Abstract
Ring chromosome 22, a rare cytogenetic finding, was first described by Weleber et al. in 1968. Since then approximately 50 patients have been reported in the medical literature. We describe five previously unreported subjects with ring chromosome 22 syndrome, summarize the clinical findings of reported patients from the literature and discuss the involvement of the ring chromosome and clinical outcome. Our subjects demonstrated the prominent features of this syndrome including mental retardation, hypotonia, motor delay, lack of speech, full eyebrows, and large ears. In addition, two of our subjects had central nervous system malformations and regression. The lack of consistent physical abnormalities in our subjects further supports no consistent phenotype manifestations in this cytogenetic syndrome. The variable clinical manifestations seen in ring chromosome 22 subjects may be associated with loss of chromosome 22 sequences near the telomere or attributed to the genetic background of each subject. Similarly, recessive alleles unmasked by the deletion could also contribute to the phenotype.
Keywords: chromosome 22, mood disorders, review, ring chromosome
Ring chromosome 22 is a rare cytogenetic disorder first reported by Weleber et al. in 1968 (1). In 1991, Severien et al. (2) reviewed 36 patients from the literature with this cytogenetic abnormality. Mental retardation, delayed motor development and mild craniofacial dysmorphia were the most consistent findings in their review. Since then additional patients with this chromosomal abnormality have been reported including those with behavioral and mood disorders, specifically bipolar affective disorder, hyperactivity, self-injurious behavior, cruelty to animals and autism (3, 4).
Herein, we report the clinical findings of five additional patients with ring chromosome 22 (one subject described in the text and four summarized in tabular form). Two of the subjects were diagnosed with bipolar affective disorder, aggression and/or hyperactivity. In addition, we summarize findings of patients in the literature with ring chromosome 22. We emphasize that genes found on chromosome 22 may impact on the phenotype of subjects with ring 22 syndrome either through a deletion of the genes in the ring formation or loss of the ring chromosome during cell division
Clinical report
Subject 1
JE is a 12-year-old male first seen at age 5 1/2 years for genetic evaluation. He was born spontaneously after a full-term uncomplicated pregnancy to a 26-year-old gravida 3, para 2 woman. His medical history was complicated by bronchitis, otitis media, conjunctivitis, a hydrocele repair, and milk intolerance during early infancy. His development was described as normal until approximately 18 months of age when his development regressed after a febrile seizure. An MRI of the head showed a prominent cisterna magna with mild cerebral atrophy. He was diagnosed with mental retardation at 3 years of age.
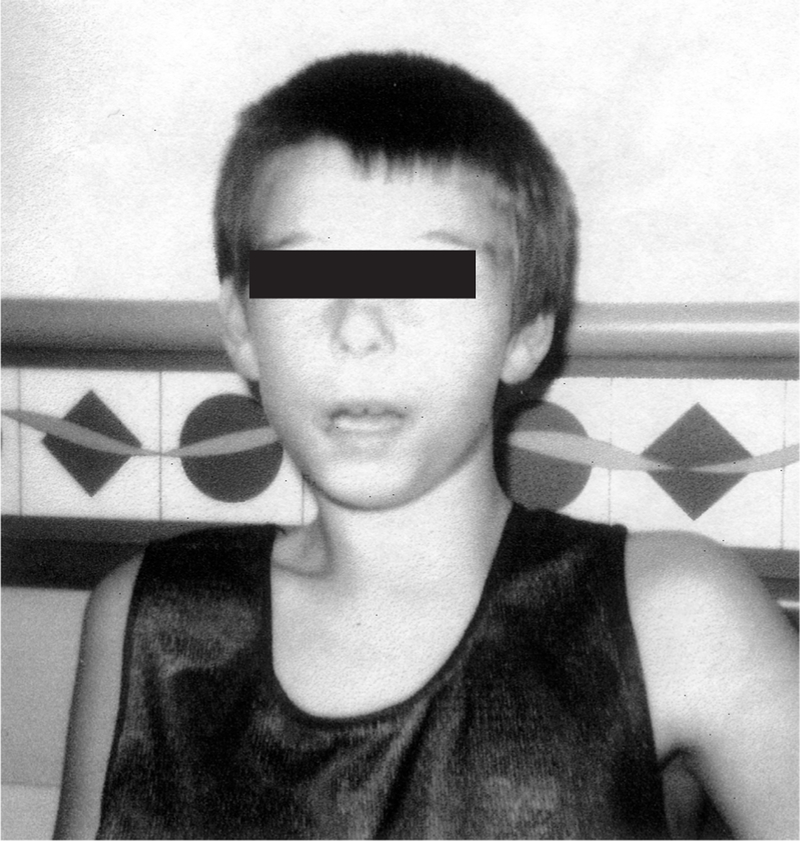
At 5 1/2 years, his head circumference and weight were at the 80th centile. His height was at the 50th centile. He had a triangular-shaped face with a broad forehead, a narrow jaw, full eyebrows and thick hair. His eyes were almond shaped with mild epicanthal folds. His nasal tip was bulbous and the philtrum was long but well defined. He had a wide mouth and large ears (95th centile) which were normally placed. He was mildly hypotonic with clonus of the right foot. His speech regressed from two word sentences at 2 years of age to a vocabulary of only a few words at 5 1/2 years (Fig. 1). A chromosome analysis revealed a de novo 46,XY,r(22)(p11.2; q13) karyotype in the 20 lymphocytes studied. Parental karyotypes were normal.
Fig. 1.
Frontal view of subject 1 at 12 years of age.
At 12 years of age, the patient showed significant behavioral changes and no meaningful speech. His behavior deteriorated and he was difficult to control. He became destructive at home, breaking most small objects in the house. After a psychiatric evaluation, bipolar affective disorder was diagnosed. He is now medically treated. A follow-up head MRI showed a giant cisterna magna and mild cerebellar atrophy. Four additional subjects (numbers 2, 3, 4, 5) with ring chromosome 22 are summarized in Table 1.
Table 1.
Clinical findings in our five subjects with ring chromosome 22
| Clinical findings | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 |
|---|---|---|---|---|---|
| Age/sex | 12y/M | 35y/M | 7y/F | 8y/M | 16y/M |
| Karyotype | 46,XY,r(22) (p11.2; q13) all lymphocytes studied showed r (22) | 46,XY,r(22) (p11.2; q13) 80% of lymphocytes studied showed r (22) | 46,XX,r(22) all lymphocytes studied showed r (22) | 46,XY,r(22) | 46,XY,r(22) (p12;q13) all lymphocytes studied showed r (22) |
| Mental retardation | + | + | + | + | + |
| Delayed motor development | − | + | + | − | + |
| Muscular hypotonia | + | + | + | − | − |
| Large ears | + | + | + | + | + |
| Epicanthal folds | + | − | − | − | − |
| Mood disorder | + | + | − | − | − |
| Lack of speech development | + | + | − | + | + |
| Full eyebrows | + | + | + | + | + |
| Microcephaly | − | − | − | − | − |
| Ataxia | − | + | − | − | + |
| Seizures | + | − | − | − | + |
| High–arched palate | − | + | − | − | − |
| Syndactyly of toes 2–3 | − | − | − | − | − |
| Flat nasal bridge | − | − | − | − | − |
| Hypertelorism | − | − | − | − | − |
| Growth retardation | − | + | − | − | − |
| Other | regression of speech; cerebellar atrophy; hydrocele | aggression; hyper–activity; height <3rd centile; no brain imaging performed | dysplastic nails; normal IgA levels; normal MRI | no brain imaging performed | mental regression; 3–4 finger syndactyly; hydrocele; cryptorchidism; cerebral and cerebellar atrophy; distal neuropathy (nerve conduction/electromyography studies showed chronic neuro– myopathic changes); low arylsulfatase A levels; IgA immune deficiency. |
Discussion
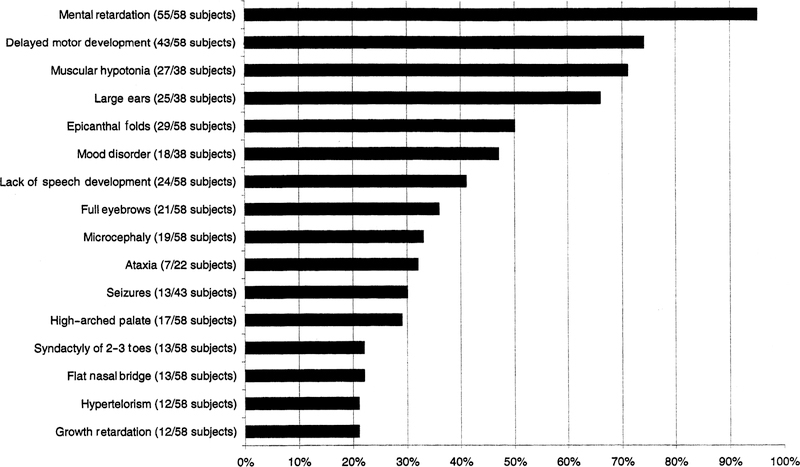
Although physical features can be seen in patients with ring chromosome 22, they are inconsistent and typically minor. Our subjects had many of the findings previously described in the literature. Hunter et al. (5) in 1977 and other subsequent reports (4, 6) have helped to delineate features seen in the ring 22 syndrome. Our report further documents the clinical features of ring chromosome 22 which have been reported in 52 individuals [36 individuals were summarized by Severien et al. (2) in 1991] and summarizes these findings in all reported patients (Fig. 2). Not all subjects reported in the literature included sufficient information to determine if the summarized feature was present.
Fig. 2.
Clinical findings in 58 patients with ring chromosome 22. The summary of clinical findings is given for 58 patients (53 from the literature and our 5 subjects) with ring chromosome 22. Not all subjects reported in the literature included sufficient information to determine if the finding was present or absent. Therefore, the percentage listed for each finding represents those subjects in which pertinent information was available to determine the presence or absence of the particular finding [e.g. 25 of 38 subjects (66%) had large ears].
Thirty-six subjects were reviewed by Severien et al. (2) An additional 17 subjects have been reported [Taalman et al. (13), Naritomi et al. (14), Watanabe et al. (15), Christodoulon et al. (16), Ritter et al. (17), Tommerup et al. (18), Petrella et al. (7), Coulter-Mackie et al. (19), Joyce et al. (20), Sovner etal. (3), Kehrer-Sawatzki et al. (21), Rubio et al. (22), Assumpcao (23), Gibbons et al. (24), MacLean et al. (4), Arnold et al. (9), De Mas et al. (25)]
Ring chromosome 22 features seen frequently (>50%) include mental retardation (55/58 subjects), delayed motor development (43/58), hypotonia (27/38), and large ears (25/38). Other common features (30%–50%) include epicanthal folds (29/58), microcephaly (19/58), full eyebrows (21/58), mood disorders (18/38), lack of speech development (24/58) and ataxia (7/22). Although ataxia was not listed as a feature in the review article by Severien et al. (2), it was a common finding in our survey of patients. Occasional traits (20%–30%) include hypertelorism (12/58), flat nasal bridge (13/58), high palate (17/58), 2–3 toe syndactyly (13/58) and seizures (13/43). Other reported findings include growth retardation, clinodactyly of 5th digit, micrognathia, cleft palate, ocular colobomas, prominent lips, low set ears, imperforate anus, and brain meningiomas. In addition, one previously reported subject had a large cisterna magna (7). This brain finding was also seen in our subject 1 but not in our subjects 3 and 5 (who had brain imaging). Approximately 10% of reported patients with ring chromosome 22 and brain-imaging studies showed central nervous system abnormalities including brain ventricle dilatation, cerebellar and cerebral atrophy, large cisterna magna, meningiomas and cerebellar tumors (2).
In 1996, Sovner et al. (3) reported that ring chromosome 22 patients had an increased risk of mood disorders. At 12 years of age, subject 1 had a significant change in his behavior with uncontrollable outbursts resulting in destruction of property at home. He had a psychological evaluation for his behavioral problems and a diagnosis of bipolar affective disorder was made. Subject 2 (Table 1) had a history of behavioral problems beginning in early childhood with aggression and hyperactivity noted as he became older. Subject 5 (Table 1) was noted to have mental and physical deterioration beginning at 12 years of age. He had an episode of status epilepticus followed by a chronic seizure disorder requiring therapy with anticonvulsants. He developed sensorimotor polyneuropathy demonstrated on nerve conduction and electromyographic studies. His brain MRI showed diffuse cerebral and cerebellar atrophy. Leukocyte arylsulfatase A levels were low, which would be suggestive of juvenile onset metachromatic leukodystrophy. Molecular confirmation of arylsulfatase A deficiency was not done. Regression, mood changes and ataxia reported in subjects with ring chromosome 22 could be attributed to gene disturbances on chromosome 22 causing central nervous system dysfunction and/or metabolic changes. Our other subjects with ring chromosome 22 had not developed behavioral problems by 7 and 8 years of age. These findings are consistent with the report by Sovner et al. (3) that patients with ring chromosome 22 are at an increased risk of mood disorders and hyperactivity. In addition, other behavioral problems and mood disorders are associated with ring chromosome 22 including autism, hyperactivity, cruelty toward animals and self-injurious behavior (3, 4, 6, 8). Mood disorders and/or behavioral problems were seen in two of our five subjects; however, the mood disorders were not reported until teenage years. The majority of reports did not mention behavioral problems although many of the patients were reported at a young age. Because no follow-up reports had been published, it is unknown whether any of the patients previously reported developed similar mood disorders as they grew older. Interestingly, a schizophrenia susceptibility locus has been mapped to chromosome 22 (22q11-q13) (9). This was suggested by linkage studies and the identification of a 22q11.2 interstitial deletion in one child among 32 patients with childhoodonset schizophrenia (10).
MacLean et al. (4) further summarized additional findings in subjects with ring chromosome 22, highlighting the association of the ring with genetic disorders involving chromosome 22. Such reports include subjects with ring chromosome 22 and multiple meningiomas, neurofibromatosis type 2, IgA immuno-deficiency, metachromatic leukodystrophy, and Opitz syndrome.
The growth retardation (which occurs in 20% of reported patients) and the other unusual manifestations seen in our subjects as well as those reported in the literature may be due in part to mis-segregation and loss of the ring chromosome during early fetal development or with age. The loss of the ring chromosome probably occurs by mitotic anaphase lag and produces a mosaic genotype (11). The resultant monosomy 22 cell line may not be viable and the total number of viable cells may diminish with repeated cell divisions compared with normal cells from normal individuals. The rate of cell division per tissue or organ varies and a resultant phenotype may occur depending on the tissue or organ most affected by the cell loss. Hence, mitotic instability of the ring can result in cells with monosomy, double rings or dicentric rings which can further affect the phenotype.
In addition, despite the apparent relationship between parental origin of the ring chromosome and phenotypes such as in ring 15 syndrome (12), the variable clinical manifestations seen in ring chromosome 22 subjects may be associated with loss of 22q sequences close to the telomere or the genetic background of each subject. Hemizygous expression of genes close to the telomere of chromosome 22 that are important for growth or other reported features may be under consideration. Similarly, recessive alleles unmasked by the deletion could also contribute to the phenotype of the subject. Correlation of specific phenotypes with the parental origin of the ring may provide evidence of uniparental gene expression or other chromosomes although imprinted genes have not been reported on chromosome 22. Thus, our observations of phenotypic differences (and similarities) among subjects with ring chromosome 22 and potential genetic mechanisms that may account for the syndromic features may stimulate efforts to understand the variable clinical presentation of ring chromosome 22 and other ring chromosome syndromes. Ring chromosome 22 patients should be monitored closely for genetic conditions whose genes are known to be located on chromosome 22 and produce resultant features due to one of the genetic mechanisms described above.
In summary, we reviewed the medical literature and found 53 reported patients with ring chromosome 22 and compared them with our five subjects. Our review further delineates the major and minor features seen in this rare cytogenetic syndrome. We emphasize the importance of follow-up, psychological/behavioral testing and comprehensive medical care in order to identify psychological problems, mental/physical deterioration or findings of gene disturbances of chromosome 22.
References
- 1.Weleber RG, Hecht F, Giblett ER. Ring G chromosome a new G deletion syndrome? Am J Dis Child 1968: 115: 489–494. [DOI] [PubMed] [Google Scholar]
- 2.Severien C, Felix S, Bartholome K. Ring chromosome 22: a case report. Klin Padiatr 1991: 203: 467–469. [DOI] [PubMed] [Google Scholar]
- 3.Sovner R, Stone A, Fox C. Ring chromosome 22 and mood disorders. J Intell Dis Res 1996: 40: 82–86. [DOI] [PubMed] [Google Scholar]
- 4.MacLean JE, Teshima IE, Szatmari P, Nowaczyk MJ. Ring Chromosome 22 and autism: report and review. Am J Med Genet 2000: 90: 382–385. [PubMed] [Google Scholar]
- 5.Hunter AGW, Ray M, Wang HS, Thompson DR. Phenotypic correlations in patients with ring chromosome 22. Clin Genet 1977: 12: 239–249. [DOI] [PubMed] [Google Scholar]
- 6.Funderburk SJ, Sparkes RS, Klisak I. Phenotypic variation in two patients with a ring chromosome 22. Clin Genet 1979: 16: 305–310. [DOI] [PubMed] [Google Scholar]
- 7.Petrella R, Levine S, Wilmot P, Ashar KD, Casamassima AC, Shapiro LR. Multiple meningiomas in a patient with constitutional ring chromosome 22. Am J Med Genet 1993: 47: 184–186. [DOI] [PubMed] [Google Scholar]
- 8.Lindenbaum RH, Bobrow M, Barber L. Monozygotic twins with ring chromosome 22. J Med Genet 1973: 10: 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnold PD, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R. Velo-cardio-facial syndrome: Implications of microdeletion of 22q11 for schizophrenia and mood disorders. Am J Med Genet 2001: 105: 354–362. [DOI] [PubMed] [Google Scholar]
- 10.Yan W, Jacobsen Krasnewich LK, Guan DM et al. 2 interstitial deletions among childhood onset schizophrenia and ‘multidimensionally impaired.’ Am J Med Genet 1998: 81: 41–43. [PubMed] [Google Scholar]
- 11.Kosztolanyi G. Does ‘ring syndrome’ exist? Analysis of 207 case reports on patients with a ring autosome. Hum Genet 1987: 75: 174–179. [DOI] [PubMed] [Google Scholar]
- 12.Rogan PK, Seip JR, Driscoll DJ et al. Distinct 15q genotypes in Russell-Silver and ring 15 syndromes. Am J Med Genet 1996: 62: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taalman RD, Weemales CR, Hustinx TJ, Scheres JC, Clement JE, Stoelinga GA. Chromosome studies in IgA-deficient patients. Clin Genet 1987: 32: 81–87. [DOI] [PubMed] [Google Scholar]
- 14.Naritomi K, Hirayama K. Determination of the break-points and the parental origin of a ring 22 chromosome: an analysis by high-resolution banding technique, quinacrine and silver stainings. Jpn J Human Genet 1988: 33: 67–73. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe H, Yamanaka T. Ring chromosome 22, 46,XX,r (22)(p11.2→q13.3) presenting with leukemoid reaction. Clin Genetics 1998: 34: 206–208. [DOI] [PubMed] [Google Scholar]
- 16.Christodoulon J, Loughnan P. Ring chromosome 22 karyotype in a patient with Opitz(BBBG) syndrome. Am J Med Genet 1990: 37: 422–424. [DOI] [PubMed] [Google Scholar]
- 17.Ritter CL, Steele MW, Wenger SL, Cohen BA. Chromosome mosaicism in hypomelanosis of Ito. Am J Med Genet 1990: 35: 14–17. [DOI] [PubMed] [Google Scholar]
- 18.Tommerup N, Warburg M, Gieselmann V, Hansen BR, Koch J, Petersen GB. Ring chromosome 22 and neurofibromatosis. Clin Genet 1992: 42: 171–177. [DOI] [PubMed] [Google Scholar]
- 19.Coulter-Mackie MB, Rip J, Ludman MD, Beis J, Cole DE. Metachromatic leukodystrophy (MLD) in a patient with a constitutional ring chromosome 22. J Med Genet 1995: 32: 787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joyce CA, Zorich B, Pike SJ, Barber JK, Dennis NR. Williams-Beuren syndrome: phenotypic variability and deletions of chromosomes 7, 11 and 22 in a series of 52 patients. J Med Genet 1996: 33: 986–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kehrer-Sawatzki H, Udart M, Krone W et al. Mutational analysis and expression studies of the neurofibromatosis type 2 (NF2) gene in a patient with a ring chromosome 22 and NF2. Hum Genet 1997: 100: 67–74. [DOI] [PubMed] [Google Scholar]
- 22.Rubio A. Four year old girl with ring chromosome 22 and brain tumor. Brain Path 1997: 7: 1027–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assumpcao FB. Brief Report: a case of chromosome 22 alteration associated with autistic syndrome. J Autism Dev Dis 1998: 28: 253–256. [DOI] [PubMed] [Google Scholar]
- 24.Gibbons B, Tan Sy, Tam Py. Ring chromosome 22 resulting in partial monosomy in a mentally retarded boy. Singapore Med J 1999: 40: 273–275. [PubMed] [Google Scholar]
- 25.De Mas PD, Chassaing N, Chaix Y et al. Molecular characterization of a ring chromosome 22 in a patient with severe language delay: a contribution to the refinement of the subtelomeric 22q deletion syndrome. J Med Genet 2002: 39: e17. [DOI] [PMC free article] [PubMed]