does not satisfy this requirement.
Life-threatening obesity was once thought inevitable and incurable in PWS. Lower caloric need in persons with this syndrome has been documented and successful weight loss has been well and repeatedly demonstrated.24 A major goal in early diagnosis of PWS is obesity prevention.20 This goal is now frequently reached, hence the provision of obesity in the absence of intervention in this diagnostic criteria.
Characteristic Facial Features.
The facies typical of PWS are illustrated in the Figure. The head shape is often dolichocephalic and the mouth appears small in infancy.
Figure.
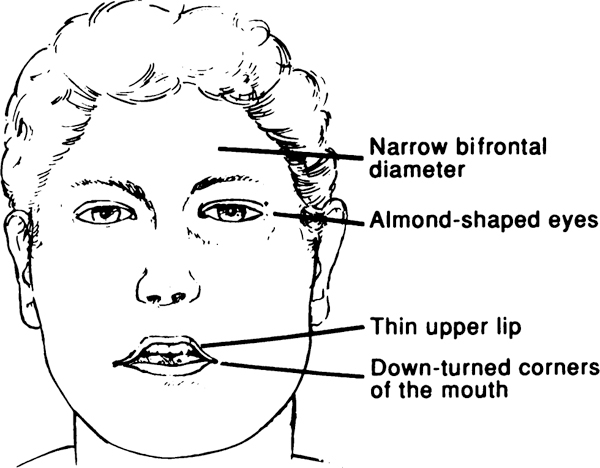
Composite drawing of facial features in Prader-Willi syndrome.
Hypogonadism.
The manifestations of hypogonadism vary in the two sexes.
Males. In the male, penile length and testicular size should be evaluated and compared to age norms.25,26 Penile length—measured stretched with a ruler resting on the pubic bone—less than the fifth percentile for age satisfies this criterion. So does truly undescended testes, often accompanied by a flat, underdeveloped scrotum, which has thin skin and is poorly rugated and pigmented. If one or both testes are palpable in the inguinal canal or in the scrotal sac, their size is best evaluated by estimating volume using an orchiometer. Norms for testicular volume and length are available.25,26
In the postadolescent male with PWS, virilization is incomplete unless hormonal replacement has been provided. Successful sexual development with testosterone treatment has been described27; therefore, “in the absence of intervention” is stipulated in the wording of this criterion.
Females. In females, the size of labia minora and clitoris is admittedly difficult to assess but norms for clitoral size are available.28 In postadolescent females, estrogen treatment is sometimes deemed appropriate.27 Breast development might be difficult to assess in obese older preadolescent and adolescent females because fat accumulation may simulate true breast tissue. Therefore, the extent of breast development is not included as a criterion for diagnosis in PWS.
Developmental Delay and Mental Retardation.
Children younger than 6 years of age show delayed motor, cognitive, and language development. The average intelligence quotient of older individuals is in the upper range of mild mental retardation, in the high 60s.29,30 Many have borderline or low-normal cognition but demonstrate learning disabilities.
Food-Related Behavior Problems.
Excessive appetite and overeating (hyperphagia), seemingly absent sense of satiation, food seeking (from raiding of garbage cans to shoplifting in food stores to pilfering money at home and in school to buy food), and obsession with food and eating are examples of food-related behavior problems found in children and adults with PWS. Such behaviors are seldom found in young children.
Abnormalities of the Prader-Willi Chromosome Region on the Proximal Part of the Long Arm of Chromosome 15.
Besides the 15q11–13 deletion, other cytogenetic abnormalities involving chromosome 15 have also been encountered. Any documented cytogenetic or molecular abnormality in the Prader-Willi chromosome region satisfies this criterion. Such findings may eventually provide a biological marker for this condition.12
History of Decreased Fetal Movements, Weak Cry, and Lethargy.
Fetal inactivity is probably directly related to the degree of hypotonia and is quite subjective. Typically, parents describe the cry in their infant with PWS as squeaky, kitten-like, or sometimes absent. Hypoarousal (inactivity and listlessness) are often noted in infants. The abnormal cry is the most reliable of these symptoms.
Behavioral Characteristics.
In addition to the food-seeking behaviors described above, older children and adults with PWS have other behavioral traits peculiar to the syndrome. Initially, the parents frequently complain of “stubbornness” in their young child with other symptoms listed in the Table emerging with age. Verbal perseverance on favorite topics is one of the most common and annoying behavioral characteristics in PWS.
Sleep Disturbances.
Excessive daytime sleeping frequently occurs in older children and adults with PWS.31 The sleep apnea may be obstructive or central or both. In some individuals, these symptoms abate with weight loss, although in most they persist.
Short Stature.
A typical pattern of growth has been demonstrated in PWS: normal length at birth, deceleration of linear growth during the first few months of life, relatively steady length growth during child-hood, and a fall-off in growth in adolescence.32,33 Mean adult height is approximately 147 cm for females and 155 cm for males.30,32,33 Stature for genetic background can be assessed using parent-specific adjustment for height available on US population.34 Another method utilizes target height. In females: mother’s height plus father’s height minus 13 cm divided by 2 is plotted for age 19 on the female growth chart; in males: father’s height plus mother’s height plus 13 cm divided by 2 is plotted at age 19 on the male growth chart; two standard deviations is ±8.5 cm.35 Charts are also available to directly plot the heights for midparental height of children aged 2 to 9 years.35
Growth hormone deficiency has been documented in PWS.36 Synthetic growth hormone is now available and is beginning to be used successfully.37 Thus the fourth minor criterion is worded “in the absence of growth hormone.” It is assumed that if intervention has been deemed necessary, this criterion is fulfilled.
Hypopigmentation.
Fairer skin and lighter hair compared to other family members is a relatively recent observation in about 50% of patients.38 This phenomenon is difficult to appreciate in younger children, who normally tend to have lighter coloring than their parents. Most hypopigmented children have a cytogenetic deletion.38
Small Hands and Feet.
Published standards are available and should be consulted before determining that small hands and feet are present.25,26 Their size should be compared to height age, not chronological age, standards. Hands and feet may be of normal size in younger children.33,39,40
Narrow Hands With Straight Ulnar Border.
Decrease in hand breadth has been demonstrated in PWS.33 The presence of straight ulnar border is admittedly a subjective finding, but the combination of the two findings is distinctive.
Eye Abnormalities.
Esotropia may be present at any age. Refractive errors, hyperopia, and nonfamilial myopia are often encountered in older children.
Thick, Viscous Saliva.
A peculiar feature of PWS, which has not been well studied, is the common presence of thick viscous saliva which tends to crust at the corners of the mouth and often covers the teeth.20 Recently, it has been pointed out that this symptom might be a diagnostic clue in infancy.41
Speech Articulation Defect.
Clinically the most prominent speech articulation defect in PWS is hypernasal speech.42
Skin Picking.
Self-mutilating habits, especially picking at minor skin lesions, are common behaviors in older children and adults with PWS. The cause of these behaviors, which often are annoying to parents and other caretakers, is poorly understood.
High Pain Threshold.
Parents and caretakers frequently notice that people with PWS seem to have a surprisingly high tolerance for pain. Scrapes, burns, and bruises are often not brought to their attention and only noted incidentally.
Decreased Vomiting.
A high threshold for vomiting is another peculiar and unexplained observation in many individuals with PWS. When the whole family has gastroenteritis, the person with PWS may get the diarrhea but may not vomit.
The combination of the last two symptoms (high tolerance for pain and high threshold for vomiting) in the same person literally can be deadly. One of the authors had a patient with PWS who died of a perforated ulcer and knows of another one who died of a perforated appendix because the lack of normal physiological responses prevented the detection of these conditions.
Temperature Instability and Altered Temperature Sensitivity.
Temperature instability has been noted in infants with this syndrome. Occasionally a history of hospitalization for “fever of unknown origin” is obtained.43,44 Some older individuals with PWS do not appear to react to cold with shivering and discomfort and tolerate heat without being uncomfortable. Again, the basis for these peculiar physiological phenomena remains unexplained.
Scoliosis and Kyphosis.
Scoliosis occurs to some degree in more than 85% of individuals with PWS, occasionally in its infantile form.45 Kyphosis is also common in older persons but the prevalence has not been documented.
Early Adrenarche.
Isolated precocious adrenarche is sometimes found in children with a variety of neurological impairments and is often encountered in PWS.46 True precocious puberty has also been described but is unusual.47
Osteoporosis.
Fractures following minor trauma in people with PWS have been described.8 It has recently been demonstrated that osteoporosis frequently is present at all ages in this condition,27 explaining this earlier observation.
Unusual Skills With Jigsaw Puzzles.
Children and adults with PWS also have some strengths. In many, strong visual-perceptual-motor skills have been documented which may explain the common observation of exceptional proficiency with jigsaw puzzles.
CONCLUSION
In spite of recent increase in research activity in cytogenetic and molecular genetics, a consistent biological marker is not yet available for PWS. Until now, clinicians have used their personal experience to determine whether an individual patient has PWS.
The proposed and tested diagnostic criteria presented here are purposefully stringent. It is acknowledged that PWS may eventually be diagnosed by molecular testing in some patients excluded by these criteria. However, for the present, these criteria will identify those in need of additional laboratory studies and ensure uniform clinical diagnosis for laboratory and clinical research. In addition, these criteria will continue to alert clinicians to a suspicion of PWS in hypotonic infants and obese, mildly retarded, and behaviorally disturbed older children and adults.
Diagnostic criteria have been developed by consensus of informed clinicians for several disorders such as neurofibromatosis, Rett syndrome, and tuberous sclerosis, all of which currently lack undisputable diagnostic biological markers.17–19 Prader-Willi Syndrome now joins this list. Other conditions will likely undergo similar attempts at organizing symptomatology in fields of medicine that still are more art than science.
ACKNOWLEDGMENT
We are grateful to the Prader-Willi Syndrome Association for their financial support as well as for their encouragement of and cooperation in this endeavor.
ABBREVIATION.
- PWS
Prader-Willi syndrome
REFERENCES
- 1.Prader A, Labhart A, Willi H. Ein Syndrom von Adipositas, Kleinwuchs, Kryptorchismus und Oligophrenie nach myatonieartigem Zustand im Neugeborenenalter. Schweiz Med Wochenschr. 1956;86:1260–1261 [Google Scholar]
- 2.Laurance BM. Hypotonia, obesity, hypogonadism and mental retardation in childhood. Arch Dis Child. 1961;36:690 [Google Scholar]
- 3.Evans PR. Hypogenital dystrophy with diabetic tendency. Guy’s Hosp Rep. 1964;113:207–222 [PubMed] [Google Scholar]
- 4.Forssman H, Hagberg B. Prader-Willi syndrome in a boy of ten with prediabetes. Acta Paediatr. 1964;53:70–78 [DOI] [PubMed] [Google Scholar]
- 5.Zellweger H, Schneider HJ. Syndrome of hypotonia-hypomentia-hypogonadism-obesity (HHHO) or Prader-Willi syndrome. AJDC. 1968;115:588–598 [DOI] [PubMed] [Google Scholar]
- 6.Dunn HG. The Prader-Labhart-Willi syndrome: review of the literature and report of nine cases. Acta Paediatr Scand. 1968(suppl 186): 1–38 [DOI] [PubMed] [Google Scholar]
- 7.Hall BD, Smith DW. Prader-Willi syndrome: a resume of 32 cases including an instance of affected first cousins, one of whom is of normal stature and intelligence. J Pediatr. 1972;81:286–293 [DOI] [PubMed] [Google Scholar]
- 8.Holm VA. The diagnosis of Prader-Willi syndrome In: Holm VA, Sulzbacher S, Pipes PL, eds. The Prader-Willi Syndrome. Baltimore, MD: University Park Press; 1981:27–36 [Google Scholar]
- 9.Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan BS, Crawford JD. Deletion of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med. 1981;304:325–329 [DOI] [PubMed] [Google Scholar]
- 10.Butler MG. Prader-Willi syndrome: current understanding of cause and diagnosis. Am J Med Genet. 1990;35:319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls RD, Knoll JH, Butler MG, Karam S, Lalande M. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature. 1989;16:281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson WP, Bottari A, Yagang X, et al. Clinical, molecular and cytogenetic survey of potential Prader-Willi syndrome patients In: Cassidy SB, ed. Prader-Willi Syndrome and Other Chromosome 15q Deletion Disorders. Heidelberg, Germany: Springer-Verlag; 1992:53–58. NATO ASI Series, Vol 61 [Google Scholar]
- 13.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome. Am J Med Genet. 1986;23:793–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donlon TA. Similar molecular deletions of chromosome 15q11.2 are encountered in both the Prader-Willi and Angelman syndromes. Hum Genet. 1988;80:322–328 [DOI] [PubMed] [Google Scholar]
- 15.Knoll JH, Nicholls RD, Magenis RE, et al. Angelman and Prader-Willi syndromes share a common chromosome 15 deletion but differ in parental origin of the deletion. Am J Med Genet. 1989;32:285–290 [DOI] [PubMed] [Google Scholar]
- 16.The Rett Syndrome Diagnostic Criteria Work Group. Diagnostic criteria for Rett syndrome. Ann Neurol. 1988;23:425–428 [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. Neurofibromatosis: National Institutes of Health Consensus Development Conference Statement. Bethesda, MD: National Institutes of Health; July 1987:6 [Google Scholar]
- 18.Gomez MR. Tuberous Sclerosis. 2nd ed New York, NY: Raven Press; 1988 [Google Scholar]
- 19.Holm VA, Cassidy SB, Butler MG, et al. Diagnostic criteria for Prader-Willi syndrome In: Cassidy SB, ed. Prader-Willi Syndrome and Other Chromosome 15q Deletion Disorders. Heidelberg, Germany: Springer-Verlag; 1992:105–115. NATO ASI Series, Vol 61 [Google Scholar]
- 20.Cassidy SB. Prader-Willi syndrome. Curr Probl Pediatr. 1984;14:1–55 [DOI] [PubMed] [Google Scholar]
- 21.Diagnosis Zellweger H. and therapy in the first phase of Prader-Willi syndrome In: Holm VA, Sulzbacher S, Pipes PL, eds. The Prader Willi Syndrome. Baltimore, MD: University Park Press; 1981:55–68 [Google Scholar]
- 22.Dubowitz V The Floppy Infant. 2nd ed London, England: Spastic International Medical Publications/William Heinemann; 1980. Clinics in Developmental Medicine No. 76 [Google Scholar]
- 23.Hamill PVV, Drizd TA, Johnson CL, et al. NCHS growth curves for children birth-18 years, United States Vital and Health Statistics: Data From the National Health Survey. Series 11, No 165. Hyattsville, MD: National Center for Health Statistics; 1977. US Dept of Health, Education, and Welfare publication (PHS) 78–1650 [PubMed] [Google Scholar]
- 24.Holm VA, Pipes PL. Food and children with Prader-Willi syndrome. AJDC. 1976;130:1063–1067 [DOI] [PubMed] [Google Scholar]
- 25.Jones KL, Smith S. Smith’s Recognizable Patterns of Human Malformation. 4th ed Philadelphia, PA: WB Saunders; 1988 [Google Scholar]
- 26.Hall JG, Froster-Iskenius UG, Allanson JE. Handbook of Normal Physical Measurements. New York, NY: Oxford University Press; 1989 [Google Scholar]
- 27.Rubin K, Cassidy SB. Hypogonadism and osteoporosis In: Management of Prader-Willi Syndrome. Greenswag LR, Alexander RC, eds. New York, NY: Springer-Verlag; 1988:23–33 [Google Scholar]
- 28.Sane K, Pescovitz OH. The clitoral index: a determination of clitoral size in normal girls and in girls with abnormal sexual development. J Pediatr. 1992;120:264–266 [DOI] [PubMed] [Google Scholar]
- 29.Crnic KA, Sulzbacher S, Snow J, Holm VA. Preventing mental retardation associated with gross obesity in the Prader-Willi syndrome. Pediatrics. 1982;66:787–789 [PubMed] [Google Scholar]
- 30.Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol. 1987;29:145–152 [DOI] [PubMed] [Google Scholar]
- 31.Cassidy SB, McKillop JA, Morgan WJ. Sleep disorders in Prader-Willi syndrome. Dysmorph Clin Genet. 1990;4:13–17 [Google Scholar]
- 32.Holm VA, Nugent JK. Growth in the Prader-Willi syndrome. Birth Defects. 1982;18(No. 3B):93–100 [PubMed] [Google Scholar]
- 33.Butler MG, Meaney FJ. Standards for selected anthropometric measurements in Prader-Willi syndrome. Pediatrics. 1991;88:853–860 [PMC free article] [PubMed] [Google Scholar]
- 34.Himes JH, Roche AF, Thissen D, Moore WM. Parent-specific adjustment for evaluation of recumbent length and stature of children. Pediatrics. 1985;75:304–313 [PubMed] [Google Scholar]
- 35.Tanner JM, Goldstein H, Whitehause RH. Standards for children’s height at ages 2–9 years allowing for heights of parents. Arch Dis Child. 1970;45:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costeff H, Holm VA, Ruvalcaba R, Shaver J. Growth hormone secretion in Prader-Willi syndrome. Acta Paediatr Scand. 1990;79:1059–1062 [DOI] [PubMed] [Google Scholar]
- 37.Angulo MA, Castro-Magaña M, Uy J. Pituitary evaluation and growth hormone treatment in Prader-Willi syndrome. J Pediatr Endocrinol. 1991;4:167–173 [Google Scholar]
- 38.Butler MG. Hypopigmentation: a common feature of Prader-Labhart-Willi syndrome. Am J Hum Genet. 1989;45:140–146 [PMC free article] [PubMed] [Google Scholar]
- 39.Chitayat D, Davis ED, McGillivray BC, Hayden MR, Hall JG. Perinatal and first year follow-up of patients with Prader-Willi syndrome: normal size of hands and feet. Clin Genet. 1989;35:161–166 [DOI] [PubMed] [Google Scholar]
- 40.Hudgins L, Cassidy SB. Hand and foot length in Prader-Willi syndrome. Am J Med Genet. 1991;41:5–9 [DOI] [PubMed] [Google Scholar]
- 41.Stephenson JBP. Neonatal presentation of Prader-Willi syndrome. AJDC. 1992;146:151–152 [DOI] [PubMed] [Google Scholar]
- 42.Speech Branson C. and language characteristics of children with Prader-Willi syndrome In: Holm VA, Sulzbacher S, Pipes PL, eds. The Prader-Willi Syndrome. Baltimore, MD: University Park Press; 1981:179–183 [Google Scholar]
- 43.Cassidy SB, McKillop JA. Temperature regulation in Prader-Willi syndrome. Am J Med Genet. 1991;41:528 [Google Scholar]
- 44.Wise MS, Zoghbi H, Edwards M, Byrd LK, Guttmacher AE, Greenberg F. Hyperthermia in infants with Prader-Willi syndrome. Am J Med Genet. 1992;42:262–263 [Google Scholar]
- 45.Holm VA, Laumen EL. Prader-Willi syndrome and scoliosis. Dev Med Child Neurol. 1981;23:192–201 [DOI] [PubMed] [Google Scholar]
- 46.Kauli R, Prager-Lewin R, Laron Z. Pubertal development in the Prader-Labhart-Willi syndrome. Acta Paediatr Scand. 1978;67:763–767 [DOI] [PubMed] [Google Scholar]
- 47.Vanelli M, Bemasconi S, Caronna N, Virdis R, Terzi C, Giovannelli G. Precocious puberty in a male with Prader-Labhart-Willi syndrome. Helv Paediatr Acta. 1984;39:373–377 [PubMed] [Google Scholar]


