Abstract
Pulmonary hypertension (PH) and its severe subtype pulmonary arterial hypertension (PAH) encompass a set of multifactorial diseases defined by sustained elevation of pulmonary arterial pressure and pulmonary vascular resistance leading to right ventricular failure and subsequent death. Pulmonary hypertension is characterized by vascular remodeling in association with smooth muscle cell proliferation of the arterioles, medial thickening, and plexiform lesion formation. Despite our recent advances in understanding its pathogenesis and related therapeutic discoveries, PH still remains a progressive disease without a cure. Nevertheless, development of drugs that specifically target molecular pathways involved in disease pathogenesis has led to improvement in life quality and clinical outcomes in patients with PAH. There are presently more than 12 Food and Drug Administration-approved vasodilator drugs in the United States for the treatment of PAH; however, mortality with contemporary therapies remains high. More recently, there have been exuberant efforts to develop new pharmacologic therapies that target the fundamental origins of PH and thus could represent disease-modifying opportunities. This review aims to summarize recent developments on key signaling pathways and molecular targets that drive PH disease progression, with emphasis on new therapeutic options under development.
Keywords: pulmonary artery hypertension, molecular pathology, vascular function, proliferation, inflammation, metabolism, DNA damage, epigenetics
Introduction
Pulmonary Hypertension
Pulmonary hypertension (PH) is defined as an abnormal elevation in resting mean pulmonary arterial pressure (mPAP) above 25 or 30 mm Hg with exercise.1 It is a debilitating disease with poor outcomes, an incompletely identified underlying pathophysiology, and limited treatment options.2 Pulmonary vascular remodeling is a prominent feature of PH.3,4 On the cellular level, it is characterized by endothelial dysfunction and elevated contractility of the small pulmonary arteries (muscularization), resulting in altered intimal, medial, and adventitial activity that still remains incompletely understood.2–5
Clinical Classification of PH (World Health Organization Classification: Groups 1–5)
The World Health Organization (WHO) classifies PH into 5 groups based on pathophysiological clinical and therapeutic considerations (Table 1).6 Pulmonary arterial hypertension (PAH: WHO group 1) is caused by vasoconstriction and stiffening of the small arteries in the lungs secondary to cell proliferation, fibrosis, as well as the development of in situ thrombi or plexiform lesions.3 The ensuing rise in the pulmonary vascular resistance (PVR) leads to increased right ventricular (RV) afterload. Without effective therapeutic treatment, patients with PAH eventually die from RV heart failure.8 Causes of PAH can range from idiopathic, heritable, or associated secondary diseases such as connective tissue disease, HIV infection, drug/toxin exposure, among others. The are other subtypes of PH are PH-driven left ventricular heart disease (WHO group 2), chronic lung diseases and/or hypoxia (WHO group 3), chronic thromboembolic PH (CTEPH, WHO group 4), and miscellaneous or multifactorial etiologies (WHO group 5).
Table 1.
| Group 1: Pulmonary arterial hypertension (PAH) |
| 1.1 Idiopathic |
| 1.2 Heritable (BMPR2, ALK-1, ENG, SMAD9, CAV1, KCNK3) |
| 1.3 Drugs or toxin induced |
| 1.4 Associated with connective tissue disease, HIV infection, portal hypertension, congenital heart disease, and schistosomiasis |
| Group 2: PH—left heart disease |
| 2.1 Left ventricular systolic dysfunction |
| 2.2 Left ventricular diastolic dysfunction |
| 2.3 Valvular disease |
| 2.4 Congenital heart disease |
| Group 3: PH—lung diseases and/or hypoxia |
| 3.1 COPD |
| 3.2 Interstitial lung disease |
| 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern |
| 3.4 Sleep disorder |
| 3.5 Alveolar hypoventilation |
| 3.6 Chronic exposure to high altitude |
| 3.7 Developmental lung diseases |
| Group 4: Chronic thromboembolic pulmonary hypertension (CTEPH) |
| Group 5: PH—multifactorial mechanisms |
| 5.1 Hematologic disorders (chronic hemolytic anemia, myeloproliferative disorders, splenectomy) |
| 5.2 Systemic disorders (sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis) |
| 5.3 Metabolic disorders (glycogen storage disease, Gaucher disease, thyroid disorders) |
| 5.4 Others (tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH) |
Abbreviations: ALK-1, activin receptor-like kinase 1; BMPR2, bone morphogenetic protein receptor 2; CAV1, caveolin 1; ENG, endoglin; KCNK3, potassium channel subfamily K member 3; PH, pulmonary hypertension; SMAD9, mothers against decapentaplegic homolog 9.
Adapted from Simonneau et al6 with permission from the publisher. Copyright 2013, Journal of the American College of Cardiology
Nice, 2013; Simonneau et al., 2019.7
Pathological Features of PH
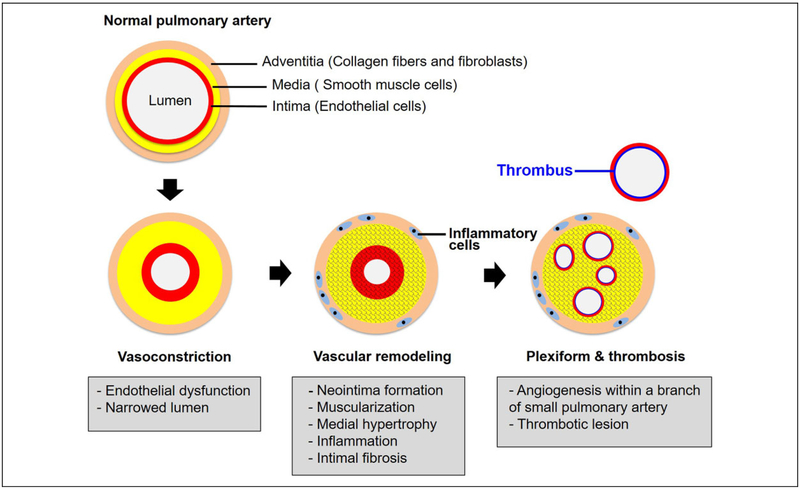
The PAH has a multifactorial pathobiology that affects the small pulmonary arteries (diameter <500 μm). A wide array of cellular alterations can all be observed in PAH, ranging from medial hypertrophy, intimal proliferative, and fibrotic changes, to adventitial thickening with moderate perivascular inflammatory infiltrates (which may be more pronounced and organized in tertiary lymphoid tissue), complex lesions (plexiform, dilated lesions), and thrombotic lesions.2–4 Figure 1 highlights some key pathological abnormalities in PAH.
Figure 1.
Pathogenesis of PAH. Multiple vascular cell types, endothelial cells, smooth muscle cells, and fibroblasts are involved in pulmonary arterial pathobiology. Healthy endothelium modulates the balance between vasodilation and vasoconstriction and inhibits smooth muscle cell proliferation in order to maintain a low-resistance pulmonary vasculature. Pulmonary vasoconstriction has long been regarded as an early event, and excessive pulmonary vasoconstriction has been related to endothelium dysfunction characterized by reduced production of vasodilators (nitric oxide and prostacyclin), along with overexpression of vasoconstrictors (endothelin-1). Abnormal proliferation of smooth muscle cells is the earliest pathobiological features of vascular remodeling, leading to muscularization of peripheral pulmonary arteries and medial hypertrophy in pulmonary muscular arteries. Recruitment of inflammatory cells and progressive migration of smooth muscle cells further results in intimal fibrosis. In the late stage of disease progression, formation of plexiform lesions and in situ thrombus occlude the vessel lumen leads to progressive reduction of the blood flow, thus establishing PAH. PAH indicates pulmonary arterial hypertension.
Current Therapeutic Targets for PH
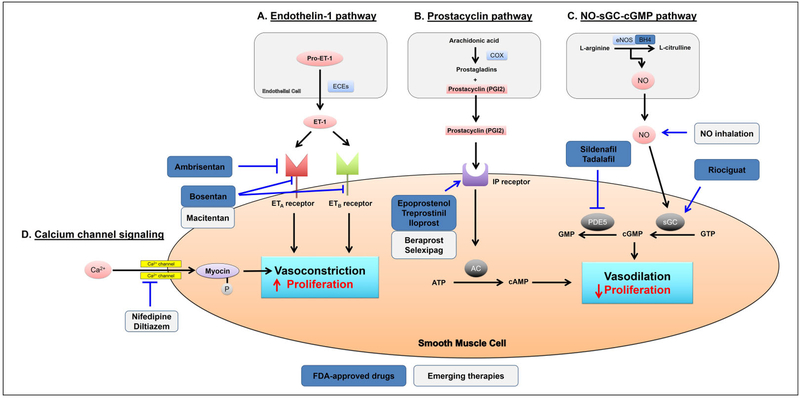
The current mainstays of pharmacologic therapy for PAH are focused on 4 major pathways (Figure 2): the nitric oxide-cyclic guanosine monophosphate signaling pathway (NO-cGMP enhancers), prostacyclin (PGI2) signaling pathway (PGI2 pathway agonists), endothelin (ET) signaling pathway (ET receptor antagonists), and calcium channel signaling pathway (calcium-channel blockers [CCBs]).
Figure 2.
Current targets and therapies in PAH. The 4 major pathways, endothelin-1, prostacyclin, NO-sGC-cGMP, and calcium channel signaling involved in the regulation of pulmonary vasomotor tone, are shown. These pathways represent the targets of all currently approved and emerging PAH therapies. A, Endothelin-1 (ET-1) stimulates vasocontraction and proliferation through activation of both ETA and ETB receptors on smooth muscle cells. Either selective (ambrisentan) or nonselective (bosentan and macitentan) ET-I receptor antagonists can block the ET-I pathway. B, Prostacyclin activates vasodilation and inhibits proliferation of smooth muscle cells through cAMP-dependent pathway. Prostacyclin and its derivatives (epoprostenol, treprostinil, iloprost, and beraprost) and l-prostanoid (IP) receptor (selexipag) provide therapeutic benefit in PAH. C, Nitric oxide (NO) activates vasodilation and antiproliferation of smooth muscle cells through cGMP-dependent pathway. Inhalation of NO and administration of soluble guanylyl cyclase (sGC) stimulator (riociguat) or phosphodiesterase 5 (PDE5) inhibitors (sildenafil and tadalafil) have been shown effective in the treatment of PAH. D, Subsequent activation of calcium (Ca2+) channels and an increase in cytosolic Ca2+ free concentration in smooth muscle cells lead to vasoconstriction and proliferation. Calcium channel blockers (nifedipine and diltiazem) have provided therapeutic benefit in patients with PAH who demonstrate a positive response to the vasoreactivity test. AC indicates adenylyl cyclase; BH4, tetrahydrobiopterin; cGMP, guanosine monophosphate; COX, cyclooxygenase; ECEs, endothelin-converting enzymes; eNOS, endothelial NO synthase; FDA, Food and Drug Administration; PAH, pulmonary arterial hypertension.
Calcium-channel blockers were the first vasodilator agents to gain popular acceptance for the treatment of PAH in the early 1980s.9–11 High-dose CCBs were later found to have survival benefit for patients with idiopathic PAH who respond to acute intravascular administration of pulmonary vasodilators during invasive right heart catheterization.11,12 Based on those data, long-acting nifedipine and diltiazem continue to be prescribed as first-line therapy in appropriate patients with PAH.11 The CCB verapamil is not recommended by some because of greater negative inotropic effects.13
Therapies targeting the NO-cGMP signaling pathway have also been used as pulmonary vasodilators to improve hemodynamics, functional status, and symptomatology in patients with PAH. These medications include nitrovasodilators (inhaled NO), phosphodiesterase 5 (PDE5) inhibitors (sildenafil and tadalafil),14 and cGMP agonists (riociguat).15–17 Ultimately, all of these drugs work to increase the functional concentrations of cGMP, thus decreasing vascular smooth muscle contraction and thus vasomotor tone in pulmonary arterioles.
Beyond the NO-cGMP pathway, it is also known that PGI2 synthase declines in the pulmonary arteries of patients with idiopathic PAH, accompanied by an apparent reduction in PGI2 release and a marked increase in thromboxane-α2.18,19 Thus, supplementation with PGI2 and its analogs has been employed as an effective therapy to induce pulmonary artery dilation. The currently available drugs worldwide that target the PGI2 pathway are epoprostenol (synthetic PGI2), iloprost (PGI2 analog), treprostinil (PGI2 analog), beraprost (PGI2 analog), and selexipag (I-prostanoid receptor agonist).20 A variety of intravenous (IV), subcutaneous, inhaled, and oral formulations of these PGI2 drugs are available for treatment in PAH. Furthermore, there is growing evidence that these medications also inhibit vascular smooth muscle cell proliferation as well as platelet activation and aggregation.21 Such pleiotropic and potentially synergistic activities correlate with existing outcomes data that indicate IV PGI2s lengthen the time to disease worsening22 and suggest PGI2s may have disease-modifying properties beyond simply vasodilation.
Endothelin-1 (ET-1), a protein secreted from pulmonary endothelial cells (ECs), has been shown to be a notable vascular mediator in PAH.3 Bosentan and ambrisentan are ET receptor antagonists that facilitate a significant improvement in pulmonary hemodynamics, exercise capacity (6-minute walk distance; 6MWD), and other symptoms. Improvement in hemodynamics, clinical status, and composite clinical outcome of patients with PAH treated with macitentan (Opsumit [Actelion]), the latest generation of ET receptor antagonist, highlights the significance of this pathway in disease therapy.23
Taken together, the past few years have seen a remarkable increase in our understanding of the cellular and molecular mechanisms responsible for the pathobiology of PH. In total, 14 Food and Drug Administration (FDA)-approved drugs for the treatment of PAH and CTEPH are currently available in the United States; however, mortality with the current therapies remains high. Based on the most recent findings of the US-based REVEAL registry, the 5-year survival rates by WHO functional classes I to IV in patients with newly diagnosed PAH are 72.2%, 71.7%, 60.0%, and 43.8%, respectively.24 Thus, there is still an unmet need for novel and disease- modifying therapeutic agents for the treatment of PH, beyond simply pulmonary vasodilation. This review focuses on the emerging therapeutic possibilities and how they relate to specific mechanisms involved in pathogenesis.
Emerging Therapeutic Strategies and Targets for PH
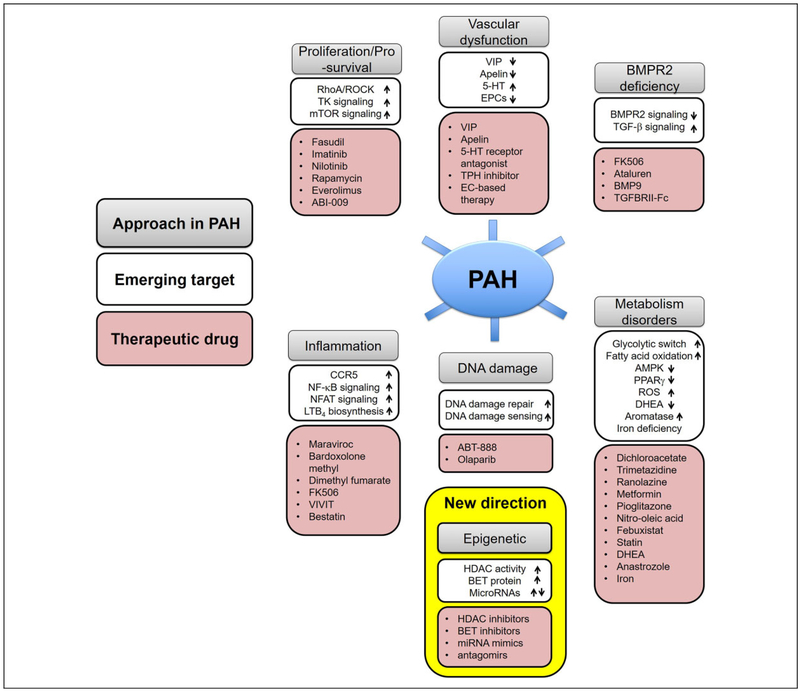
Several key molecular, cellular, and genetic abnormalities that are vital for the vascular remodeling of PH have recently been identified. Therapeutic approaches targeting pulmonary arterial homeostasis and proliferation, bone morphogenetic protein receptor 2 (BMPR2) signaling deficiency, inflammation, DNA damage, and mitochondrial/metabolic dysfunction may be particularly promising and will be discussed (Figure 3).
Figure 3.
Emerging therapeutic targets in PAH. An improved understanding of the molecular and genetic mechanisms leading to PAH have provided translational opportunities for the development and testing of novel therapeutics agents. A number of emerging pathways amenable to therapeutic manipulation are summarized. 5-HT indicates serotonin; AMPK, AMP-activated protein kinase; BET, bromodomain and extra terminal domain; BMP9, bone morphogenetic protein 9; BMPR2, bone morphogenetic protein receptor type 2; CCR5, C-C chemokine receptor 5; DHEA, dehydroepiandrosterone; EC, endothelial cell; EPCs, endothelial progenitor cells; HDAC, histone deacetylases; LTB4, leukotriene B4; miRNA, microRNA; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor kappa light chain enhancer of activated B cells; PAH, pulmonary arterial hypertension; PPARγ, peroxisome proliferator-activated receptor γ; ROS, reactive oxygen species; TK, tyrosine kinase; TPH, tryptophan hydroxylase; VIP, vasoactive intestinal peptide.
Promotion of Vascular Homeostasis
Apelin.
The endogenous peptide apelin, which has vasodilatory effects on the pulmonary vasculature, is related at least in part to the regulation of endothelial NO and NO synthase (NOS) levels.25 Apelin is also a key downstream target of BMPR2 signaling, mediated through the actions of β-catenin and peroxisome proliferator-activated receptor γ (PPARγ).26 Previous studies have revealed that administration of apelin prevents PH in preclinical rodent models.26,27 Furthermore, clinical trials have shown that short-term infusion of apelin decreases PVR and increases cardiac output and stoke volume in patients with PAH with short-term hemodynamic effects.28,29 Although there was no alteration in mPAP and systemic vascular resistance, the long-term effects of apelin in PAH remain undefined.
Serotonin receptor antagonist and tryptophan hydroxylases inhibitor.
Serotonin (5-HT) is a signaling molecule that plays a major role in the pathogenesis of PAH. The 5-HT is released from endothelium and further stimulates smooth muscle cell proliferation and vessel constriction directly through 5-HT receptor or 5-HT transporter (SERT).30,31 Patients with idiopathic PAH carry high levels of circulating 5-HT.32 In preclinical models of disease, increased SERT expression in pulmonary artery smooth muscle cells (PASMCs) drove the development of PH.33 Of the 14 distinct 5-HT receptors, the 5-HT2A, 5-HT2B, and 5-HT1B receptors are especially related to PAH,34,35 and numerous inhibitors and antagonists of 5-HT receptors have been studied in this disease. Treatment with 5-HT1B receptor antagonist LY393558, alone or in combination with fluoxetine (selective 5-HT reuptake inhibitor) and SB224289 (5-HT1B receptor antagonist), reduced 5-HT-induced pulmonary vasoconstriction in rats exposed to hypoxia for 2 weeks.31 Additionally, C-22, an antagonist of the 5-HT2B receptor, can lower pulmonary pressures as well as repress RV hypertrophy and pulmonary vascular remodeling in a monocrotaline-induced PAH model.36 Terguride is an oral antagonist of 5-HT2A and 5-HT2B receptors. In a monocrotaline rat model, it attenuates PAH development through blockading the signaling action of serotonin to the 5-HT2A and 5-HT2B receptors on the vasculature.37 A selective 5-HT2B receptor antagonist, PRX-08066, has been tested in a phase II clinical trial (), displaying efficacy in inhibiting a rise in PAP in humans exposed to hypoxia.
Inhibition of 5-HT synthesis may be another potential therapeutic strategy for the treatment of PAH.38 Two tryptophan hydroxylases (TPH1 and TPH2) are critical for 5-HT synthesis. Genetic TPH1 deficiency has been shown to protect mice from experimentally induced PAH.39–41 Moreover, a recent study demonstrated that TPH1 inhibition by KAR5585 and KAR5418 ameliorates PAH in both moncrotaline (MCT)- induced and SU5416-hypoxia rat models.42 In aggregate, inhibition of either 5-HT synthesis or 5-HT receptors would be a promising therapeutic strategy against PAH; however, due to the pleiotropic effects of 5-HT in other tissues and organ systems, ameliorating untoward side effects and enhancing specificity of action may be key requirements for the future utility of such drugs in this disease.
Vasoactive intestinal polypeptide.
Vasoactive intestinal polypeptide (VIP) is a neuropeptide (28 amino acids) hormone that inhibits smooth muscle cell proliferation and platelet aggregation, scavenges free radical oxygen, and induces pulmonary vasodilation via VPAC1, VPAC2, and PAC1 receptor activation.43 Activation of these receptors stimulates both adenylyl and guanylyl cyclase-signaling pathways. Genetic deficiency of VIP in mice causes spontaneous development of PH44 due to increased expression of genes associated with proinflammation and pulmonary vascular remodeling, as well as decreased expression of genes involved in antiproliferation.45
The expression and receptor-binding affinity of VPAC2 is elevated in PASMCs from patients with PAH. However, VIP levels in the lung and serum are low in PAH.46 As such, supplementation of VIP via nebulization (200 μg daily) was found to improve pulmonary hemodynamics in patients with PAH, resulting in lower PVR and improvement of 6MWD.47 In a clinical study, 20 patients with PAH who inhaled a single dose of 100-μg VIP analog (aviptadil) showed significantly reduced PAP with minimal side effects. In 6 patients, a decrease in PVR of >20% was also reported.48 Furthermore, a combination treatment with VIP and the ET receptor antagonist bosentan has been evaluated in preclinical models of PH, with robust reversal of pulmonary vascular remodeling and improvement of hemodynamics, leading to mortality prevention for at least 45 days. Thus, adding VIP to current vasodilator therapy may be effective,49 but this concept awaits verification in larger clinical trials.
Endothelial progenitor cells.
Endothelial progenitor cells (EPCs) originate from hemangioblasts in bone marrow or mesodermal stem cells and are enriched in peripheral blood.50,51 Circulating in plasma, they gravitate to sites of ischemia or endothelial injury, then differentiate into mature ECs in situ, contributing to revascularization and vascular homeostasis.50 Because loss of or damage to the pulmonary vascular endothelium is central to development of PH, cell-based therapy using EPCs (EC-based therapy) has been considered a potential therapeutic option for PH.52–54
Administration of EPCs has been shown to improve PH. Treatment with umbilical cord blood-derived or autologous EPC has been found to reverse and prevent PH in preclinical rodent models, with improvement of hemodynamics and enriched medial thickening of the small pulmonary arteries.53–55 The EC-based therapy also improved hemodynamic and histologic PH in rodent models, particularly by EPCs transfected with genes such as endothelial NOS (eNOS) that inhibits smooth muscle cell contraction and proliferation.54,56 A combination treatment of EPCs with sildenafil has resulted in synergistic effects on PH manifestation, as compared to EPCs therapy alone.57 Moreover, EC-based therapy may have preventative activity in PH via promoting vascular endothelial repair58 through paracrine stimulation.59,60
Two pilot studies have been reported of patients with idiopathic PAH including adults and children were administrated a single IV infusion of autologous mononuclear cells that provide support for the therapeutic potential of EC-based therapy in human patients.61,62 The PHACeT trial assessing the safety of administrating autologous, cultured, eNOS-transduced mononuclear cells in patients with PAH demonstrated that such delivery was tolerated hemodynamically by these patients and there was evidence of short-term hemodynamic improvement associated with longer term benefits in quality of life and function.63 However, the complete actions of EC-based therapy for PAH remain uncertain, specifically whether the complex behavior of progenitor cells in lung tissue is beneficial or harmful, on balance. For example, in hypoxia, inflammatory and progenitor cells have been found to contribute, rather than ameliorate, to pulmonary vascular remodeling64; but it is unknown whether this directly applies to PAH.
Restoration of BMPR2 Signaling
Heterozygous loss-of-function mutations in BMPR2 have been detected in more than 70% of patients with hereditary PAH as well as in 10% to 40% of patients with sporadic idiopathic PAH.65 The BMPR2 is a member of the transforming growth factor β (TGF-β) receptor family and has been indicated to play a critical role in pathogenesis of PAH. Based on cell and animal findings as well as genetic and pharmacologic data (as summarized in66), activation of the BMPR2 signaling in various vascular cell types prevents pulmonary vascular remodeling and attenuates the severity of pulmonary vascular growth and proliferation.67,68 More recently, additional genetic polymorphisms related to bone morphogenetic protein (BMP) signaling have been linked to heritable PAH, including Smad9, activin A receptor-like type 1, endoglin, and BMP9.69 As such, the putative therapeutic benefits targeting BMPR2 signaling have been investigated substantially and have now progressed into a number of clinical trials.
Most recently, enhancement of endothelial BMPR2 signaling has been explored via delivery of a small peptide mimetic of BMP9.68 Such a ligand has been shown to prevent apoptosis and enhance the integrity of both blood outgrowth ECs and pulmonary artery endothelial cells (PAECs) from patients with PAH carrying BMPR2 mutations. Furthermore, BMP9 delivery reverses PAH in mice with heterozygous R899X mutation, as well as SU5416-hypoxia and monocrotaline models of PAH in rats. A drug discovery company (Morphogen-IX, Cambridge, UK) has recently been founded to develop BMPs as a novel therapy for PAH.
Because loss of function in BMPR2 leads to enhanced TGF-β signaling associated with PH pathology,70,71 therapies that suppress TGF-β signaling may also be effective in ameliorating PAH. The immunoglobulin-Fc fusion protein of TGF-β (TGFBRII-Fc), a selective TGF-β inhibitor targeting TGF-β 1/3, has been examined in 3 PH animal models (monocrotaline-exposed rats as well as SU5416-hypoxiaexposed mice and rats). Administration of TGFBRII-Fc improved vascular remodeling, pulmonary hemodynamics, and overall survival in these models.72 Two ligand traps (sotatercept and luspatercept) were successful in phase I clinical trials and are currently being exam ined in phase II trials ( and ) in a diverse population of patients with PH.
Beyond designing specific drugs targeting molecules directly involved in BMPR2 signaling, repurposing of specific existing drugs that affect BMP signaling has also been pursued. Based on in vitro drug screening coupled with cell culture and animal modeling, FK506 (tacrolimus), a calcineurin inhibitor, was reported to promote expression and activity of BMPR2, prevent PAH development in mice with a deletion of BMPR2 in endothelium, and reverse PAH in monocrotaline and SU5416-hypoxia rat models.73 In a recent, single-center 16-week clinical trial in 20 patients with PAH, while FK506 was found to increase BMPR2 expression as well as improvement of 6MWD and echocardiographic parameters of heart failure in some patients, these changes were not significant.74 Nonetheless, FK506 was generally well tolerated and thus suggest the utility of supporting the study of FK506 in a phase IIb/III efficacy trial. More recently, another repurposed drug, enzastaurin, was found to improve experimental PH based on its effects on the fragile histidine triad, a novel BMPR2 modifier.75
Finally, ataluren is a drug that interacts with ribosomes to enable the readthrough of premature stop codons (such as those found in a majority of BMPR2 mutations that predispose to PAH) which is already undergoing phase III clinical trials in cystic fibrosis. An in vitro study has shown that treatment with ataluren restores BMPR2 expression in lung vascular and peripheral blood cells from patients carrying nonsense mutations of BMPR2 and Smad9.76 Further human studies of this drug in hereditary PAH are pending. If successful, such therapy would provide a clear impetus to expand the use of genetic testing in order to define BMPR2 mutation status in all patients with PAH.
Inhibition in Proliferation and Prosurvival Signaling
Rho kinase inhibitors.
RhoA (Ras homolog), a small monomeric G-protein that comprises several Cdc42, Rac, and Rho subfamilies, and their downstream effectors (eg, ROCK) interact with each other and further stimulate other intracellular signaling pathways to regulate cell adhesion, migration, proliferation, contraction, apoptosis, and hypertrophy.77,78 RhoA/ROCK signaling has been implicated in PH pathogenesis through vascular remodeling and vasoconstriction.79–81 The first ROCK inhibitor, fasudil, was approved for the treatment of subarachnoid hemorrhage-induced brain vessel vasospasm s.82,83 Chronic fasudil treatment has also been found to improve pathologic parameters of PH and pulmonary artery remodeling in both hypoxia- and monocrotaline-induced rodent models of PH.84–86 In patients with PH, low IV administration of fasudil caused modest acute decreases in PVR.87,88 Fasudil may also show efficacy in a rat model of end-stage PH due to left ventricular heart disease.89 Despite these promising results, the roles and mechanisms of RhoA/ROCK signaling in the development and progression of PH remain unknown. The combination of fasudil with certain pulmonary vasodilators may show synergistic effects in animal models, but no clinical trial has evaluated the long-term efficacy of fasudil in PAH.90
Receptor tyrosine kinase inhibitors.
Multiple growth factors have been associated with the abnormal proliferation and migration of pulmonary artery vascular smooth muscle cells (PAVSMCs) in PH such as epidermal growth factor, fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF).91–94 As a potent mitogen, PDGF forms homodimers or heterodimers and exerts its effects via 2 receptor tyrosine kinases (RTKs): PDGFR-α and PDGFR-β.95 As shown in cancer therapeutics, blocking these RTKs may have substantial effects in mitigating cellular proliferation. Because of the growing appreciation of molecular similarities of cancer and vascular cells seen in PH,96 the possibility of repurposing RTK inhibitors has been pursued.
Imatinib is a tyrosine kinase inhibitor (TKI) that was first used to treat chronic myelogenous leukemia.97 In experimental PH, imatinib was found to inhibit proliferation of PASMCs and reverse neointima formation in rodents through inhibition of PDGFR phosphorylation and downstream signaling.91 Moreover, the effects of imatinib in patients with PAH were studied in a randomized, double-blind, placebo-controlled phase II study.98 Fifty-nine patients (WHO functional classes II-IV) who followed over 3 months of PAH-specific therapy (PGI2 analogs, ERAs or PDE5 inhibitors) were administered an oral imatinib (400 mg daily) or placebo. Upon 24 weeks of treatment, the study failed to meet its primary efficacy end point of improvement in 6MWD. However, some secondary end points such as PVR were improved. A post hoc analysis, which stratified patients according to baseline median PVR, showed improvements in hemodynamics and 6MWD in patients treated with imatinib who had PVR baseline more than 1000 dyn s/cm5. In a phase III trial of imatinib in PAH, 202 patients with elevated PVR (>800 dyn s/cm5) receiving imatinib showed significant improvement in 6MWD, along with decreased mean PAP and PVR.99 However, this trial also called into question the safety of using imatinib in patients with PAH, particularly an elevated risk of subdural hematoma, for those receiving imatinib and anticoagulation. Approval for use of imatinib in patients with PAH was not granted, but nonetheless these results may suggest a benefit for this type of therapy if a more specific PAH population with a more favorable risk-benefit profile could be identified. Alternatively, other TKIs may be of interest, such as nilotinib, a second-generation TKI with 30-fold more potency than imatinib which was found to robustly reverse pulmonary vascular remodeling in experimental PH.100 Related approaches such as FGF2 small interfering RNAs and a pharmacologic FGFR1 inhibitor (SU5402) have been shown to reverse experimental PH.101
Mammalian target of rapamycin inhibitors.
Beyond RTKs, mammalian target of rapamycin (mTOR) is a serine/threonine kinase that plays an important role in cell proliferation, survival, and metabolism in response to various environmental factors. The mTOR signaling pathway has been implicated in pulmonary vascular remodeling,102 and thus mTOR signaling may present an attractive potential therapeutic target for PAH associated with severe pulmonary vascular remodeling.
Rapamycin and its analogs are currently the only FDA-approved mTOR inhibitors to predominantly suppress the mammalian target of rapamycin complex 1 (mTORC1), a kinase complex that regulates survival, growth, and metabolism in response to available cellular nutrients and energy. Rapamycin is in clinical use as an immunosuppressor to prevent transplant rejection and as an antiproliferative agent applied to coronary stents to reduced local restenosis.103 Rapamycin has been shown to inhibit proliferation in 3 types of PA vascular cells (EC, SMC, and fibroblast) by suppression of mTORC1. Moreover, rapamycin treatment over a 24-hour period triggers apoptosis in primary human ECs, human umbilical vein ECs, and aortic ECs, by inhibition of mTORC2.104,105 All together, these studies indicate that rapamycin may be applicable as a proapoptotic drug for human pulmonary vascular cell types in PAH as well. In preclinical studies, rapamycin treatment reduced the proliferation of PASMCs from monocrotaline-exposed PH model of rats106 and PAVSMCs from patients with idiopathic PAH.107 In hypoxia-induced PH m ice, rapamycin attenuated thickening and proliferation of the pulmonary vasculature as well as RV hypertrophy to prevent vascular remodeling.108 In a small clinical study, a second mTOR inhibitor, everolimus, improved PVR and 6MWD in 8 of the 10 patients with PAH or CTEPH.109 An albumin-bound mTOR inhibitor (ABI-009) is also now being evaluated in patients with severe PAH ().
Anti-Inflammatory Mechanisms
Chronic inflammation is increasingly considered an important and causative factor of various subtypes of PH, contributing to structural pulmonary vessel remodeling and stiffening.110,111 Among inflammatory mediators, cytokines and chemokines, which mediate innate and acquired immune cell (T cells, B cells, leukocytes, microphages, dendritic cells, and mast cells) trafficking, result in great degrees of perivascular inflammatory infiltrates occurring in resident vascular cells.112–115 In PH, cytokines and chemokines are mainly secreted by various immune cells, as well as by resident vascular cells such as pulmonary vascular endothelial and smooth muscle cells.110 Recent findings have specifically linked myelopoiesis, monocytes, and interstitial macrophages to particular key roles in PAH pathogenesis.116–119
While future therapeutic endeavors are poised to target specific immune cell compartments, more developed pipelines have explored the control of expression and production of cytokines and chemokines, such as interleukins-1β (IL-1β), IL-6, IL-8, monocyte chemoattractant protein 1, chemokine C-C m otif ligand 5 (CCL5/RANTES), and tumor necrosis factor α, all dysregulated in severe PH (predominately within the pulmonary vascular lesions).112 Their actions, at least in part, can drive pulmonary vascular remodeling through promoting proliferation, migration, and differentiation of vascular cells.112
Nuclear factor-κB inhibition.
Nuclear factor kappa light chain enhancer of activated B cells (NF-κB) is a ubiquitous transcription factor that is regulated by various stimuli, including growth factors, inflammatory mediators (cytokines), chemotherapeutic drugs, and many others. It is a crucial regulator of fundamental cell functions such as survival, proliferation, and mobility.120,121 Several studies have identified NF-κB as crucial for PAH pathogenesis via activation of inflammatory and proliferative stimuli in PASMCs and mediating cytokine-induced release of the vasoconstrictor ET-1.122 In addition, activation of NF-κB is induced in monocrotaline PH rats, and pharmacologic NF-κB blockade has been found to ameliorate PH.123,124 Furthermore, by study of transgenic mice with cardiac-specific overexpression of a dominant-negative IκBα gene (an inhibitory binding partner of NF-κB), inhibition of NF-κB transcriptional activity was sufficient to prevent RV hypertrophy induced in monocrotaline PH rats.125 Lastly, NF-κB has been reported to be highly activated in pulmonary lymphocytes, macrophages, ECs, and PASMCs from patients with end-stage idiopathic PAH,126 suggesting that NF-κB may be a potential therapeutic target for PAH.
Bardoxolone methyl (CDDO-Me) is an orally available semisynthetic triterpenoid that inhibits NF-κB activation but also acts as a nuclear factor erythroid 2-related factor 2 (Nrf2) inducer. CDDO-Me exhibits anti-inflammatory effects by activation of Nrf2/Keap1 pathway and downregulation of NF-κB activity.127 In preclinical studies, it was found to increase endothelial NO bioavailability, improve metabolic dysfunction, and inhibit smooth muscle cell proliferation.127–129 Currently, a phase II clinical trial is examining its efficacy in patients with PH ().130 Another NF-κB relevant drug, dimethyl fumarate (DMF),131 has been recently examined as a therapeutic agent for PAH using patient-derived cells and mice models. The DMF works through inhibition of NF-κB but also signal transducer and activator of transcription 3 (STAT3) and c-jun (an activator protein-1 transcriptional factor subunit) signaling, leading to degradation of the pro-fibrogenic mediators: specificity protein 1 (Sp1), transcriptional coactivator with PDZ-binding motif (TAZ), and β-catenin.132 In rodent models of PAH, DMF treatment was effective in reducing inflammation, oxidative damage, fibrosis, and hemodynamic reversal.132 Advancement of DMF to clinical trial in PAH has not yet been explored, but given its favorable safety profile and FDA approval as Tecfidera for the treatment of multiple sclerosis,133 repurposing for PAH may be reasonable in the near future.
Nuclear factor of activated T cells inhibition.
Nuclear factor of activated T cells (NFAT), a calcium (Ca2 +)-dependent transcription factor, is active in diseased pulmonary vasculature.110,134 The NFAT is highly activated in PASMCs and circulating leukocytes in both human and animal instances of PAH.134 Another study indicated that dysregulation of miR-204 ultimately activates NFATc2 and NFAT signaling, leading to hyperproliferative PASMCs and ultimate development of PH in rodents.135 The NFAT inhibition, by cyclosporine (an NFATc2 inhibitor) or via the selective peptide VIVIT that interferes with the interaction between NFAT and calcineurin, was shown to reverse monocrotaline-induced rat model of PH.134 While the selectivity of VIVIT is attractive for further clinical testing, challenges with delivery and stability have substantially limited the use of VIVIT in humans.136
Leukotriene B4 inhibitor.
Leukotriene B4 (LTB4) is a pro-inflammatory lipid mediator produced from arachidonic acid by consecutive activities of 5-lipoxygenase, 5-lipoxygenase-activating protein, and leukotriene A4 hydrolase.137 Several studies have suggested that LTB4 contributes substantially to PAH initiation and progression.
Use of an LTB4 receptor antagonist (ONO4057) reduced RV hypertrophy in monocrotaline-exposed rats and prevented these animals from developing PH.138 More recently, bestatin (ubenimex), a nonspecific inhibitor of leukotriene A4 hydrolase, was shown to reverse established PH in both SU5416-exposed athymic rats and monocrotaline-exposed rats.139 The efficacy of bestatin in patients with PAH was recently studied in a 24-week phase II clinical trial (, LIBERTY trial) which included 61 patients with PAH across 45 clinical sites in North America. Although the trial results await publication, preliminary reports from the trial sponsor (Eiger Biopharmaceuticals, Palo Alto, CA, USA) have indicated that treatment failed to improve PVR in comparison to placebo and was unable to improve exercise capacity of patients, as measured by 6MWD. It is possible that the nonspecificity of this particular drug may have played a role in these negative trial results. Nonetheless, in the wake of these results, it remains to be seen if targeting LTB4 or other leukotriene metabolites still has a viable path forward in clinical trial for improving the inflammatory component of this deadly disease.
CCR5 antagonist.
HIV infection is a key risk factor for developing PAH,140 but the pathogenic mechanisms remain enigmatic. CCR5 is a G-protein-coupled receptor that acts as a coreceptor critical for HIV entry into cells; thus, it is a common therapeutic target for HIV infection.141,142 Recently, CCR5 has been implicated as a potential therapeutic target for PAH. The CCR5 is activated upon stimulation by CCL3, CCL4, and CCL5 (CCR5 ligands) and is highly expressed in the principal cell types implicated in PH pathogenesis, for example, macrophages, T cells, ECs, and smooth muscle cells.113,114,143,144 Increased CCL5 expression has been observed within the pulmonary vascular wall in patients with idiopathic PH.145 It has been also shown that elevated CCR5 level is found in lung tissues isolated from patients with PAH, in mice with hypoxia-exposed PH, and in HIV-infected macaques.146 Furthermore, CCR5-knockout mice manifested less severe phenotypes of PH compared to wild-type mice upon chronic hypoxic exposure.147 Maraviroc, a pharmacologic CCR5 antagonist, prevented the development of PH in hypoxic mice146 and partially reversed PH in a mouse model of disease driven by metabolic syndrome.147 As of yet, no clinical trial targeting CCR5/CCL5 in HIV-PAH has been reported.
Targeting Metabolism
Mitochondria are double-membrane-bound organelles that generate most cells’ adenosine triphosphate (ATP) supply from pyruvate via the glycolysis pathway, or fatty acids via β-oxidation. In addition to supplying cellular energy, they are involved in other essential cellular activities, such as reactive oxygen species and diffusible metabolite production, Ca2+ signaling, and regulation of autophagy and apoptosis,148 among others. Profound mitochondrial dysfunction and metabolic reprogramming have been identified as driving causes of various type of PH, in which oxidative mitochondrial function is generally suppressed in PASMCs, RV cardiomyocytes, and ECs. As a result, there is a switch in oxidative phosphorylation toward glycolysis (the Warburg effect).149 Upregulation of glycolysis is associated with resistance to apoptosis, whereas lower oxidative activity in mitochondria ultimately engenders greater cell proliferation.150 Based on halting or reversing aspects of the Warburg effect, a numbers of agents may hold promise in preclinical and early clinical therapy for PAH.
Pyruvate dehydrogenase kinase inhibitor.
Pyruvate dehydrogenase (PDH) is a key enzyme that converses pyruvate into acetylcoenzyme A (acetyl-CoA) as a substrate for the Krebs cycle, and this process is suppressed in PAH. To do so, pyruvate dehydrogenase kinase (PDK), an enzymatic PDH inhibitor, is able to be activated through the tyrosine kinase axis151 and hypoxia-inducible transcription factor, such as HIF-1α.152 PDH is also repressed when there is decreased mitochondrial Ca2+ signaling,153 a striking feature of PAH.
Dichloroacetate (DCA) is a small molecule PDK inhibitor under study in investigator-led clinical trials for various cancers and PAH.154 In PAH, inhibition of PDK via DCA attenuated and reversed pulmonary vascular remodeling in monocrotaline and hypoxic rats with PH.155,156 In these studies, DCA induced apoptosis and inhibited proliferation through depolarizing mitochondria, which resulted in the release of H2O2 and cytochrome c, leading to the subsequent restoration of Kv channel expression and function.155,156 In addition to the effects on mitochondria function, DCA was found to inhibit NFAT, suppress HIF-1α, as well as upregulate copper/zinc superoxide dismutase (Cu/Zn SOD; also called SOD1) activity in experimental PH models.157,158 A recent phase I clinical study included 20 patients with PAH treated with chronic oral DCA. Findings were complicated, demonstrating that DCA administration led to an improvement in PAP, PVR, and functional capacity in some, but not all, of the patients.159 Intriguingly, resistance to DCA resistance correlated with the presence of carrying genetic variants of sirtuin-3 (SIRT3) and uncoupling protein 2 (UCP2), both of which predict decreased protein activity and have been related to the development of PAH.160 Thus, these findings give impetus to further study of DCA as well as potentially using genetic screening as a guide for defining appropriate responders and trial participants.
Fatty acid oxidation inhibitor.
Fatty acid and glucose metabolism are inversely associated with RV homeostasis and dysfunction. In the failing RV with PAH, inhibition of glucose oxidation is accompanied by increased fatty acid oxidation, a process known as the Randle cycle.161 As such, inhibition of fatty acid oxidation (and thus the Randle cycle) has been offered as a putative therapeutic possibility to improve RV function and to promote glucose oxidation. A key regulatory enzyme for fatty acid oxidation in the RV is malonyl-CoA decarboxylase (MCD). The MCD-deficient mice have displayed increased glucose oxidation and a decreased tendency to develop hypoxic PH,162 thus offering preclinical support for this hypothesis.
Trimetazidine and ranolazine are both small molecules that partially inhibit fatty acid oxidation via restoring PDH activity and glucose oxidation, thereby accelerating ATP levels.163 They have been shown to reduce elevated RV glycogen levels in the hypertrophic rat RV, to increase exercise capacity and cardiac output, and to attenuate exertional lactic acidemia in pulmonary artery banding.163 In addition, trimetazidine has been found to normalize intracellular calcium levels in PASMCs from patients with PAH and to improve PAPs, RV thickness, and pulmonary vascular remodeling in animal models of PAH.162 Ranolazine is a structurally related drug, originally marketed as an antianginal drug for coronary artery disease,164 but now being studied as a therapeutic that can reverse the Randle cycle specifically in the diseased RV.163 A numbers of clinical trial studies on the use of ranolazine in PH have recently completed. A safety study of ranolazine in patients with PAH appeared safe without worsening hemodynamics ().165 An open-label ranolazine study in patients with PAH with angina or an anginal equivalent showed improvement in exercise capacity,166 RV function and RV structure (reduced size), but did not alter hemodynamic parameters ().166 A phase IV study investigating the efficacy of ranolazine in 10 patients with PH and diastolic dysfunction (WHO group 2 PH) is ongoing but has not yet been published (). Two other clinical trials are in progress, studying the effects of ranolazine on RV ejection fraction and change in metabolic and microRNA (miRNA) profiles in non-WHO patients with group 2 PH with RV dysfunction ( and ).
Metformin.
AMP-activated protein kinase (AMPK) is a serine/threonine kinase that positively regulates NO production via eNOS, whereupon it suppresses vascular smooth muscle cell proliferation and remodeling.167 The AMPK has been reported to be downregulated in the pulmonary arteries from patients with PAH and hypoxia-induced PH m ice.168 Furthermore, endothelial-specific AMPK knockout mice are predisposed to develop PH upon hypoxic exposure,168 suggesting that AMPK could be potential novel therapeutic targets of PH.
The antidiabetic agent metformin is an AMPK activator. Thus, it has been hypothesized as a new therapeutic approach for PH, given the importance of AMPK in PH and the association of metabolic syndrome and diabetes in WHO group 2 PH (namely, PH associated with heart failure with preserved ejection fraction, HFpEF).169 Metformin has been shown to protect against the development of PH in hypoxia- and monocrotaline-exposed models of PH in rats170 as well as in a preclinical rodent model of PH-HFpEF.169 A phase II clinical trial evaluating the efficacy of metformin on pulmonary vascular function in patients with PAH is currently underway ().
Peroxisome proliferator-activated receptor γ agonist.
The PPARγ is a nuclear receptor that predominantly regulates lipid and glucose metabolism in adipocytes and is crucial for cardiovascular homeostasis. Evidence indicates that PPARγ serves as a protective modulator in PAH,171 particularly in diseased PASMCs172,173 and PAECs.174,175 The level of PPARγ expression was observed to be low in lungs from SU5416-hypoxiaand chronic hypoxia-exposed rats, as well as in lungs from patients with severe PAH and with chronic obstructive pulmonary disease.176,177 Mice carrying endothelial-deficient PPARγ were found to suffer more severe PH compared to wild-type mice under hypoxia exposure.175 Similarly, mice with PPARγ; gene deletion in arterial smooth muscle cells developed PH.178 In part, this may be driven by PPARγ alterations of TGF/BMP signaling with downstream effects on cell proliferation and glucose metabolism.173 The miRNA 130/301 family was also found to target PPARγ in multiple vascular cell types, leading to an array of consequences on proliferation, vasomotor tone, and vascular matrix stiffening in PH.179,180 In aggregate, these studies provide a mechanistic foundation for the essential role of PPARγ deficiency for PAH disease development, thus prompting speculation about the potential for PPARγ agonists in this disease.
The PPARγ activators (ligands) such as rosiglitazone and pioglitazone (thiazolidinedione, TZD) have indeed shown benefits in animal models of PAH.172,181 Rosiglitazone attenuated hypoxia-induced models of PH in mice and rats.177,182,183 Treatment with rosiglitazone reversed PAH by inhibition of TG Fp1-Stat3-FoxO 1 cascade pathways in TGFβ1-overexpressing mice.173 Despite these promising attributes, the clinical potential of rosiglitazone was stymied by cardiovascular safety concerns in larger phase III trials in diabetic patients.184 On the other hand, pioglitazone, another TZD-class insulin sensitizer, has been studied for therapeutic revival in a number of cardiovascular diseases, given its beneficial safety profile versus rosiglitazone185–187 and even beneficial effects on diastolic left ventricular function.188 In preclinical studies, treatment with pioglitazone reversed PAH and prevented RV failure in the SU5416-hypoxia rats through regulating distinct messenger RNAs (mRNAs) and miRNAs, renovating mitochondrial function (Food and Agriculture Organization induction), and preventing lipid accumulation in myocyte.181 Taken together, next-generation PPARγ-activating drugs may, in fact, be potent agents for the treatment of PAH, RV failure, and abnormality in altered lipid/glucose metabolism, which are characteristic features of cardiopulmonary remodeling.
Beyond TZD drugs, an electrophilic nitroalkene-containing fatty acid nitro-oleic acid (NO2-OA) is a novel and endogenous PPARγ ligand that directly adducts the Cys285 residue in the PPARγ ligand-binding domain, thus leading to potent agonist for PPARγ.189 The NO2-OA has displayed beneficial metabolic effects by induction of PPARγ transcriptional activity189 and attenuated hypoxia-induced PH in mice by inhibiting the proliferation of smooth muscle cells to reduce muscularization of small pulmonary vessels.190 At present, NO2-OA has cleared preclinical toxicology and testing in FDA-approved human safety trials of both oral and IV formulations (IV IND 122583; oral IND 124524), and a phase II human trial with this molecule is underway in PAH.
Antioxidants.
A number of recent human studies have implicated increased oxidative stress (ie, the rapid depletion of small molecule antioxidants, lipid peroxidation, and DNA oxidation) in patients with PH.191–194 It is reasonable to suggest that redox imbalance may contribute to the pathological conditions associated with idiopathic PAH. Several small molecules with antioxidant properties have been reported to have therapeutic outcomes in animal models of PH. In preclinical studies, several antioxidants have been demonstrated to inhibit PH and/or RV dysfunction. Examples include N-acetylcysteine, probucol, tempol, allicin, pyrrolidine dithiocarbamate, superoxide dismutase, allopurinol, sulfur dioxide, and resveratrol.194 More recently, new specific oxidase inhibitors are available, such as febuxostat (a putative inhibitor of xanthine oxidase) and statins (inhibitors of HMG-CoA reductase), which have broad effects on modulation of inflammatory vascular oxidases, but have not been tested rigorously in clinical human PAH.
Targeting sex hormones.
A gender predisposition has long been appreciated in PAH. The initial National Institutes of Health registry identified a ratio of 1.7:1 female:male patients with PAH,195 and a recent French registry for multinational treatment trials reported a ratio of 4:1.196 However, men with PAH tend to suffer from more severe disease, and male sex has been identified as a risk factor for mortality in 2 individual PAH registries.197,198 A recent study has reported that sex hormone levels are associated with the risk of PAH in men. Specifically, an increase in estradiol (E2) level and a decrease in dehydroepiandrosterone (DHEA) level were linked to more severe hemodynamic burden.199 In particular, elevated circulating E2 level is linked to a high risk of PAH in men.
Although the biogenesis and metabolism of E2 and DHEA are complex, findings have indicated the importance of aromatase, one of the several biogenesis enzymes for E2 production, in the development of PH. Namely, the occurrence of a gain-of-function single-nucleotide polymorphism in the aromatase promoter region is not only associated with a higher level of circulating E2 in patients with PH but also with an increased risk of developing PAH with cirrhosis.200 Administration of the aromatase inhibitor anastrozole has been found to reduce PAPs, pulmonary vascular remodeling, and indices of RV hypertrophy in hypoxia-exposed mice and Su5416-hypoxia PH models in rats.201 A phase II clinical trial evaluating the safety and efficacy of anastrozole in patients with PAH has been completed, and it demonstrated daily treatment with 1 mg anastrozole for 3 months significantly lowered E2 levels in plasma by 40% and increased 6MWD capacity.202
The DHEA is a cholesterol-derived steroid hormone secreted mainly from the adrenal cortex. It is a precursor for the synthesis of estrogen and testosterone203 and has been found to inhibit hypoxia-induced vasoconstriction by stimulating potassium channels via sGC activation204 and to improve endothelial function by increased NO synthesis.205 Administration of DHEA has been observed to partially reverse PAH in both preclinical and clinical studies. The DHEA treatment protects against monocrotaline-induced PH in pneumonectomized rats206 and ameliorates severe PAH in SU5416-hypoxia rats by protecting the RV against fibrosis and apoptosis.207 A recent small clinical trial demonstrated an improvement of 6MWD as well as pulmonary hemodynamics in patients associated with chronic obstructive lung disease.208 Taken together, DHEA is advancing as a possible new drug for the treatment of PH; it remains to be seen if male patients in particular may derive benefits from DHEA hormone therapy.
Other metabolic processes.
There continue to be advancing insights into the profound metabolic reprogramming and dys-regulation seen in PH. Iron deficiency in patients with PH has been observed,209–212 accompanied by some mechanistic insights into the role of iron in PH.213–215 Moreover, deficiency of iron-sulfur clusters, critical for function of oxidative phosphorylation and electron transport, has been causatively linked to PH via both genetic and acquired triggers.216,217 Clinical trials are currently ongoing to evaluate whether iron supplementation can improve manifestations of PAH (). However, these trials will need to tread cautiously, given other data in sickle cell-induced PH that iron overload can also worsen PH.218 Furthermore, dysregulation of transcriptional factors such as the Yes-associated protein (YAP) and TAZ has been linked to alterations of glycolysis and glutamine metabolism, driving a pathogenic and hyperpro-liferative state.219,220 Inhibitors of YAP/TAZ and glutaminolysis have been examined in rat models of PH and have shown promise.
DNA Damage
Cells have an evolutionally conserved pathway, termed the DNA damage response, that senses and transduces signaling and repairs damaged DNA lesions in order to maintain genomic integrity. Successful DNA repair permits cells to continue the process of proliferation. Conversely, failure of DNA repair processes causes accumulation of DNA damage and induction of apoptosis.221
DNA damage and repair have been associated with disease progression of PAH. Whole-exome sequencing has recently resulted in the identifying mutations in topoisomerase DNA II binding protein 1 (TOPBP1), a key player in DNA damaging signaling, that are relevant to PAH susceptibility.222 Variation in TOPBP1 expression has been localized in situ in PAECs isolated from lungs of patients with idiopathic PAH.222 In addition, BMPR2 deficiency impaired DNA damage responses in PAH PAECs, with lower breast cancer 1 (BRCA1) expression and increased susceptibility to outright genomic lesions and instability.223 Beyond TOPBP1, loss of Ku70 (a subunit of the Ku protein complex involved in the nonhomologous end-joining pathway of DNA double-strand break repair) in mice, displays visual similarities to plexiform lesions in patients with PAH lung.224 Moreover, DNA damage in PAH has been found to be associated with poly [ADP-ribose] polymerase 1 (PARP1) overexpression in PASMCs due to miR-223 down-regulation, a feature of PAH-PASMCs contributing to the cancer-like phenotype of these cells.225,226 Inhibition of PARP1 by ABT-888 has been revealed to reverse PH in monocrotaline and SU5416-hypoxia rats.225 An early phase I clinical trial to investigate the safety of PARP1 inhibitor (olaparib) on pulmonary vascular function in patients with PAH is currently enrolling (), to be followed by a multicenter phase II trial that will be conducted in recognized PAH programs throughout Canada.
New Perspectives: Epigenetic Modulation
Recent emphasis has been placed on investigating epigenetic modulation as a therapeutic approach for PH. Histone modifications (eg, methylation, acetylation, phosphorylation) lead to an alteration in chromatin structure and facilitation of transcriptional gene expression. The balance of deacetylation and acetylation is modulated by histone deacetylases (HDACs) and the histone acetyltransferases, respectively. Increased level of HDACs is found in lung tissues of both patients with PAH and rats exposed to hypoxia,227 indicating that elevated HDAC activity might contribute to pathology of PAH associated with high proliferative potential.
Inhibition of HDAC activity by valproic acid (VPA) and suberoylanilide hydroxamic acid (a relatively broad-spectrum HDAC inhibitor) has been shown to reduce PA pressure without altering systemic blood pressure in chronic hypoxia-exposed rats, as well as inhibiting proliferation and inflammation in the lung.227 Specifically, inhibition of HDAC1 repressed proliferation and migration of PASMCs.228 Inhibition of HDAC4 and HDAC5 by MC1568 (a selective class II HDAC inhibitor) reversed PH in multiple PH rat models.229 More recently, therapeutic targeting of HDAC2A was found to improve experimental PH.230 However, the therapeutic potential of existing HDAC inhibitors remains controversial in PAH due to their lack of specificity. Although these inhibitors have shown promising results in left ventricle (LV) remodeling, broad-spectrum HDAC inhibition with trichostatin A has also been associated with dysfunction and worsened remodeling of the RV via antiangiogenic and/or proapoptotic effects,231 suggesting the intriguing possibility of differential modulation of histone acetylation in the RV versus the LV.
Lysine acetylation of histones alters the electrostatic properties on chromatins and creates docking sites for the bromo-domain and extra terminal domain (BET) proteins, leading to initiation of transcriptional processes that result in significant gene expression changes.232 In PH, a member of the BET family protein, BRD4, has been reported to be upregulated in the lungs, distal pulmonary arteries, and PASMCs from patients with PAH.233 Furthermore, pharmacological inhibition of BRD4 by the small molecule JQ1 reversed PAH in SU5416-hypoxia-exposed rats,233 as well as reduced human pulmonary microvascular EC proliferation and migration by inhibiting NF-κB p65 protein recruitment to the IL-6 and IL-8 promoters.234 In aggregate, these studies suggest that BET inhibitors may have valuable therapeutic potential for the treatment of PAH, potentially in combination and synergy with HDAC inhibitors.
Noncoding RNAs such as miRNAs can also epigenetically regulate transcriptional and post-transcriptional events, and there is a growing literature regarding their influence on PH pathogenesis.235 The miRNAs are small noncoding RNAs that incorporate into the RNA-induced silencing complex of proteins, leading to binding of mRNA target transcripts and either degradation or inhibition of protein translation. In consequence, they influence many biological processes, including cell differentiation, survival, and proliferation.236 A growing number of miRNAs are dysregulated in the pulmonary vessels and RV tissue in PAH. Some are detectable as extracellular molecules in circulating plasma, making them potential biomarkers and therapeutic targets as well.235,237,238 Although the study of miRNAs has offered a wealth of knowledge regarding the fundamental pathogenesis of PH, therapeutic strategies to modulate miRNAs have been more challenging. In general, 2 main techniques have been explored in more depth: administration of miRNA mimics (in the form of oligonucleotides or lentiviruses) or delivery of oligonucleotide inhibitors (ie, antagomirs). Various animal studies have independently reported success in these methods of transfer, but the transition to clinical study has been marked by hurdles of safety, efficacy, as well as tissue specificity of delivery. The latter issue is particularly important for miRNA therapies, given each miRNA’s typical pleiotropic and complex biology in a variety of organ systems. When coupling that pleiotropy with the historic difficulties of delivering a compound specifically to the pulmonary vasculature, the challenges of clinical targeting of miRNA biology in PH become evident. Nonetheless, if other therapeutic niches can be solved with RNA therapeutics, the path forward may become much more obvious. In aggregate, the potential is growing for therapies, and even diagnostics, that target epigenetic regulation and gene expression repositories in PAH.
Conclusions
Pulmonary hypertension is a devastating disorder and largely remains a disease without a cure. Apart from the current FDA-approved palliative therapies, recent studies provide hope that multiple molecular targets exist that may truly influence disease progression. These targets influence proliferation, inflammation, DNA damage, and mitochondrial/metabolic function. Substantial progress in our knowledge of epigenetic regulation and miRNA function in PH may also provide further translational options for this disease. However, with the advent of so many novel therapeutic agents, effective testing of these drugs presents new challenges as well. The pool of available patients for clinical study is relatively small, and eligible patients with PAH for clinical trials are typically already on multiple drugs, a fact which can confound results from already small patient populations. Likely, to expedite drug development, research efforts for future clinical trials will have to begin to include other historically neglected global PH centers, such as those in Asia, in order to recruit sufficient numbers of patients. It is unclear whether this next generation of drugs will necessitate improvements in local pulmonary vascular delivery, as these novel drugs may carry powerful and unwanted off-target side effects if delivered systemically. Finally, trial design itself for the new generation of PH drugs will also necessitate innovation. It remains unclear whether the standard indices of evaluating pulmonary vasodilators (ie, invasive hemodynamic testing) should be used for evaluating disease-modifying drugs that act in other ways beyond vasodilation alone. More specific and useful assessments (ie, imaging or functional endpoint points) may need to be developed to determine drug efficacy. It also remains a distinct possibility that genetic testing or genomic sequencing may play a role in recruiting the optimal patients for a specific trial drug—those that have the highest chance of responding favorably to the therapy.239 If those innovations come to bear, it is possible that drug efficacy may be adequately tested in the future without necessitating long-term patient outcome data, thus obviating the need for lengthy and costly trials that, in their current form, would certainly slow, if not prevent entirely, the advent of these new therapies. Thus, there is great optimism in the PH community, with a large number of new potential therapies currently under investigation in the preclinical or early-stage clinical trials. With appropriate innovation in late-stage clinical trial design, we expect that the landscape of PH therapies will evolve closer to a cure over the next decades.
Acknowledgments
The authors thank Dr Steven R. Woodcock for helpful commons on the manuscript. This work was supported by R01 HL124021, HL 122596, HL 138437, and UH2 TR002073 as well as the AHA grant 18EIA33900027 (SYC).
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. S.Y.C. has served as a consultant for Actelion (Significant), Gilead, Aerpio, Pfizer, and Vivus (Modest). Patent applications (S.Y.C.) have been filed regarding targeting metabolism in pulmonary hypertension.
References
- 1.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016;4(4):306–322. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122(12):4306–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008;44(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Morrell NW, Archer SL, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;430(suppl 12):S13–S24. [DOI] [PubMed] [Google Scholar]
- 5.Tuder RM, Lee SD, Cool CC. Histopathology of pulmonary hypertension. Chest. 1998;114(1):1S–6S. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(suppl 25):D34–D41. [DOI] [PubMed] [Google Scholar]
- 7.Simonneau G, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J1. 2019;53(1):pii:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3):549–555. [DOI] [PubMed] [Google Scholar]
- 9.Camerini F, Alberti E, Klugmann S, Salvi A. Primary pulmonary hypertension: effects of nifedipine. Br Heart J. 1980;44(3): 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin LJ, Nicod P, Hillis L, Firth BG. Treatment of primary pulmonary hypertension with nifedipine: a hemodynamic and scintigraphic evaluation. Ann Intern Med. 1983;99(4):433–438. [DOI] [PubMed] [Google Scholar]
- 11.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327(2):76–81. [DOI] [PubMed] [Google Scholar]
- 12.Sitbon O, Humbert M, Jaїs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation. 2005;111(23):3105–3111. [DOI] [PubMed] [Google Scholar]
- 13.Opie LH. Calcium channel antagonists in the treatment of coronary artery disease: fundamental pharmacological properties relevant to clinical use. Prog Cardiovasc Dis. 1996;38(4):273–290. [DOI] [PubMed] [Google Scholar]
- 14.Francis SH, Busch JL, Corbin JD. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 2010;62(3):525–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghofrani H-A, Galie N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330–340. [DOI] [PubMed] [Google Scholar]
- 16.Rubin LJ, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J. 2015;45(5):1303–1313. [DOI] [PubMed] [Google Scholar]
- 17.Ghofrani H-A, Grimminger F, Grunig E, et al. Predictors of long-term outcomes in patients treated with riociguat for pulmonary arterial hypertension: data from the PATENT-2 open-label, randomised, long-term extension trial. Lancet Respir Med. 2016; 4(5):361–371. [DOI] [PubMed] [Google Scholar]
- 18.Tuder RM, Cool C, Geraci MW, et al. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med. 1999;159(6): 1925–1932. [DOI] [PubMed] [Google Scholar]
- 19.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med. 1992;327(2): 70–75. [DOI] [PubMed] [Google Scholar]
- 20.Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev. 2012;21(126):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004;351(14):1425–1436. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–1482. [DOI] [PubMed] [Google Scholar]
- 23.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. [DOI] [PubMed] [Google Scholar]
- 24.Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL registry. Chest. 2015;148(4): 1043–1054. [DOI] [PubMed] [Google Scholar]
- 25.Jongmin K Apelin-APJ signaling: a potential therapeutic target for pulmonary arterial hypertension. Mol Cells. 2014;37(3): 196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alastalo T-P, Li M, de Jesus Perez V, et al. Disruption of PPARγ/β-catenin-mediated regulation of apelin impairs BMP-induced mouse and human pulmonary arterial EC survival. J Clin Invest. 2011;121(9):3735–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falcao-Pires I, Goncalves N, Henriques-Coelho T, et al. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol-Heart C. 2009;296(6): H2007–H2014. [DOI] [PubMed] [Google Scholar]
- 28.Brash L, Barnes G, Brewis M, et al. Apelin improves cardiac output in patients with pulmonary arterial hypertension. Eur Respir J. 2015;46(suppl 59):PA2107. [Google Scholar]
- 29.Brash L, Barnes GD, Brewis MJ, et al. Short-term hemodynamic effects of apelin in patients with pulmonary arterial hypertension. JACC Basic Transl Sci. 2018;3(2):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Tian HY, Yan XL, et al. Serotonin inhibits apoptosis of pulmonary artery smooth muscle cell by pERK1/2 and PDK through 5-HT1B receptors and 5-HT transporters. Cardiovasc Pathol. 2013;22(6):451–457. [DOI] [PubMed] [Google Scholar]
- 31.Morecroft I, Loughlin L, Nilsen M, et al. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313(2): 539–548. [DOI] [PubMed] [Google Scholar]
- 32.Hervè P, Launay J-M, Scrobohaci M-L, et al. Increased plasma serotonin in primary pulmonary hypertension. Am J Med. 1995; 99(3):249–254. [DOI] [PubMed] [Google Scholar]
- 33.Guignabert C, Izikki M, Tu LI, et al. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ Res. 2006;98(10): 1323–1330. [DOI] [PubMed] [Google Scholar]
- 34.Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT (1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ Res. 2001;89(12):1231–1239. [DOI] [PubMed] [Google Scholar]
- 35.Launay JM, Hervè P, Peoc’h K, et al. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. [DOI] [PubMed] [Google Scholar]
- 36.Zopf DA, Neves LA, Nikula KJ, Huang J, Senese PB, Gralinski MR. C-122, a novel antagonist of serotonin receptor 5-HT2B, prevents monocrotaline-induced pulmonary arterial hypertension in rats. Eur J Pharmacol. 2011;670(1):195–203. [DOI] [PubMed] [Google Scholar]
- 37.Dumitrascu R, Kulcke C, Konigshoff M, et al. Terguride ameliorates monocrotaline-induced pulmonary hypertension in rats. Eur Respir J. 2011;37(5):1104–1118. [DOI] [PubMed] [Google Scholar]
- 38.Matthes S, Bader M. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol Sci. 2018;39(6):560–572. [DOI] [PubMed] [Google Scholar]
- 39.Morecroft I, Dempsie Y, Bader M, et al. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia- induced pulmonary hypertension. Hypertension. 2007;49(1): 232–236. [DOI] [PubMed] [Google Scholar]
- 40.Dempsie Y, Morecroft I, Welsh DJ, et al. Converging evidence in support of the serotonin hypothesis of dexfenfluramine-induced pulmonary hypertension with novel transgenic mice. Circulation. 2008;117(22):2928–2937. [DOI] [PubMed] [Google Scholar]
- 41.Rothman RB, Cadet JL, Dersch CM, et al. Altered gene expression in pulmonary tissue of tryptophan hydroxylase-1 knockout mice: implications for pulmonary arterial hypertension. PLoS One. 2011;6(3):e17735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aiello RJ, Bourassa P-A, Zhang Q, et al. Tryptophan hydroxylase 1 inhibition impacts pulmonary vascular remodeling in two rat models of pulmonary hypertension. J Pharmacol Exp Ther. 2017; 360(2):267–279. [DOI] [PubMed] [Google Scholar]
- 43.Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Recept Channel. 2002;8(3–4):137–153. [PubMed] [Google Scholar]
- 44.Said SI, Hamidi SA, Dickman KG, et al. Moderate pulmonary arterial hypertension in male mice lacking the vasoactive intestinal peptide gene. Circulation. 2007;115(10):1260–1268. [DOI] [PubMed] [Google Scholar]
- 45.Hamidi SA, Prabhakar S, Said SI. Enhancement of pulmonary vascular remodelling and inflammatory genes with VIP gene deletion. Eur Respir J. 2008;31(1):135–139. [DOI] [PubMed] [Google Scholar]
- 46.Petkov V, Mosgoeller W, Ziesche R, et al. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J Clin Invest. 2003;111(9):1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians. New Concepts Exp Ther. 2010;121(18):2045–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leuchte HH, Baezner C, Baumgartner RA, et al. Inhalation of vasoactive intestinal peptide in pulmonary hypertension. Eur Respir J. 2008;32(5):1289–1294. [DOI] [PubMed] [Google Scholar]
- 49.Hamidi SA, Lin RZ, Szema AM, Lyubsky S, Jiang YP, Said SI. VIP and endothelin receptor antagonist: an effective combination against experimental pulmonary arterial hypertension. Resp Res. 2011;12(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoder MC. Human endothelial progenitor cells. Csh Perspect Med. 2012;2(7):a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin R-Z, Dreyzin A, Aamodt K, Dudley AC, Melero-Martin JM. Functional endothelial progenitor cells from cryopreserved umbilical cord blood. Cell Transplant. 2011;20(4):515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol. 2010;43(5): 546–554. [DOI] [PubMed] [Google Scholar]
- 53.Yip H-K, Chang L-T, Sun C-K, et al. Autologous transplantation of bone marrow-derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit Care Med. 2008;36(3):873–880. [DOI] [PubMed] [Google Scholar]
- 54.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96(4):442–450. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi M, Nakamura T, Toba T, Kajiwara N, Kato H, Shimizu Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004;10(5–6):771–779. [DOI] [PubMed] [Google Scholar]
- 56.Nagaya N, Kangawa K, Kanda M, et al. Hybrid cell-gene therapy for pulmonary hypertension based on phagocytosing action of endothelial progenitor cells. Circulation 2003;108(7):889–895. [DOI] [PubMed] [Google Scholar]
- 57.Diller G-P, van Eijl S, Okonko DO, et al. Circulating endothelial progenitor cells in patients with eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation. 2008; 117(23):3020–3030. [DOI] [PubMed] [Google Scholar]
- 58.Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93(2):e17–e24. [DOI] [PubMed] [Google Scholar]
- 59.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–742. [DOI] [PubMed] [Google Scholar]
- 60.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11): 1204–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang XX, Zhang FR, Shang YP, et al. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a pilot randomized controlled trial. J Am Coll Cardiol. 2007;49(14): 1566–1571. [DOI] [PubMed] [Google Scholar]
- 62.Hui ZJ, Xiang WX, Rong ZF, et al. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Pediatr Transplant. 2008;12(6):650–655. [DOI] [PubMed] [Google Scholar]
- 63.Granton J, Langleben D, Kutryk MB, et al. Endothelial NO-synthase gene-enhanced progenitor cell therapy for pulmonary arterial hypertension. Circ Res. 2015;117(7):645–654. [DOI] [PubMed] [Google Scholar]
- 64.Yeager ME, Frid MG, Stenmark KR. Progenitor cells in pulmonary vascular remodeling. Pulm Circ. 2011;1(1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aldred MA, Jairam V, Victoria J, et al. BMPR2 gene rearrangements account for a significant proportion of mutations in familial and idiopathic pulmonary arterial hypertension. Hum Mutat. 2006;27(2):212–213. [DOI] [PubMed] [Google Scholar]
- 66.Hensley MK, Levine A, Gladwin MT, Lai YC. Emerging therapeutics in pulmonary hypertension. Am J Physiol-Lung C. 2018; 314(5):L769–L781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reynolds AM, Xia W, Holmes MD, et al. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol-Lung C. 2007;292(5): L1182–L1192. [DOI] [PubMed] [Google Scholar]
- 68.Long L, Ormiston ML, Yang X, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma L, Chung WK. The role of genetics in pulmonary arterial hypertension. J Pathol. 2017;241(2):273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morrell NW, Yang X, Upton PD, et al. Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-b1 and bone morphogenetic proteins. Circulation. 2001;104(7): 790–795. [DOI] [PubMed] [Google Scholar]
- 71.Long L, Crosby A, Yang X, et al. Altered bone morphogenetic protein and transforming growth factor-b signaling in rat models of pulmonary hypertension. Circulation. 2009;119(4):566–576. [DOI] [PubMed] [Google Scholar]
- 72.Yung LM, Nikolic I, Paskin-Flerlage SD, Pearsall RS, Kumar R, Yu PB. A selective transforming growth factor-b ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care. 2016;194(9):1140–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spiekerkoetter E, Tian X, Cai J, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123(8):3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spiekerkoetter E, Sung YK, Sudheendra D, et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017; 50(3): pii:1602449. [DOI] [PubMed] [Google Scholar]
- 75.Prosseda SD, Tian X, Kuramoto K, et al. Fragile histidine triad (FHIT), a novel modifier gene in pulmonary arterial hypertension. Am J Respir Crit Care. 2018;0(ja):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drake KM, Dunmore BJ, McNelly LN, Morrell NW, Aldred MA. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol. 2013;49(3):403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riento K, Ridley AJ. ROCKs: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Bio. 2003;4:446–456. [DOI] [PubMed] [Google Scholar]
- 78.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21(1):247–269. [DOI] [PubMed] [Google Scholar]
- 79.Oka M, Fagan KA, Jones PL, McMurtry IF. Therapeutic potential of RhoA/Rho kinase inhibitors in pulmonary hypertension. Br J Pharmacol. 2008;155(4):444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robertson TP, Michelle D, Ward JP, Aaronson PI, Mark EA. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000;131(1):5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robertson TP, Aaronson PI, Ward JPT. Ca2+ sensitization during sustained hypoxic pulmonary vasoconstriction is endothelium dependent. Am J Physiol-Lung C. 2003;284(6):L1121–L1126. [DOI] [PubMed] [Google Scholar]
- 82.Hisaoka T, Yano M, Ohkusa T, et al. Enhancement of Rho/Rhokinase system in regulation of vascular smooth muscle contraction in tachycardia-induced heart failure. Cardiovasc Res. 2001; 49(2):319–329. [DOI] [PubMed] [Google Scholar]
- 83.Yamashita K, Kotani Y, Nakajima Y, et al. Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res. 2007; 1154:215–224. [DOI] [PubMed] [Google Scholar]
- 84.Abe K, Shimokawa H, Morikawa K, et al. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94(3): 385–393. [DOI] [PubMed] [Google Scholar]
- 85.Abe K, Tawara S, Oi K, et al. Long-term inhibition of Rho-kinase ameliorates hypoxia-induced pulmonary hypertension in mice. J Cardiovasc Pharm. 2006;48(6):280–285. [DOI] [PubMed] [Google Scholar]
- 86.Li F, Xia W, Li A, Zhao C, Sun R. Long-term inhibition of Rho kinase with fasudil attenuates high flow induced pulmonary artery remodeling in rats. Pharmacol Res. 2007;55(1):64–71. [DOI] [PubMed] [Google Scholar]
- 87.Fukumoto Y, Matoba T, Ito A, et al. Acute vasodilator effects of a Rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91(3):391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishikura K, Yamada N, Ito M, et al. Beneficial acute effects of Rho-kinase inhibitor in patients with pulmonary arterial hypertension. CircJ. 2006;70(2):174–178. [DOI] [PubMed] [Google Scholar]
- 89.Zhuang R, Wu J, Lin F, et al. Fasudil preserves lung endothelial function and reduces pulmonary vascular remodeling in a rat model of end-stage pulmonary hypertension with left heart disease. Int J Mol Med. 2018;42(3):1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Wu S. Effects of fasudil on pulmonary hypertension in clinical practice. Pulm Pharmacol Ther. 2017;46:54–63. [DOI] [PubMed] [Google Scholar]
- 91.Schermuly RT, Dony E, Ghofrani HA, et al. Reversal of experimental pulmonary hypertension by PDGF inhibition. J Clin Invest. 2005;115(10):2811–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol. 1997;150(4):1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 93.Ushio-Fukai M, Griendling KK, Becker PL, Hilenski L, Halleran S, Alexander RW. Epidermal growth factor receptor transactivation by angiotensin II requires reactive oxygen species in vascular smooth muscle cells. Arterioscler Thromb VascBiol. 2001; 21(4):489–495. [DOI] [PubMed] [Google Scholar]
- 94.Xin X, Johnson AD, Scottburden T, Engler D, Casscells W. The predominant form of fibroblast growth factor receptor expressed by proliferating human arterial smooth muscle cells in culture is type I. Biochem Bioph Res Co. 1994;204(2):557–564. [DOI] [PubMed] [Google Scholar]
- 95.Barst RJ. PDGF signaling in pulmonary arterial hypertension. J Clin Invest. 2005;115(10):2691–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension from cancer biology to new pulmonary arterial hypertension therapeutics targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care. 2017;195(4):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Redner RL. Why doesn’t imatinib cure chronic myeloid leukemia?. Oncologist. 2010;15(2):182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ghofrani HA, Morrell NW, Hoeper MM, et al. Imatinib in pulmonary arterial hypertension patients with inadequate response to established therapy. Am J Respir Crit Care. 2010;182(9): 1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoeper M, Barst R, Bourge R, et al. Imatinib improves exercise capacity and hemodynamics at 24 weeks as add-on therapy in symptomatic pulmonary arterial hypertension patients: the IMPRES study. Chest. 2011;140(4):1045A. [Google Scholar]
- 100.Pullamsetti SS, Berghausen EM, Dabral S, et al. Role of Src tyrosine kinases in experimental pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2012;32(6):1354–1365. [DOI] [PubMed] [Google Scholar]
- 101.Izikki M, Guignabert C, Fadel E, et al. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119(3):512–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goncharova EA. mTOR and vascular remodeling in lung diseases: current challenges and therapeutic prospects. FASEB J. 2013;27(5):1796–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346(23): 1773–1780. [DOI] [PubMed] [Google Scholar]
- 104.Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22(2):159–168. [DOI] [PubMed] [Google Scholar]
- 105.Barilli A, Visigalli R, Sala R, et al. In human endothelial cells rapamycin causes mTORC2 inhibition and impairs cell viability and function. Cardiovasc Res. 2008;78(3):563–571. [DOI] [PubMed] [Google Scholar]
- 106.Houssaini A, Abid S, Mouraret N, et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol. 2013;48(5):568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goncharov DA, Kudryashova TV, Ziai H, et al. Mammalian target of rapamycin complex 2 (mTORC2) coordinates pulmonary artery smooth muscle cell metabolism, proliferation, and survival in pulmonary arterial hypertension. Circulation. 2014; 129:864–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Paddenberg R, Stieger P, von Lilien AL, et al. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Resp Res. 2007;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seyfarth H-J, Hammerschmidt S, Halank M, Neuhaus P, Wirtz HR. Everolimus in patients with severe pulmonary hypertension: a safety and efficacy pilot trial. Pulm Circ. 2013;3(3):632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hassoun PM, Mouthon L, Barbera JA, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol. 2009;54(suppl 1):S10–S19. [DOI] [PubMed] [Google Scholar]
- 111.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109(8):867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115(1):165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schober A Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28(11):1950–1959. [DOI] [PubMed] [Google Scholar]
- 114.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006; 354(6):610–621. [DOI] [PubMed] [Google Scholar]
- 115.Stacher E, Graham BB, Hunt JM, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care. 2012; 186(3):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan L, Chen X, Talati M, et al. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2016;193(8):898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pugliese SC, Kumar S, Janssen WJ, et al. A time- and compartment-specific activation of lung macrophages in hypoxic pulmonary hypertension. J Immunol. 2017;198(12): 4802–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kumar R, Mickael C, Kassa B, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017; 8:15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Florentin J, Coppin E, Vasamsetti SB, et al. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol. 2018;200(10):3612–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Karin M Nuclear factor-[kappa]B in cancer development and progression. Nature. 2006;441(7092):431–436. [DOI] [PubMed] [Google Scholar]
- 121.Inoue JI, Gohda J, Akiyama T, Semba K. NF-κB activation in development and progression of cancer. Cancer Sci. 2007;98: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wort SJ, Ito M, Chou PC, et al. Synergistic induction of endothelin-1 by tumor necrosis factor α and interferon γ is due to enhanced NF-κB binding and histone acetylation at specific κB sites. J Biol Chem. 2009;284(36):24297–24305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kimura S, Egashira K, Chen L, et al. Nanoparticle-mediated delivery of nuclear factor κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension. 2009;53(5):877–883. [DOI] [PubMed] [Google Scholar]
- 124.Huang J, Kaminski PM, Edwards JG, et al. Pyrrolidine dithiocarbamate restores endothelial cell membrane integrity and attenuates monocrotaline-induced pulmonary artery hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(6): L1250–L1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kumar S, Wei C, Thomas CM, et al. Cardiac-specific genetic inhibition of nuclear factor-κB prevents right ventricular hypertrophy induced by monocrotaline. Am J Physiol-Heart C. 2012; 302(8):H1655–H1666. [DOI] [PubMed] [Google Scholar]
- 126.Price LC, Caramori G, Perros F, et al. Nuclear factor κ-B is activated in the pulmonary vessels of patients with end-stage idiopathic pulmonary arterial hypertension. PLoS One. 2013; 8(10):e75415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang YY, Yang YX, Zhe H, He ZX, Zhou SF. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Des Dev Ther. 2014;8:2075–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heiss EH, Schachner D, Werner ER, Dirsch VM. Active NF-E2-related factor (Nrf2) contributes to keep endothelial NO synthase (eNOS) in the coupled state: role of reactive oxygen species (ROS), eNOS, and heme oxygenase (HO-1) levels. J Biol Chem. 2009;284(46):31579–31586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chan SY, Rubin LJ. Metabolic dysfunction in pulmonary hypertension: from basic science to clinical practice. Eur Respir Rev. 2017;26(146):170094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hu J, Xu Q, McTiernan C, Lai YC, Osei-Hwedieh D, Gladwin M. Novel targets of drug treatment for pulmonary hypertension. Am J Cardiovasc Drug. 2015;15(4):225–234. [DOI] [PubMed] [Google Scholar]
- 131.Gill AJ, Kolson DL. Dimethyl fumarate modulation of immune and antioxidant responses: application to HIV therapy. Crit Rev Immunol. 2013;33(4):307–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grzegorzewska AP, Seta F, Han R, et al. Dimethyl fumarate ameliorates pulmonary arterial hypertension and lung fibrosis by targeting multiple pathways. Sci Rep UK. 2017;7:41605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miclea A, Leussink VI, Hartung HP, Gold R, Hoepner R. Safety and efficacy of dimethyl fumarate in multiple sclerosis: a multi-center observational study. J Neurol. 2016;263(8):1626–1632. [DOI] [PubMed] [Google Scholar]
- 134.Bonnet S, Rochefort G, Sutendra G, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007;104(27): 11418–11423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Courboulin A, Paulin R, Giguere NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011; 208(3):535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu H, van Berkel TJ, Biessen EA. Therapeutic potential of VIVIT, a selective peptide inhibitor of nuclear factor of activatedT cells, in cardiovascular disorders. Cardiovasc Drug Rev. 2007;25(2):175–187. [DOI] [PubMed] [Google Scholar]
- 137.Samuelsson B, Dahlen S, Lindgren J, Rouzer C, Serhan C. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237(4819):1171–1176. [DOI] [PubMed] [Google Scholar]
- 138.Tian W, Jiang X, Sung YK, Qian J, Yuan K, Nicolls MR. Leukotrienes in pulmonary arterial hypertension. Immunol Res. 2014;58(0):387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tian W, Jiang X, Tamosiuniene R, et al. Blocking macrophage leukotriene B4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5(200): 200ra117–200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jarrett H, Barnett C. HIV-associated pulmonary hypertension. Curr Opin HIV AIDS. 2017;12(6):566–571. [DOI] [PubMed] [Google Scholar]
- 141.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381(6584):667–673. [DOI] [PubMed] [Google Scholar]
- 142.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates ofHIV-1. Nature. 1996;381(6584): 661–666. [DOI] [PubMed] [Google Scholar]
- 143.Schecter AD, Calderon TM, Berman AB, et al. Human vascular smooth muscle cells possess functional CCR5. J Biol Chem. 2000;275(8):5466–5471. [DOI] [PubMed] [Google Scholar]
- 144.Ishida Y, Kimura A, Kuninaka Y, et al. Pivotal role of the CCL5/CCR5 interaction for recruitment of endothelial progenitor cells in mouse wound healing. J Clin Invest. 2012;122(2):711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dorfmuller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care. 2002;165(4):534–539. [DOI] [PubMed] [Google Scholar]
- 146.Amsellem V, Lipskaia L, Abid S, et al. CCR5 as a treatment target in pulmonary arterial hypertension. Circulation. 2014; 130(11):880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Combadiėre C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolem ic mice. Circulation. 2008;117(13): 1649–1657. [DOI] [PubMed] [Google Scholar]
- 148.Dromparis P, Michelakis ED. Mitochondria in vascular health and disease. Annu Rev Physiol. 2013;75(1):95–126. [DOI] [PubMed] [Google Scholar]
- 149.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115(1):148–164. [DOI] [PubMed] [Google Scholar]
- 150.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hitosugi T, Fan J, Chung TW, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44(6):864–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. [DOI] [PubMed] [Google Scholar]
- 153.Pan X, Liu J, Nguyen T, et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol. 2013;15:1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Whitehouse S, Randle PJ. Activation of pyruvate dehydrogenase in perfused rat heart by dichloroacetate (Short Communication). BiochemJ. 1973;134(2):651–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Michelakis ED, McMurtry MS, Wu XC, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002; 105(2):244–250. [DOI] [PubMed] [Google Scholar]
- 156.McMurtry MS, Bonnet S, Wu X, et al. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ Res. 2004;95(8): 830–840. [DOI] [PubMed] [Google Scholar]
- 157.Guignabert C, Tu L, Izikki M, et al. Dichloroacetate treatment partially regresses established pulmonary hypertension in mice with SM22alpha-targeted overexpression of the serotonin transporter. FASEB J 2009;23(12):4135–4147. [DOI] [PubMed] [Google Scholar]
- 158.Li B, Yan J, Shen Y, Liu Y, Ma Z. Dichloroacetate prevents but not reverses the formation of neointimal lesions in a rat model of severe pulmonary arterial hypertension. Mol Med Rep. 2014;10: 2144–2152. [DOI] [PubMed] [Google Scholar]
- 159.Michelakis ED, Gurtu V, Webster L, et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci Transl Med. 2017;9(413): pii:eaao4583. [DOI] [PubMed] [Google Scholar]
- 160.Paulin R, Dromparis P, Sutendra G, et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014; 20(5):827–839. [DOI] [PubMed] [Google Scholar]
- 161.Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem. 1994;55(0):1–11. [DOI] [PubMed] [Google Scholar]
- 162.Sutendra G, Bonnet S, Rochefort G, et al. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci Transl Med. 2010;2(44): 44ra58–44ra58. [DOI] [PubMed] [Google Scholar]
- 163.Fang YH, Piao L, Hong Z, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle’s cycle. J Mol Med. 2012;90(1):31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rayner-Hartley E, Sedlak T. Ranolazine: a contemporary review. J Am Heart Assoc. 2016;5(3):e003196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gomberg-Maitland M, Schilz R, Mediratta A, et al. Phase I safety study of ranolazine in pulmonary arterial hypertension. Pulm Circ. 2015;5(4):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khan SS, Cuttica MJ, Beussink-Nelson L, et al. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: a pilot study. Pulm Circ. 2015;5(3):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105: 114–127. [DOI] [PubMed] [Google Scholar]
- 168.Omura J, Satoh K, Kikuchi N, et al. Protective roles of endothelial AMP-activated protein kinase against hypoxia-induced pulmonary hypertension in mice. Circ Res. 2016;119:197–209. [DOI] [PubMed] [Google Scholar]
- 169.Goncharov DA, Goncharova EA, Tofovic SP, et al. Metformin therapy for pulmonary hypertension associated with HFpEF versus PAH. Am J Respir Crit Care. 2018;0(ja):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Agard C, Rolli-Derkinderen M, Dumas-de-La-Roque E, et al. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br J Pharmacol. 2009;158(5):1285–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hansmann G, Zamanian RT. PPARγ activation: a potential treatment for pulmonary hypertension. Sci Transl Med. 2009;1(12): 12ps14–12ps14. [DOI] [PubMed] [Google Scholar]
- 172.Hansmann G, de Jesus Perez VA, Alastalo TP, et al. An anti-proliferative BMP-2/PPARg/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118(5):1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Calvier L, Chouvarine P, Legchenko E, et al. PPARg links BMP2 and TGFβ1 pathways in vascular smooth muscle cells, regulating cell proliferation and glucose metabolism. Cell Metab. 2017;25(5):1118–1134.e1117. [DOI] [PubMed] [Google Scholar]
- 174.Diebold I, Hennigs Jan K, Miyagawa K, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21(4):596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Guignabert C, Alvira CM, Alastalo T-P, et al. Tie2-mediated loss of peroxisome proliferator-activated receptor-g in mice causes PDGF receptor-b-dependent pulmonary arterial muscularization. Am JPhysiol-Lung C. 2009;297(6):L1082–L1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ameshima S, Golpon H, Cool CD, et al. Peroxisome proliferator-activated receptor gamma (PPARg) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ Res. 2003;92(10):1162–1169. [DOI] [PubMed] [Google Scholar]
- 177.Kim EK, Lee JH, Oh YM, Lee YS, Lee SD. Rosiglitazone attenuates hypoxia induced pulmonary arterial hypertension in rats. Respirology. 2010;15(4):659–668. [DOI] [PubMed] [Google Scholar]
- 178.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator activated receptor-g activation. Circulation. 2007;115(10):1275–1284. [DOI] [PubMed] [Google Scholar]
- 179.Bertero T, Lu Y, Annis S, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J Clin Invest. 2014;124(8):3514–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bertero T, Cottrill Katherine A, Lu Y, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell Rep. 2015;13(5):1016–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Legchenko E, Chouvarine P, Borchert P, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci Transl Med. 2018; 10(438):eaao0303. [DOI] [PubMed] [Google Scholar]
- 182.Liu Y, Tian XY, Huang Y, Wang N. Rosiglitazone attenuated endothelin-1-induced vasoconstriction of pulmonary arteries in the rat model of pulmonary arterial hypertension via differential regulation of ET-1 receptors. PPAR Res. 2014;2014:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nisbet RE, Bland JM, Kleinhenz DJ, et al. Rosiglitazone attenuates chronic hypoxia-induced pulmonary hypertension in a mouse model. Am JRespir Cell Mol. 2010;42(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457–2471. [DOI] [PubMed] [Google Scholar]
- 185.Soccio Raymond E, Chen Eric R, Lazar Mitchell A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014;20(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Breunig IM, Shaya FT, McPherson ML, Snitker S. Development of heart failure in medicaid patients with type 2 diabetes treated with pioglitazone, rosiglitazone, or metformin. J Manag Care Pharm. 2014;20(9):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374(14):1321–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hughes AD, Park C, March K, et al. A randomized placebo controlled double blind crossover study of pioglitazone on left ventricular diastolic function in type 2 diabetes. Int J Cardiol. 2013;167(4):1329–1332. [DOI] [PubMed] [Google Scholar]
- 189.Schopfer FJ, Cole MP, Groeger AL, et al. Covalent peroxisome proliferator-activated receptor g adduction by nitro-fatty acids: selective ligand activity abd anti-diabetic signaling actions. J Biol Chem. 2010;285(16):12321–12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Klinke A, Moller A, Pekarova M, et al. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. Am J Respir Cell Mol. 2014;51(1):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Cracowskic J-L, Cracowskic C, Bessardb G, et al. Increased lipid peroxidation in patients with pulmonary hypertension. Am J Respir Crit Care. 2001;164(6):1038–1042. [DOI] [PubMed] [Google Scholar]
- 192.Bowers R, Cool C, Murphy RC, et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care. 2004;169(6): 764–769. [DOI] [PubMed] [Google Scholar]
- 193.Preston IR, Tang G, Tilan JU, Hill NS, Suzuki YJ. Retinoids and pulmonary hypertension. Circulation. 2005;111(6):782–790. [DOI] [PubMed] [Google Scholar]
- 194.Wong C-M, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Sign. 2013;18(14):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension: a national prospective study. Ann Intern Med. 1987;107(2):216–223. [DOI] [PubMed] [Google Scholar]
- 196.Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med. 2015;373(9):834–844. [DOI] [PubMed] [Google Scholar]
- 197.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. [DOI] [PubMed] [Google Scholar]
- 198.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation. 2010;122(2):164–172. [DOI] [PubMed] [Google Scholar]
- 199.Ventetuolo CE, Baird GL, Barr RG, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care. 2016;193(10):1168–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care. 2009;179(9):835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mair KM, Wright AF, Duggan N, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care. 2014;190(4):456–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kawut SM, Archer-Chicko CL, DeMichele A, et al. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care. 2017;195(3): 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22(3): 185–212. [DOI] [PubMed] [Google Scholar]
- 204.Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca2+- activated K+-channel opener. Am J Physiol-Lung C. 1998; 274(2):L186–L195. [DOI] [PubMed] [Google Scholar]
- 205.Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003;144(8):3449–3455. [DOI] [PubMed] [Google Scholar]
- 206.Homma N, Nagaoka T, Karoor V, et al. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol-Lung C. 2008;295(1): L71–L78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alzoubi A, Toba M, Abe K, et al. Dehydroepiandrosterone restores right ventricular structure and function in rats with severe pulmonary arterial hypertension. Am J Physiol-Heart C. 2013;304(12):H1708–H1718. [DOI] [PubMed] [Google Scholar]
- 208.Dumas DLRE, Savineau J, Metivier A, et al. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol (Paris). 2012;73(1):20–25. [DOI] [PubMed] [Google Scholar]
- 209.Rhodes CJ, Howard LS, Busbridge M, et al. Iron deficiency and raised hepcidin in idiopathic pulmonary arterial hypertension: clinical prevalence, outcomes, and mechanistic insights. J Am Coll Cardiol. 2011;58(3):300–309. [DOI] [PubMed] [Google Scholar]
- 210.Ruiter G, Lankhorst S, Boonstra A, et al. Iron deficiency is common in idiopathic pulmonary arterial hypertension. Eur Respir J. 2011;37(6):1386–1391. [DOI] [PubMed] [Google Scholar]
- 211.Soon E, Treacy CM, Toshner MR, et al. Unexplained iron deficiency in idiopathic and heritable pulmonary arterial hypertension. Thorax. 2011;66(4):326–332. [DOI] [PubMed] [Google Scholar]
- 212.van Empel VPM, Lee J, Williams TJ, Kaye DM. Iron deficiency in patients with idiopathic pulmonary arterial hypertension. Heart Lung Circul. 2014;23(3):287–292. [DOI] [PubMed] [Google Scholar]
- 213.Rouault TA, Tong WH. Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol. 2005; 6:345–351. [DOI] [PubMed] [Google Scholar]
- 214.Ghosh Manik C, Zhang DL, Jeong Suh Y, et al. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2a. CellMetab. 2013;17(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cotroneo E, Ashek A, Wang L, et al. Iron homeostasis and pulmonary hypertension: Iron deficiency leads to pulmonary vascular remodeling in the rat. Circ Res. 2015;116:1680–1690. [DOI] [PubMed] [Google Scholar]
- 216.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.White K, Lu Y, Annis S, et al. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol Med. 2015; 7(6):695–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kato GJ, Taylor JG. Pleiotropic effects of intravascular haemolysis on vascular homeostasis. Br J Haematol. 2010;148(5): 690–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang W, Xiao ZD, Li X, et al. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015; 17:490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mo JS, Meng Z, Kim YC, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17(4):500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2015;16(1):20–33. [DOI] [PubMed] [Google Scholar]
- 222.Perez VADJ, Yuan K, Lyuksyutova MA, et al. Whole-exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care. 2014; 189(10):1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li M, Vattulainen S, Aho J, et al. Loss of bone morphogenetic protein receptor 2 is associated with abnormal DNA repair in pulmonary arterial hypertension. Am J Respir Cell Mol. 2014; 50(6):1118–1128 [DOI] [PubMed] [Google Scholar]
- 224.Ngo J, Matsuyama M, Kim C, et al. Bax deficiency extends the survival of Ku70 knockout mice that develop lung and heart diseases. Cell Death Dis. 2015;6(3):e1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Meloche J, Pflieger A, Vaillancourt M, et al. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation. 2014;129(7):786–797. [DOI] [PubMed] [Google Scholar]
- 226.Meloche J, Guen ML, Potus F, et al. miR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol-Cell Physiol. 2015;309(6):C363–C372. [DOI] [PubMed] [Google Scholar]
- 227.Zhao L, Chen CN, Hajji N, et al. Histone deacetylation inhibition in pulmonary hypertension. Circulation. 2012;126(4): 455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Galletti M, Cantoni S, Zambelli F, et al. Dissecting histone deacetylase role in pulmonary arterial smooth muscle cell proliferation and migration. Biochem Pharmacol. 2014;91(2): 181–190 [DOI] [PubMed] [Google Scholar]
- 229.Kim J, Hwangbo C, Hu X, et al. Restoration of impaired endothelial myocyte enhancer factor 2 function rescues pulmonary arterial hypertension. Circulation. 2015;131(2): 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sofer A, Lee S, Papangeli I, et al. Therapeutic engagement of the HDAC IIA-MEF2 axis improves experimental pulmonary hypertension. Am J Respir Crit Care. 2018;198(10):1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bogaard HJ, Mizuno S, Hussaini AAA, et al. Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. Am J Respir Crit Care. 2011; 183(10):1402–1410. [DOI] [PubMed] [Google Scholar]
- 232.Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nat Rev Drug Discov. 2014; 13:337–356. [DOI] [PubMed] [Google Scholar]
- 233.Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535 [DOI] [PubMed] [Google Scholar]
- 234.Mumby S, Gambaryan N, Meng C, et al. Bromodomain and extra-terminal protein mimic JQ1 decreases inflammation in human vascular endothelial cells: implications for pulmonary arterial hypertension. Respirology. 2017;22(1): 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Negi V, Chan SY. Discerning functional hierarchies of micro RNAs in pulmonary hypertension. JC I Insight. 2017;2(5): e91327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Farh KK-H, Grimson A, Jan C, et al. The widespread impact of mammalian microRNAs on mRNA repression and evolution. Science. 2005;310(5755):1817–1821. [DOI] [PubMed] [Google Scholar]
- 237.Kheyfets VO, Sucharov CC, Truong U, et al. Circulating miRNAs in pediatric pulmonary hypertension show promise as biomarkers of vascular function. OxidMed Cell Longev. 2017;2017:4957147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension translating microRNA biology in pulmonary hypertension it will take more than “miR” words. Am J Respir Crit Care. 2017;195(2): 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Newman JH, Rich S, Abman SH, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach. A Joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop Report. Am J Respir Crit Care. 2017;195(12):1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]