Abstract
Background:
Program utilization patterns are described within a large network of harm reduction service providers in Ukraine. The relationship between utilization patterns and HIV incidence is determined among people who inject drugs (PWID) controlling for oblast-level HIV incidence and treatment/syringe coverage.
Methods:
Data were extracted from the network’s monitoring and evaluation database (Jan 2011-Sep 2014, n=327,758 clients). Latent profile analysis was used to determine harm reduction utilization patterns using the number of condoms, syringes, and services (i.e., testing, information and counseling sessions) received monthly over a year. Cox proportional hazards regression determined the relations between HIV seroconversion and utilization class membership.
Results:
In the final 4-class model, class 1 (34.0% of clients) received 0.1 HIV tests, 1.3 syringes, 0.6 condom and minimal counseling and information sessions per month; class 2 (33.6%) received 8.6 syringes, 3.2 condoms, and 0.5 HIV tests and counseling and information sessions; class 3 (19.1%) received 1 HIV test, 11.9 syringes, 4.3 condoms, and 0.7 information and counseling sessions; class 4 (13.3%) received 1 HIV test, 26.1 syringes, 10.3 condoms, and 1.8 information and 1.9 counseling sessions. Class 4 clients had significantly decreased risk for HIV seroconversion as compared to those in class 1 after controlling for oblast-level characteristics.
Conclusions:
Injection drug use continues to be a major mode of HIV transmission in Ukraine, making evaluation of harm reduction efforts in reducing HIV incidence among PWID critical. These analyses suggest that receiving more syringes and condoms decreased risk of HIV. Scaling up HIV testing and harm reduction services is warranted.
Introduction
Ukraine has one of the highest burdens of HIV among European countries, with an epidemic that is primarily concentrated among people who inject drugs (PWID) (Zaller et al., 2015). There were approximately 325,000 to 425,000 PWID in Ukraine in 2012 (G. Berleva et al., 2012). HIV prevalence was estimated to be 19.7% in the 2013 bio-behavioral survey conducted among PWID from 29 cities (O. Balakirieva, Bondar, Loktieva, Sazonova, & Sereda, 2014). Studies had suggested that the HIV epidemic among PWID was slowing in Ukraine (O. M. Balakirieva, Bondar, & Denysuk, 2007; O. M. Balakirieva, Bondar, Sereda, & Sazonova, 2012; Degenhardt et al., 2014; Pohorila, Taran, Kolodiy, & Diyeva, 2009; Vitek et al., 2014); Vitek et al. (2014) reported that among PWID aged 25 years and younger annual HIV case reports decreased by 78% between 2005 and 2012 and prevalence in integrated biobehavioral surveys (IBBS) significantly decreased between 2009 and 2013 as well (9.9% to 3.6%, p=0.01). However, HIV prevalence increased to 21.9% among PWID in 2015 (Ukraine UNAIDS Global AIDS Response Progress Reporting [GARPR], 2016).
Complicating the HIV epidemic in Ukraine is an economic downturn (Iwanski, 2015) and armed conflict with the Russian occupation of Crimea in 2014 and an ongoing separatist movement in the Donbass region (Crawford, 2015). The current political and economic situation in Ukraine threatens to turn back the progress that has been made with respect to the HIV epidemic (Mackey & Strathdee, 2015). In its latest Global AIDS Response Progress Report to UNAIDS (Ukraine UNAIDS GARPR, 2016) Ukraine reported that 25% of people living with HIV (PLHIV) were on antiretroviral treatment (ART) and approximately 45,000 PWID live in the regions in conflict. Empirical data on the impact of the economic crisis and ongoing armed conflict in the Donbass region are not readily available. Indeed, the most recent GARPR report to UNAIDS (2016) consistently reported a lack of data for most indicators in most of the conflict areas (i.e., Crimea, Sevastopol City, Donetsk, and Lugansk). In addition, there has been loss of purchasing power for ART related to the devaluation of the hryvnia, difficulties with ART procurement, and delays in service provision.
The WHO has guidelines to delineate a comprehensive package of evidence-based HIV-related recommendations for key populations (Dutta, Wirtz, Baral, Beyrer, & Cleghorn, 2012; World Health Organization, 2014). Essential health sector interventions include condom and lubricant programming, harm reduction services for substance use (i.e., needle and syringe programs [NSP] and medication-assisted treatment [MAT]), behavioral interventions, HIV testing and counseling, HIV treatment and care, prevention and management of co-infections and comorbidities, and sexual and reproductive health interventions. For PWID, four interventions are critical for the prevention and control of HIV and AIDS: NSP, MAT, antiretroviral treatment (ARV), and HIV counseling and testing. Ukraine’s first NSP opened in Odessa in 1996 (“Police block needle exchange program,” 1997).
With respect to MAT, buprenorphine has been available in Ukraine since 2004 and methadone maintenance since 2007 (Dvoriak et al., 2014). However, coverage has been low with approximately 8,800 patients receiving MAT as of September 2016 (Ukrainian Center for Disease Control, 2016). Access to MAT in Ukraine requires named-based registration with the national narcology service (Bojko et al., 2015; Makarenko et al., 2016), which is an important structural barrier to uptake. Since 2002, MAT has been financially supported by the Global Fund to Fight AIDS, Tuberculosis and Malaria; in November 2016 it was announced that MAT would be fully funded by the Ukrainian government’s budget starting in 2017 (UNAIDS, 2016).
Alliance for Public Health (Alliance Ukraine), is a leading organization in Ukraine’s response to the HIV/AIDS epidemic and works closely with civil society organizations, the Ukrainian Ministry of Health, and other governmental organizations. Alliance Ukraine manages prevention programs as well as provides technical assistance and financial support to local organizations. According to their 2013 annual report, Alliance Ukraine (2014) supported 76 non-governmental organizations (NGOs) throughout Ukraine which served an 63.4% (196,400) of the estimated total number of PWID in the country. Direct services for PWID included syringe distribution and exchange and pharmacy-based syringe exchange, among others. Although studies have described MAT and ARV coverage for PWID in Ukraine (Bruce, Dvoryak, Sylla, & Altice, 2007; Degenhardt et al., 2014; Zaller et al., 2015), there is a dearth of data on harm reduction service coverage and utilization.
This study aims to describe program utilization patterns within the large network of Alliance Ukraine-affiliated harm reduction NGOs and determine the relationship between utilization patterns and HIV incidence while taking into account relevant oblast-level measures of HIV prevalence and incidence and coverage of MAT, ARV, and syringes. Because resources for HIV testing, ARV (Wolfe, Carrieri, & Shepard, 2010), and MAT (Bojko et al., 2015; Wolfe et al., 2010; Zaller et al., 2015) are limited, this analysis also seeks to identify which venues for harm reduction services may be more effective in reducing the HIV burden among PWID.
Methods
Study population and data collection
Data for these analyses were extracted from Alliance Ukraine’s SYREX database (Alliance Ukraine, 2015). SYREX was developed to register clients at harm reduction NGOs and collect data on the commodities (i.e., syringes and condoms) and services (i.e., counseling and information) provided. At each client visit, the number of syringes and condoms distributed is recorded, as well as whether HIV testing, information, or counseling were provided. Given that Alliance Ukraine is the only harm reduction provider in the country, the database includes data on all harm reduction clients in Ukraine. However, it should be noted that syringes are available for purchase in pharmacies, and most PWID buy syringes as well.
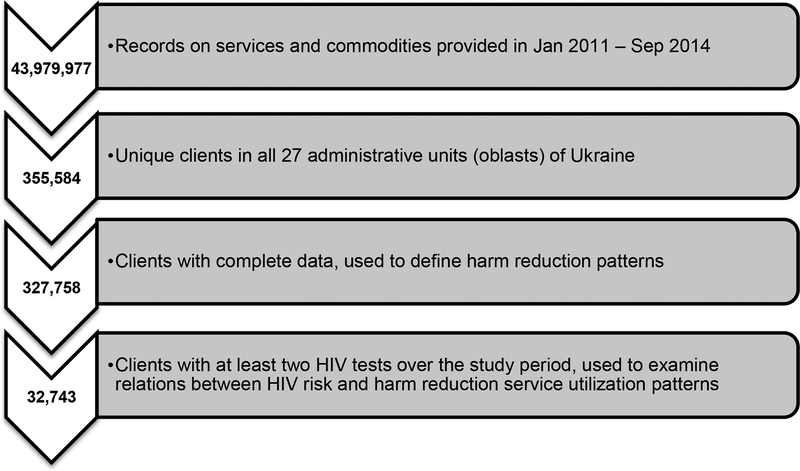
The raw SYREX dataset included 43,979,977 records on services and commodities provided in Jan 2011 – Sep 2014. After aggregation by type of service, provision place/venue/modality and date, the resulting dataset included 26,350,972 episodes of service provision. Although there is guidance that prevention services have to be provided as a package (World Health Organization, 2014), numbers of episodes of individual services differed – there were 6,123,164 episodes of syringe provision, 5,064,058 episodes of condom provision, 3,003,768 episodes of informational materials provision, 3,203,871 episodes of counseling, and 249,046 HIV tests recorded. These five services were included in the analysis. The final dataset for analyzing patterns of harm reduction utilization included 327,758 clients from the 24 oblasts (i.e., regions), one autonomous republic (the Autonomous Republic of Crimea) and two cities with special status (Kyiv and Sevastopol) of Ukraine (Figure 1).
Figure 1.
Data Extraction and Study Sample
For the final dataset, frequency variables were constructed for each unique client in all 27 administrative units of Ukraine. Frequencies were calculated for the number of commodities (i.e., syringes and condoms) and services received (i.e., information and counseling sessions) on average per month across the study period.
The main outcome of interest was HIV seroconversion. In any given year, there were enough rapid HIV test kits to test approximately 40% of clients. However, HIV testing was not randomly distributed (i.e., those at highest risk [e.g., received more condoms and needles] tended to be tested more frequently (Denisiuk et al., 2014)). Thus, 32,743 clients had a first negative HIV test result and at least one follow-up test; this was the sample for analyses of HIV risk (Figure 1).
In addition to client-level data, oblast-level indicators characterizing the status of HIV epidemic and coverage of treatment and prevention programs were included in the analyses (Table 1). These included number of newly registered cases per 100,000 population, proportion of PWID among newly diagnosed cases from Ukraine Ministry of Health official statistics for 2014 (Ukraine Ministry of Health, 2015), and HIV prevalence among PWID aged 25 years and younger (Balakireva, Bondar, Loktieva, Sazonova, & Sereda, 2014). The total number of syringes distributed in 2011–2014 and number of patients on MAT at of the end of 2013 were extracted from SYREX. Using the estimated PWID population size (G Berleva et al., 2012), the oblast-level number of syringes per PWID per year and proportion of PWID on MAT were calculated. The 2013 IBBS provided data on self-reported ART coverage, access to clean needles, and prevention program coverage. Each oblast-level variable was categorized into tertiles as reported in table 1.
Table 1.
Summary of oblast-level measures for 27 Ukrainian oblasts
| Data source | Mean | SD | Range | |
|---|---|---|---|---|
| Estimated size of PWID population | SYREX | 11,955·6 | 10650·7 | 1400 – 42000 |
| New registered HIV cases per 100,000 population1 | Ukraine Ministry of Health | 39·1 | 28·0 | 6·6 – 109·4 |
| HIV prevalence in pregnant women1 | Ukraine Ministry of Health | 0·325 | 0·198 | 0·07 – 0·74 |
| HIV prevalence among PWID aged <25 years | Ukraine Ministry of Health | 0·048 | 0·063 | 0 – 0·283 |
| Percent of PWID among newly diagnosed HIV cases | Ukraine Ministry of Health | 24·2% | 54·3% | 1·2 – 39·8% |
| Total number of syringes distributed | SYREX | 2,835,902 | 3,718,133 | 295,363 – 76,569,348 |
| Syringes per PWID2 | SYREX | 200·4 | 96·4 | 55·3 – 422·9 |
| Proportion of PWID on MAT1,2 | SYREX | 0·028 | 0·022 | 0·008 – 0·086 |
| Self-reported ART coverage of PWID | IBBS | 34·2% | 17·3% | 1·5 – 70·0% |
| Self-reported receipt of clean syringes/needles | IBBS | 49·2% | 19·4% | 15·5 – 97·1% |
| Self-reported client of a prevention program | IBBS | 34·7% | 21·9% | 6·1 – 96·1% |
N=25; data missing from the Autonomous Region of Crimea and Sebastopol City
Calculated variable
ARV = antiretroviral therapy; MAT = medication-assisted therapy; PWID = people who inject drugs
Harm reduction service venues were defined as the venue in which a client received most of their needles. Possible venues included (1) secondary exchange or distribution through (2) street venues, (3) mobile van, (4) pharmacy, (5) office, (6) health care facility, and (7) another source.
The study protocol was approved by IRB #1 of the Ukrainian Institute on Public Health Policy.
Data analysis
First, a latent profile analysis (LPA; a latent class analysis for continuous variables) was conducted on the mean number of commodities (i.e., condoms and/or syringes) and services (i.e., testing, information and counseling sessions) PWID received monthly for the entire population of clients with data for the study period. In this way service utilization patterns can be determined for all clients and not just those who had multiple HIV tests.
The data were not normally distributed and could not be log-transformed into a normal distribution. As a result, a two-part mixture model was constructed, which is designed for data with a zero-inflated distribution. In two-part models, one item is coded into two variables. The first variable was dichotomous, indicating whether or not participants received a commodity or service. The second variable was continuous, and the values of this variable are the mean number of commodities/services received monthly, with those who did not receive any coded as missing. The model was then run three times: the first time was an LPA with dichotomous items only, the second time was with continuous items only, and the third time the two model results were combined into a final model result. In each model, each participant was assigned to one class. Syringes and condoms per month were correlated in the model, as these two commodities tended to be provided together. The LPA was conducted with MPlus software version 7.11 (Muthén & Muthén, 1998–2015).
For each latent class solution, a class indicator variable was created. Cox proportional hazard regression models were constructed to determine the risk of HIV seroconversion by service utilization class, with adjustment for oblast-level variables. Only clients with at least two HIV tests were included in these models (n=32,743). Stratified multivariable Cox proportional hazard regression analyses were conducted with STATA 14.1 (StataCorp, College Station, TX).
Eight oblasts (i.e., Cherkasska, Dnipropetrovska, Donetska, Khersonska, Kyiv City, Mykolaivska, Odesska, Sumska, and Ternopilska) are in the highest tertile of syringes per PWID per year. Six of these oblasts (i.e., Cherkasska, Dnipropetrovska, Donetska, Khersonska, Mykolaivska, and Odesska) are also in the highest tertile of newly registered HIV cases along with Kyivska. In other words, oblasts with high HIV-burden tend to distribute more syringes to the PWID in their communities. Because of this type of multicollinearity among the oblast-level variables, interaction terms between oblast level variables were not tested and a multivariable Cox proportional hazard model was not constructed.
Role of the funding sources: The funding sources were not involved in the study design; collection, analysis, or interpretation of the data; manuscript writing; or the decision to submit the paper for publication.
Results
Over the course of the study period, the 327,758 PWID received 9.09 syringes (range 0–1442.45), 3.45 condoms (range 0–884.5), 0.57 information sessions (range 0–152.5), and 0.55 counseling sessions (range 0–152.5) per month on average. Approximately one-third (34.32%) of PWID were tested for HIV at least once and 10.53% were tested at least twice.
LPA models of 1 to 6 classes were constructed for the number of commodities and services each participant received every month. Table 2 provides model fit indices for all the LPA models. Based on the Vuong-Lo-Mendell-Rubin likelihood ratio tests (LRT) and Lo-Mendell-Rubin adjusted LRT (Table 2), the 4-class model is not better than the 3-class model, as indicated by the non-significant p-values. However, the parametric bootstrapped LRT suggests that four classes are better than three classes. Nylund et al. (2007) suggest that the bootstrap method may be more reliable. The 4-class model also has the clearest classification (entropy=0·.864).
Table 2.
Model fit indices for latent profile analyses of commodity and service patterns among 327,758 harm reduction clients in Ukraine, 2011–2014
| 1 class | 2 classes | 3 classes | 4 classes | 5 classes | 6 classes | |
|---|---|---|---|---|---|---|
| Number of Free Parameters | 17 | 28 | 39 | 50 | 61 | 72 |
| Loglikelihood | ||||||
| H0 Value | −2380830·52 | −2152133·602 | −2081795·283 | −2026755·576 | −1986706·155 | −1910175·937 |
| H0 Scaling Correction Factor for MLR | 1·1569 | 1·1807 | 1·4011 | 1·7655 | 1·6852 | 2·5581 |
| Information Criteria | ||||||
| AIC | 4761695·041 | 4304323·204 | 4163668·566 | 4053611·151 | 3973534·311 | 3820495·873 |
| BIC | 4761876·941 | 4304622·805 | 4164085·868 | 4054146·153 | 3974187·013 | 3821266·275 |
| Sample-Size Adjusted BIC (n* = (n + 2) / 24) | 4761822·915 | 4304533·82 | 4163961·924 | 4053987·25 | 3973993·152 | 3821037·456 |
| VUONG-LO-MENDELL-RUBIN LIKELIHOOD RATIO TEST FOR 1 (H0) VERSUS X CLASSES | ||||||
| H0 Loglikelihood Value | −2380830·52 | −2152133·602 | −2081795·285 | −2024390·907 | −2034810·19 | |
| 2 Times the Loglikelihood Difference | 457393·837 | 140676·638 | 110079·418 | 75369·503 | 30935·587 | |
| Difference in the # of Parameters | 11 | 11 | 11 | 10 | 11 | |
| Mean | 24·645 | 171·177 | 1023·008 | 341·417 | 145·522 | |
| Standard Deviation | 23·809 | 236·321 | 1633·437 | 1454·091 | 323·518 | |
| p value | <0·001 | <0·001 | 0·333 | <0·001 | <0·001 | |
| LO-MENDELL-RUBIN ADJUSTED LRT TEST | ||||||
| Value | 454143·000 | 139676·806 | 109297·050 | 74780·680 | 30715·718 | |
| p value | <0·001 | <0·001 | 0·3333 | <0·001 | <0·001 | |
| PARAMETRIC BOOTSTRAPPED LIKELIHOOD RATIO TEST FOR 1 (H0) VERSUS X CLASSES | ||||||
| H0 Loglikelihood Value | −2380830·52 | −2152133·602 | −2081795·285 | −2024390·907 | −1929141·322 | |
| 2 Times the Loglikelihood Difference | 457393·837 | 140676·638 | 110079·418 | 75369·503 | 37930·771 | |
| Difference in the # of Parameters | 11 | 11 | 11 | 10 | 11 | |
| Approximate p value | <0·001 | <0·001 | <0·001 | <0·001 | 0·051 | |
| Successful Bootstrap Draws | 5 | 5 | 5 | 5 | 5 | |
| Entropy | 0·851 | 0·803 | 0·864 | 0·826 | 0·833 | |
H0 = null hypothesis; MLR = Maximum likelihood ratio; AIC = Akaike information criterion; BIC = Bayesian information criterion
Table 3 summarizes the means and standard deviations of monthly commodity and service receipt for each class of the 2- to 6-class LPA models. Inspection of the 5- and 6-class models reveals that some of the classes are not substantially different from each other. For example, in the 5-class model, classes 1 and 2 have a low mean number of annual HIV tests and receive very few syringes and condoms. The same can be said of classes 1, 3, and 4 in the 6-class model. These results further support the choice of the 4-class model.
Table 3.
Latent profile analysis models of commodity and service patterns among 327,758 harm reduction clients in Ukraine, 2011–2014
| 2-class model | 3-class model | 4-class model | 5-class model | 6-class model | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Class 1 | n (%) | 142,372 (43.4%) | 142,372 (43.4%) | 110,112 (33 – 6%) | 89,723 (27.4%) | 96,517 (29.4%) | |||||
| # of HIV tests, annually | 0.188 | 0.469 | 0.529 | 0.774 | 0·409 | 0·807 | 0.239 | 0.426 | 0.105 | 0.348 | |
| # syringes, monthly | 1.698 | 2.059 | 5.939 | 6.600 | 8·622 | 12·463 | 2.769 | 2.431 | 0.818 | 1.202 | |
| # condoms, monthly | 0.721 | 1.008 | 2.155 | 2.166 | 3·158 | 5·404 | 1.143 | 1.213 | 0.354 | 0.470 | |
| # information, monthly | 0.090 | 0.100 | 0.333 | 0.198 | 0·507 | 0·522 | 0.155 | 0.106 | 0.039 | 0.073 | |
| # counseling, monthly | 0.081 | 0.690 | 0.311 | 0.286 | 0·425 | 0·472 | 0.098 | 0.121 | 0.005 | 0.022 | |
| Class 2 | n (%) | 95,222 (29.1%) | 95,222 (29.1%) | 62,663 (19 – 1%) | 54,733 (16.7%) | 48,836 (14.9%) | |||||
| # of HIV tests, annually | 0.729 | 1.011 | 0.141 | 0.410 | 1·000 | 0·000 | 0.077 | 0.266 | 0.592 | 0.863 | |
| # syringes, monthly | 14.270 | 24.687 | 1.029 | 1.262 | 11·862 | 19·045 | 0.549 | 0.681 | 6.902 | 7.331 | |
| # condoms, monthly | 5.361 | 12.400 | 0.458 | 0.711 | 4·325 | 8·880 | 0.246 | 0.367 | 2.529 | 2.979 | |
| # information, monthly | 0.905 | 1.715 | 0.048 | 0.057 | 0·699 | 0·927 | 0.018 | 0.034 | 0.392 | 0.351 | |
| # counseling, monthly | 0.883 | 1.881 | 0.055 | 0.146 | 0·729 | 0·866 | 0.056 | 0.195 | 0.310 | 0.236 | |
| Class 3 | n (%) | 90,164 (27.5%) | 111,280 (34 – 0%) | 90,952 (27.8%) | 94,870 (28.9%) | ||||||
| # of HIV tests, annually | 0.855 | 1.176 | 0·138 | 0·344 | 0.496 | 0.864 | 0.357 | 0.657 | |||
| # syringes, monthly | 22.585 | 33.340 | 1·300 | 1·564 | 9.909 | 13.459 | 2.733 | 7.225 | |||
| # condoms, monthly | 8.653 | 17.381 | 0·566 | 0·823 | 3.594 | 5.869 | 1.133 | 1.762 | |||
| # information, monthly | 1.493 | 2.365 | 0·066 | 0·073 | 0.590 | 0.559 | 0.148 | 0.167 | |||
| # counseling, monthly | 1.461 | 2.740 | 0·059 | 0·138 | 0.518 | 0.504 | 0.091 | 0.105 | |||
| Class 4 | n (%) | 43,703 (13 – 3%) | 52,357 (16.0%) | 13,633 (4.2%) | |||||||
| # of HIV tests, annually | 0·980 | 1·682 | 1.000 | 0.000 | 0.241 | 0.584 | |||||
| # syringes, monthly | 26·141 | 40·173 | 13.419 | 20.437 | 1.481 | 3.875 | |||||
| # condoms, monthly | 10·270 | 21·519 | 4.871 | 9.604 | 0.244 | 0.597 | |||||
| # information, monthly | 1·820 | 3·167 | 0.796 | 0.984 | 0.056 | 0.140 | |||||
| # counseling, monthly | 1·879 | 3·754 | 0.832 | 0.910 | 0.206 | 0.308 | |||||
| Class 5 | n (%) | 39,993 (12.2%) | 23,365 (7.1%) | ||||||||
| # of HIV tests, annually | 1.071 | 1.730 | 0.741 | 1.324 | |||||||
| # syringes, monthly | 27.441 | 41.721 | 46.006 | 64.038 | |||||||
| # condoms, monthly | 10.819 | 22.399 | 19.301 | 35.723 | |||||||
| # information, monthly | 1.907 | 3.296 | 3.314 | 4.799 | |||||||
| # counseling, monthly | 1.967 | 3.909 | 3.787 | 5.626 | |||||||
| Class 6 | n (%) | 50,537 (15.4%) | |||||||||
| # of HIV tests, annually | 0.755 | 1.035 | |||||||||
| # syringes, monthly | 15.016 | 14.645 | |||||||||
| # condoms, monthly | 5.397 | 5.989 | |||||||||
| # information, monthly | 0.928 | 0.717 | |||||||||
| # counseling, monthly | 0.898 | 0.576 | |||||||||
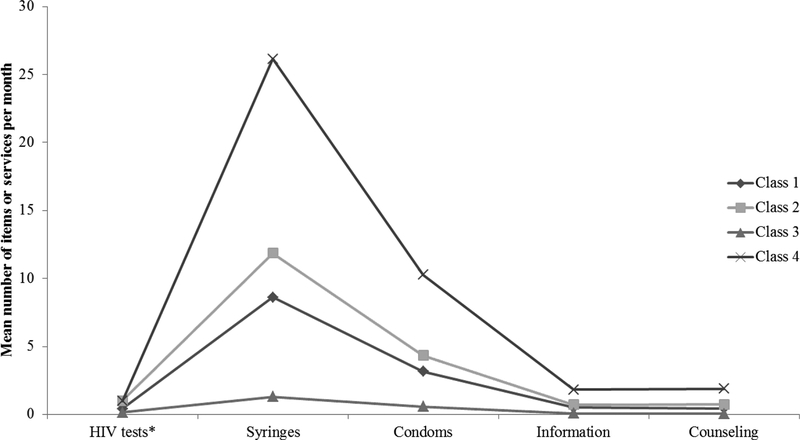
Figure 2 summarizes the 4-class LPA model. Approximately one-third (34.0%) of clients are in class 1; they received a mean of 0.138 HIV tests, 1.3 syringes, 0.566 condom and minimal counseling and information sessions per month. Another one-third (33.6%) of clients are in class 2; they received 8.622 syringes, 3.158 condoms and 0.5 HIV tests, counseling, and information sessions per month on average. In class 3, 19.1% of clients received 1 HIV test per month, 11.862 syringes, 4.325 condoms per month with 0.699 information and 0.729 counseling sessions. Finally, 13.3% of clients were in class 4, which received the most services per month: 1 HIV test, 26.141 syringes, 10.27 condoms, 1.82 information and 1.879 counseling sessions per month. Class 1 and class 2 are distinguished from each other by the difference in the mean number of syringes and condoms distributed to each class as well as the number of annual HIV tests. Thus, based on the model fit indices, entropy, interpretability of the classes, and the fact that the 4-class model is consistent with the field experiences of Alliance Ukraine, the 4-class model was chosen.
Figure 2.
Four class model of commodity and service patterns among 327,758 harm reduction clients in Ukraine, 2011–2014 *Annual HIV tests
The overall HIV incidence in the sample was 23.9 per 1,000 person-years (95% confidence interval [CI] = 22.3, 25.7) across the study period. Incidence among class 1 clients was 29.7 (95% CI=27.3, 32.4) and 17.6 per 1,000 person-years (95% CI=15.6, 19.7) among class 4 clients. Cox proportional hazards regression models were constructed with HIV seroconversion as the outcome and latent class membership as the main exposure of interest. The crude hazard ratio (HR) was 0.43 (95% CI=0.37, 0.50). Note that class 2 and 3 clients had a maximum of 1 HIV test during the study period and were therefore not included in the HIV seroconversion analyses. Additionally, 91.9% of class 1 clients and 84.5% of class 4 clients were excluded because they did not have two HIV tests.
Stratified analyses were constructed for each oblast-level variable (table 4). For most strata of each variable, class 4 clients (1 HIV test, 26.141 syringes, 10.27 condoms, 1.82 information and 1.879 counseling per month) had significantly decreased risk of HIV seroconversion relative to those in class 1 (received 0.138 HIV test, 1.3 syringes, 0.566 condom and little counseling and information per month). There are three instances where the relationship between HIV incidence and class membership was not significant: the second tertile of the total number of syringes distributed, the first tertile of number of syringes per PWID per year, and the first tertile of the proportion of newly registered HIV cases that are PWID.
Table 4.
Multivariable Cox proportional hazards regression models of the association between HIV seroconversion and harm reduction service utilization patterns among 32,743 harm reduction clients with at least two HIV tests, stratified by oblast-level characteristics, 2011–2014
| Percent | Item classes | |
|---|---|---|
| Stratified HR (95% Cl) | ||
| Class 4 vs. Class 1 (ref) | ||
| Crude HR | 0·43 (0·37, 0·50) | |
| Size of the PWID population | ||
| 1,400 – 5,500 | 9·4 | 0·58 (0·37, 0·93) |
| 5,800 – 11,700 | 18·7 | 0·38 (0·25, 0·56) |
| 12,500 – 42,000 | 72·0 | 0·44 (0·37, 0·53) |
| Harm reduction service coverage | ||
| Total number of syringes distributed | ||
| 295,363 – 837,424 | 7·9 | 0·27 (0·13, 0·58) |
| 985,583 – 2,432,788 | 20·1 | 0·76 (0·54, 1·06) |
| 2,508,041 – 13,238,151 | 72·0 | 0·37 (0·31, 0·44) |
| Number of syringes per PWID per year | ||
| 55·3 – 162·2 | 14·8 | 0·67 (0·44, 1·04) |
| 167·5 – 219 | 17·5 | 0·50 (0·33, 0·75) |
| 240·8 – 422·9 | 67·7 | 0·37 (0·31, 0·44) |
| Proportion of PWID with access to clean syringes | ||
| 15·5 – 43·9% | 17·2 | 0·21 (0·13, 0·34) |
| 44·8 – 52·1% | 44·3 | 0·50 (0·40, 0·62) |
| 52·5 – 97·1% | 38·5 | 0·44 (0·35, 0·56) |
| Proportion of PWID that are harm reduction clients | ||
| 6·1 – 19·3% | 17·9 | 0·29 (0·17, 0·47) |
| 24·0 – 42·0% | 44·4 | 0·45 (0·36, 0·57) |
| 44·4 – 96·1% | 37·8 | 0·43 (0·34, 0·54) |
| HIV prevalence and incidence | ||
| Rate of newly registered HIV cases per 100,000 population | ||
| 6·6 – 22·2 | 13·1 | 0·57 (0·36, 0·92) |
| 23·2 – 48·1 | 31·3 | 0·49 (0·38, 0·62) |
| 48·2 – 109·4 | 55·6 | 0·36 (0·29, 0·45) |
| Proportion of newly registered HIV cases that are PWID | ||
| 1·2 – 18·6% | 11·0 | 0·76 (0·48, 1·21) |
| 19·0 – 24·9% | 46·5 | 0·30 (0·22, 0·40) |
| 25·2 – 39·8% | 42·5 | 0·43 (0·36, 0·53) |
| HIV prevalence among PWID <25 years old | ||
| 0 – 1·4% | 17 – 4 | 0·61 (0·39, 0·95) |
| 1·5 – 4·4% | 34·3 | 0·32 (0·23, 0·44) |
| 5·2 – 28·3% | 48·2 | 0·42 (0·35, 0·51) |
| Drug and HIV treatment coverage | ||
| Proportion of PWID on MAT | ||
| 0·8 – 2·4% | 46·7 | 0·44 (0·27, 0·71) |
| 2·5 – 3·8% | 39·4 | 0·28 (0·16, 0·48) |
| 5·0 – 8·6% | 13·9 | 0·45 (0·38, 0·54) |
| Proportion of PWID on ARVs | ||
| 1·5 – 25·6% | 22·7 | 0·39 (0·31, 0·51) |
| 26·5 – 39·3% | 48·2 | 0·36 (0·28, 0,47) |
| 42·0 – 70·0% | 29·1 | 0·47 (0·35, 0·63) |
ARV = antiretroviral therapy; MAT = medication-assisted therapy; PWID = people who inject drugs
Two dose-response associations were observed for the relationship between class membership and HIV acquisition within strata of (1) syringes per PWID per year and (2) rate of newly registered HIV cases per 100,000 population. The HRs for HIV incidence decreased with each increase in the syringes per PWID per year. In oblasts where 55.3–162.2 syringes are distributed per PWID per year (i.e., equivalent to 1.1 to 3.1 syringes per week), class 4 clients had 33% decreased risk for HIV (stratified HR=0.67, 95% CI=0.44, 1.04) as compared to class 1 clients. For class 4 clients in oblasts where 167.5 to 219 syringes are distributed per PWID per year (i.e., equivalent to 3.2 to 4.2 syringes per week), there was a 50% decreased risk for HIV as compared to class 1 clients (stratified HR=0.50, 95% CI=0.33, 0.75). For class 4 clients living in oblasts where 240.8 to 422.9 syringes are distributed per PWID per year (i.e., equivalent to 4.8 syringes per week to 1.16 syringes per day), there was a 63% decreased risk for HIV as compared to class 1 clients (stratified HR=0.37, 95% CI=0.31, 0.44).
Stratified HRs also decreased with increasing rate of newly registered HIV cases per 100,000 population tertiles. Compared to class 1 clients, class 4 clients had 43% decreased risk for HIV acquisition if they lived in oblasts with 6.6 to 22.2 new HIV cases per 100,000 population (stratified HR=0.57, 95% CI=0.36, 0.92), 51% decreased risk in oblasts with 23.2 to 48.1 new HIV cases per 100,000 population (stratified HR=0.49, 95% CI=0.38, 0.62), and 64% decreased risk in oblasts with 48.2 to 109.4 new HIV cases per 100,000 population (stratified HR=0.36, 95% CI=0.29, 0.45).
Stratified analyses were also conducted for each harm reduction venue (table 5). Secondary exchange, street venues, pharmacies, and office-based venues were associated with significantly reduced risk of HIV incidence among class 4 versus class 1 clients. Mobile vans and other sources were not significant predictors of HIV incidence.
Table 5.
Multivariable Cox proportional hazards regression models of the association between HIV seroconversion and harm reduction service utilization patterns among 32,743 harm reduction clients with at least two HIV tests, stratified by main syringe source, 2011–2014
| Percent | Item classes | |
|---|---|---|
| Stratified HR (95% Cl) | ||
| Class 4 vs. Class 1 (ref) | ||
| Crude HR | 0·43 (0·37, 0·50) | |
| Secondary exchange | 7·9 | 0·66 (0·44, 0·99) |
| Street | 48·7 | 0·41 (0·33, 0,51) |
| Mobile | 4·2 | 0·51 (0·22, 1·16) |
| Pharmacy | 6·1 | 0·24 (0·10, 0·58) |
| Office | 29·1 | 0·34 (0·25, 0·47) |
| Health care facility | 0·3 | -- |
| Other source | 3·3 | 0·56 (0·26, 1·23) |
| Missing | 0·6 | -- |
Discussion
Analyses of data from 2005 to 2012 suggested that the Ukrainian HIV epidemic was slowing (Vitek et al., 2014) but more recent data from the Ukrainian Ministry of Health reports increased HIV prevalence among PWID in 2015 (Ukraine UNAIDS Global AIDS Response Progress Reporting [GARPR], 2016). Injection drug use continues to be a major mode of HIV transmission, making evaluation of harm reduction efforts in reducing HIV incidence among PWID critical. Some studies in the 1990s found that risk of HIV was higher among NSP users. For example, NSP users were somewhat more likely to seroconvert than non-users in Montreal and frequent NSP attendance predicted HIV serostatus in Vancouver (Bruneau et al., 1997; Strathdee et al., 1997). However, analyses of the large SYREX database in this study suggests that, across all oblasts and regardless of oblast characteristics, receiving more syringes and condoms predicted decreased risk of HIV acquisition. One of the two dose response relationships observed also seems to support this – living in an oblast that distributed more syringes per PWID per year was associated with lower risk of HIV acquisition.
With respect to harm reduction utilization patterns, the classes were best distinguished by the mean number of condoms and syringes provide each month. There was limited variation related to number of counseling and information sessions per month or the number of HIV tests over the study period. For example, class 1 received counseling approximately once every 3 months, class 2 was counseled approximately once every two months, class 3 was counseled less than once a year, and class 4 was counseled almost twice per month. This is likely congruent with the way services are provided in the NGOs – formal counseling and information sessions are provided less frequently than commodities. Daily counseling sessions would not be expected for those who received the equivalent of one or more syringes per day.
More syringes than condoms were distributed. Without having empirical data on condom sources, we can speculate that condoms are readily available outside of harm reduction programs, for free or for purchase, while syringes are more limited in their availability (i.e., NSPs and pharmacies). Another possible explanation is that injection episodes may be more frequent than sexual episodes. As a result, more syringes are needed. Data from the IBBS study in 2011 seem to support this explanation: 7.9% of PWID reported sexual contact at least every day while 30.8% report injecting 7 or more times per week.
HIV testing did not vary appreciably across classes. This is likely due to programmatic constraints - Alliance Ukraine only has funding to test 40% of its harm reduction clients each year. As a result, only 10% of clients had two or more HIV tests across the study period. HIV testing is likely not randomly offered to all clients, as high-risk and/or symptomatic clients would be more likely to undergo testing.
In terms of stratified oblast-level analyses, class 4 clients were significantly less likely to seroconvert to HIV as compared to class 1 clients within most strata of the harm reduction service coverage, HIV prevalence and incidence, and drug and HIV treatment coverage variables. Although HRs were protective, with the exception of the dose-response observed for syringes per PWID per year and newly registered HIV case rate, the strength of the associations were inconsistent across strata.
Two dose response relationships were observed, such that more syringes per PWID per year and higher rate of newly registered HIV cases per 100,000 population was associated with lower risk of HIV acquisition. In oblasts with higher incidence, the effect of syringe and condom distribution may be more pronounced. It is important to note that the Donetska and Luganska oblasts are also partially occupied by Russia. The confrontation with pro-separatist and Russian forces in this area began in April 2014 may influence public health programming (Mackey & Strathdee, 2015), including the HIV response in these regions, and might be reflected in SYREX dataset covering the period Jan 2011 – Sep 2014.
Limitations of this study should be acknowledged. SYREX was designed for monitoring and recording service provision in HIV prevention programs and not for research purposes. As a result, the number of variables available for analysis is limited. For example, without injection and sex frequency, it is unclear if the different classes represent individuals who need fewer commodities because they have sex and inject drugs less frequently or because they are not receiving the optimal level of services. Two regions have missing oblast-level variables: the Autonomous Region of Crimea and Sebastopol City. These two regions are in Russian-occupied Crimea and data are not readily available at this time. Thus, the extent to which these associations are causal cannot be fully determined. Despite these limitations, SYREX represents all PWID accessing harm reduction services in Ukraine and has a large sample size for determining patterns of harm reduction utilization and predicting the impact of harm reduction on HIV incidence.
In summary, these data suggest the effectiveness of harm reduction services for reducing HIV incidence in Ukraine – an intervention shown to be cost effective (Kim, Pulkki-Brannstrom, & Skordis-Worrall, 2014). A major barrier to providing the full recommended WHO package of harm reduction interventions (World Health Organization, 2014) for PWID is adequate resources for HIV testing, MAT, and ARVs. The recent announcement regarding the Ukrainian government fully funding MAT beginning in 2017 is a step in the right direction, assuming that this includes increasing access. The conflict in Ukraine may be turning back the progress that has been made in addressing the country’s HIV epidemic (Mackey & Strathdee, 2015). HIV testing should be scaled-up both for case-finding and for its utility as a powerful behavioral intervention (Fonner, Denison, Kennedy, O’Reilly, & Sweat, 2012; Mazhnaya et al., 2014). These data also support scaling up syringe and condom distribution levels across oblasts.
References
- Alliance Ukraine. 2014. Ânnual report of ICF “International HIV/AIDS Alliance in Ukraine” in 2013. http://www.aidsalliance.org.ua/ru/library/our/finalreport/pdf/ar_2013_en.pdf, acccessed 12 February 2016.
- Alliance Ukraine. (2015). Automated Records Management System in Harm Reduction Programs: SyrEx2. Kiev, Ukraine: ICF International AIDS Alliance in Ukraine; Retrieved from http://www.aidsalliance.org.ua/cgi-bin/index.cgi?url=/en/library/syrex/index.htm [Google Scholar]
- Balakireva OM, Bondar TV, Loktieva I, Sazonova YO, & Sereda YV (2014). Summary of the analytical report: monitoring the behaviour and HIV-infection prevalence among people who inject drugs as a component of HIV second generation surveillance. from http://www.aidsalliance.org.ua/ru/library/our/2014/arep14/zvit%20IDU_obl_eng.pdf
- Balakirieva O, Bondar T, Loktieva I, Sazonova Y, & Sereda Y 2014. Ŝummary of the analytical report: Monitoring the behaviour and HIV-infection prevalence among people who inject drugs as a component of HIV second generation surveillance, according to the results of 2013 bio-behavioral survey. http://www.aidsalliance.org.ua/ru/library/our/2014/arep14/zvit%20IDU_obl_eng.pdf, acccessed 2 April 2015.
- Balakirieva OM, Bondar TV, & Denysuk AI 2007. ^Monitoring behaviour of injecting drug users as a component of second generation epidemiological surveillance. http://www.aidsalliance.org.ua/ru/library/our/monitoring/pdf/2011report_IDUs_eng_finale_2.pdf acccessed 14 May 2015.
- Balakirieva OM, Bondar TV, Sereda YV, & Sazonova YO 2012. ^Behaviour monitoring and HIV prevalence among injecting drug users as a component of second generation sentinel surveillance. http://www.aidsalliance.org.ua/ru/library/our/2012/me/idu_en_2011.pdf, acccessed 14 May 2015.
- Berleva G, Dumchev K, Kasianchuk M, Nikolko M, Saliuk T, Shvab I, & Yaremenko O 2012. Êstimation of the Size of Populations Most-at-Risk for HIV Infection in Ukraine. http://www.aidsalliance.org.ua/ru/library/our/2013/SE_2012_Eng.pdf, acccessed
- Berleva G, Dumchev K, Kasianchuk M, Nikolko M, Saliuk T, & Yaremenko O 2012. Ânalytical report: Estimation of the size of populations most-at-risk for HIV infection in Ukraine as of 2012, based on the results of 2011 survey. http://www.aidsalliance.org.ua/ru/library/our/2013/SE_2012_Eng.pdf, acccessed 2 April 2015.
- Bojko MJ, Mazhnaya A, Makarenko I, Marcus R, Dvoriak S, Islam Z, & Altice FL (2015). “Bureaucracy & Beliefs”: Assessing the Barriers to Accessing Opioid Substitution Therapy by People Who Inject Drugs in Ukraine. Drugs (Abingdon Engl), 22(3), 255–262. doi: 10.3109/09687637.2015.1016397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce RD, Dvoryak S, Sylla L, & Altice FL (2007). HIV treatment access and scale-up for delivery of opiate substitution therapy with buprenorphine for IDUs in Ukraine--programme description and policy implications. Int J Drug Policy, 18(4), 326–328. doi: 10.1016/j.drugpo.2006.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau J, Lamothe F, Franco E, Lachance N, Desy M, Soto J, & Vincelette J (1997). High rates of HIV infection among injection drug users participating in needle exchange programs in Montreal: results of a cohort study. Am J Epidemiol, 146(12), 994–1002. [DOI] [PubMed] [Google Scholar]
- Crawford C (2015). One Year on from the Annexation of Crimea. Konrad-Adenauer-Stiftung International Reports, 6, 6–20. [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, … Beyrer C. (2014). What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy, 25(1), 53–60. doi: 10.1016/j.drugpo.2013.08.004 [DOI] [PubMed] [Google Scholar]
- Denisiuk O, Smyrnov P, Kumar AM, Achanta S, Boyko K, Khogali M, … Zachariah R. (2014). Sex, drugs and prisons: HIV prevention strategies for over 190 000 clients in Ukraine. Public Health Action, 4(2), 96–101. doi: 10.5588/pha.13.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Wirtz AL, Baral S, Beyrer C, & Cleghorn FR (2012). Key harm reduction interventions and their impact on the reduction of risky behavior and HIV incidence among people who inject drugs in low-income and middle-income countries. Curr Opin HIV AIDS, 7(4), 362–368. doi: 10.1097/COH.0b013e328354a0b5 [DOI] [PubMed] [Google Scholar]
- Dvoriak S, Karachevsky A, Chhatre S, Booth R, Metzger D, Schumacher J, … Woody G. (2014). Methadone maintenance for HIV positive and HIV negative patients in Kyiv: acceptability and treatment response. Drug Alcohol Depend, 137, 62–67. doi: 10.1016/j.drugalcdep.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonner VA, Denison J, Kennedy CE, O’Reilly K, & Sweat M (2012). Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev, 9, CD001224. doi: 10.1002/14651858.CD001224.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanski T (2015). A Ship Run Aground. Deepening Problems in the Ukrainian Economy. Center for Eastern Studies/Osrodek Studiow Wschodnic Commentary, 173, 1–8. [Google Scholar]
- Kim SW, Pulkki-Brannstrom AM, & Skordis-Worrall J (2014). Comparing the cost effectiveness of harm reduction strategies: a case study of the Ukraine. Cost Eff Resour Alloc, 12, 25. doi: 10.1186/1478-7547-12-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey TK, & Strathdee SA (2015). Responding to the public health consequences of the Ukraine crisis: an opportunity for global health diplomacy. J Int AIDS Soc, 18(1), 19410. doi: 10.7448/ias.18.1.19410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenko I, Mazhnaya A, Polonsky M, Marcus R, Bojko MJ, Filippovych S, … Altice FL. (2016). Determinants of willingness to enroll in opioid agonist treatment among opioid dependent people who inject drugs in Ukraine. Drug Alcohol Depend, 165, 213–220. doi: 10.1016/j.drugalcdep.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazhnaya A, Andreeva TI, Samuels S, DeHovitz J, Salyuk T, & McNutt LA (2014). The potential for bridging: HIV status awareness and risky sexual behaviour of injection drug users who have non-injecting permanent partners in Ukraine. J Int AIDS Soc, 17, 18825. doi: 10.7448/IAS.17.1.18825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2015). Mplus User’s Guide. Los Angeles: Muthén & Muthén. [Google Scholar]
- Nylund KL, Asparoutiov T, & Muthen BO (2007). Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Structural Equation Modeling-a Multidisciplinary Journal, 14(4), 535–569. [Google Scholar]
- Pohorila N, Taran Y, Kolodiy I, & Diyeva T 2009. ^Behaviour monitoring and HIV-infection prevalence among injecting drug users. http://www.aidsalliance.org.ua/ru/library/our/monitoring/pdf/rep_idu_eng10.pdf acccessed 14 May 2015.
- Police block needle exchange program. (1997). Kyiv Post. Retrieved from http://www.kyivpost.com/article/content/ukraine/police-block-needle-exchange-program-1776.html
- Strathdee SA, Patrick DM, Currie SL, Cornelisse PG, Rekart ML, Montaner JS, … O’Shaughnessy MV (1997). Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS, 11(8), F59–65. [DOI] [PubMed] [Google Scholar]
- Ukraine harmonized report on progress in the implementation of national response to AIDS (GARPR Ukraine): Reporting period: January 2015 - December 2015. (2016). from http://www.unaids.org/sites/default/files/country/documents/UKR_narrative_report_2016.pdf
- Ukraine Ministry of Health. 2015. ĤIV infection in Ukraine Informational Bulletin #43. http://ucdc.gov.ua/pages/diseases/hiv_aids/monitoring/information-bulletins, acccessed 11 Oct 2015.
- Ukrainian Center for Disease Control. 2016. Înformation on quantitative and qualitative characteristics of non-personal SMT patients as of 09.01.2016. http://ucdc.gov.ua/uploads/documents/ca45c0/4b2808f040b3d3885dd4086c170977ad.xlsx, acccessed 6 Nov 2016.
- UNAIDS. (2016). Ukrainian Government to fully finance opioid substitution therapy [Press release]. Retrieved from http://www.unaids.org/en/resources/presscentre/featurestories/2016/november/20161103_ukraine
- Vitek CR, Cakalo JI, Kruglov YV, Dumchev KV, Salyuk TO, Bozicevic I, … Rutherford GW (2014). Slowing of the HIV epidemic in Ukraine: evidence from case reporting and key population surveys, 2005–2012. PLoS One, 9(9), e103657. doi: 10.1371/journal.pone.0103657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, & Shepard D (2010). Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet, 376(9738), 355–366. doi: 10.1016/S0140-6736(10)60832-X [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2014. ^Policy brief: Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. http://apps.who.int/iris/bitstream/10665/128049/1/WHO_HIV_2014.8_eng.pdf?ua=1&ua=1, acccessed 12 February 2016. [PubMed]
- Zaller N, Mazhnaya A, Larney S, Islam Z, Shost A, Prokhorova T, … Flanigan T (2015). Geographic variability in HIV and injection drug use in Ukraine: implications for integration and expansion of drug treatment and HIV care. Int J Drug Policy, 26(1), 37–42. doi: 10.1016/j.drugpo.2014.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]