Abstract
Occupational and tobacco exposure to aromatic amines (AAs) including 4-aminobiphenyl (4-ABP) and 2-naphthylamine (2-NA) are associated with bladder cancer (BC) risk. Several epidemiological studies have also reported a possible role for structurally related heterocyclic aromatic amines (HAAs) formed in tobacco smoke or cooked meats with BC risk. We had screened for DNA adducts of 4-ABP, 2-NA, and several prominent HAAs formed in tobacco smoke or grilled meats including 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylmidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-9H-pyrido[2,3-b]indole (AαC) in the bladder DNA of BC patients, using liquid chromatography/mass spectrometry. We detected DNA adducts of 4-ABP, but not of adducts the other carcinogens. In this study, we have examined the capacity of RT4 cells, an epithelial human bladder cell line, to bioactivate AAs and HAAs to DNA damaging agents, which may contribute to BC. 4-ABP and AαC formed DNA adducts, but DNA adducts of 2-NA, PhIP and MeIQx were not detected. 4-ABP DNA adducts were formed at 10-fold higher levels than AαC adducts. Pretreatment of RT4 cells with α-naphthoflavone (1–10 μM), a specific cytochrome P450 1 (CYP1) inhibitor, decreased AαC adduct formation by 50 percent but did not affect the level of 4-ABP adducts. However, cell pretreatment with 8-methoxypsoralen (0.1–1 μM), a potent inhibitor of CYP2A, resulted in a 90 percent decrease of 4-ABP DNA adducts levels. These data signify that CYP2A and CYP1A isoforms expressed in the target urothelium bioactivate 4-ABP and AαC and may be a critical feature of aromatic amine-induced urinary bladder carcinogenesis. The bioactivation of other tobacco and environmental AAs by bladder CYPs and their ensuing bladder DNA damage warrants further study.
Keywords: Bladder cancer; aromatic amines; heterocyclic aromatic amines; 4-aminobiphenyl; 2-amino-9H-pyrido[2,3-b]indole; DNA adduct
Introduction
Bladder cancer (BC) is the fourth most common cancer in men in the United States and the most lethal cancer malignancy of the urinary system (Siegel et al. 2017). Genetic susceptibility such as slow N-acetyltransferase 2 (NAT2) and glutathione S-transferase mu 1 (GSTM1) null genotypes, chronic infection, and occupational and environmental exposures to chemicals including arsenic and aromatic amines (AAs) are major risk factors for BC (Burger et al. 2013). Occupational exposure to AAs including 4-aminobiphenyl (4-ABP) and 2-naphthylamine (2-NA) has been linked to the elevated BC risk in factory workers (Jemal et al. 2011). Cigarette smoking is a well-known risk factor for BC (Burger et al. 2013). 4-ABP and 2-NA are assumed to contribute to the pathogenesis of BC of smokers based on occupational exposure data and BC risk. Smokers excrete elevated levels of mutagenic compounds in urine compared to non-smokers. A high portion of this mutagenicity is associated with AAs and structurally related heterocyclic aromatic amines (HAAs) (DeMarini 2004; Peters et al. 2003). 4-ABP and 2-NA were detected in the urine of smokers and nonsmokers (Riedel et al. 2006). 2-Amino-9H-pyrido[2,3-b]indole (AαC) is the most abundant HAA formed in tobacco smoke, occurring at levels ranging from 25 to 260 ng per cigarette (Hoffmann et al. 2001). These amounts are up to 100-fold higher than the levels of 4-ABP, 2-NA, or benzo[a]pyrene (B[a]P) present in tobacco smoke (Hoffmann et al. 2001). AαC was detected in the urine of smokers, and the concentrations were positively correlated to the number of cigarettes smoked per day (Fu et al. 2014; Turesky et al. 2007). Other HAAs including 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) were also detected in the urine of smokers or omnivores (Turesky and Le Marchand 2011). Well-done cooked meats contain AαC and more than 20 other HAAs including PhIP and MeIQx (Sugimura et al. 2004). AAs and HAAs are multisite rodent carcinogens (IARC. 2004; Sugimura et al. 2004). Notably, several epidemiological studies have identified consumption of well done-cooked meats as a risk factor for BC (Balbi et al. 2001; Ferrucci et al. 2010; Lin et al. 2012; Lumbreras et al. 2008; Michaud et al. 2006).
AAs and HAAs require bioactivation by cytochrome P450 (CYP)-catalyzed N-oxidation of the exocyclic amine groups to form N-hydroxylated AAs and HAAs metabolites. Conjugation enzymes, such as NAT or sulfotransferases (SULT), catalyze the conversion of the N-hydroxylated metabolites to unstable esters, which undergo heterolytic cleavage to form the short-lived nitrenium ions that covalently adduct to DNA (Turesky and Le Marchand 2011). CYP1A2-mediated N-hydroxylation of AAs in the liver is considered one major bioactivation pathway involved in aromatic amine bladder carcinogenesis (IARC. 2004). The N-hydroxy-AA and N-hydroxy-HAA intermediates can reach extrahepatic tissues including the bladder through the bloodstream to form DNA adducts (Kaderlik et al. 1994; Radomski and Brill 1970). Alternatively, AAs and HAAs can reach the bladder and undergo bioactivation by CYPs expressed in bladder epithelium (Nakajima et al. 2006).
We recently identified DNA adducts of 4-ABP in the genome of some BC patients (Guo et al. 2018). Thus, we sought to characterize the DNA adducts of 4-ABP, 2-NA and several prototypical HAAs, including AαC, PhIP, and MeIQx, formed in RT4 cells, a well-characterized human bladder epithelial cell line (O’Toole et al. 1983), to better understand the capacity of bladder cells to bioactivate these procarcinogens. Our findings show that 4-ABP and AαC are bioactivated in RT4 cells and form DNA adducts, whereas the other compounds are inactive. The levels of 4-ABP DNA adducts were significantly higher than those of AαC, supporting the role of 4-ABP in BC etiology. We demonstrated that 4-ABP and AαC are bioactivated by CYP2A and CYP1 enzyme families, respectively, using selective CYP inhibitors and recombinant human enzymes. The chemicals in tobacco smoke, diet, and environment that damage the genome of the bladder are largely unknown. Further studies on the capacity of CYPs in bladder to bioactivate tobacco-associated and environmental AAs may help to identify other potential bladder carcinogens.
Materials and methods
Chemicals
HAAs were purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). 4-Hydroxyaminobiphenyl (HONH-4-ABP) and HONH-HAAs were prepared as described (Pathak et al. 2016). N-Hydroxy-2-naphthylamine (HONH-2-NA) was provided by the late Dr. Fred F. Kadlubar (National Center for Toxicological Research, Jefferson, AR). Dimethyl sulfoxide (DMSO), 4-ABP, 2-NA, ethoxyresorufin, α-naphtoflavone (α-NF), 8-methoxypsoralen (8-MOP), 7-hydroxycoumarin, coumarin, dilauroyl-l-α-phosphatidylcholine (DLPC), NADPH, ethylenediaminetetraacetic acid (EDTA), β-mercaptoethanol (BME), calf thymus DNA (CT-DNA), RNase A (bovine pancreas), RNase T1 (Aspergillus oryzae), proteinase K (Tritirachium album), sodium dodecyl sulfate (SDS), DNase I (type IV, bovine pancreas), alkaline phosphatase (Escherichia coli), and nuclease P1 (Penicillium citrinum) were purchased from Sigma Aldrich (St. Louis, MO, USA). Phosphodiesterase I (Crotalus adamanteus venom) was purchased from Worthington Biochemical Corp. (Newark, NJ). E. Coli expressed and purified human CYP2A6, CYP2A13, and NADPH-P450 oxidoreductase were kindly provided by Dr. L. B. Von Weymarn (University of Minnesota). N-(2′-Deoxyguanosin-8-yl)-4-aminobiphenyl (dG-C8–4-ABP), and [13C10]-dG-C8–4-ABP, N-(2′-deoxyguanosin-8-yl)-AαC (dG-C8-AαC), [13C10]-dG-C8-AαC, N-(2′-deoxyguanosin-8-yl)-MeIQx (dG-C8-MeIQx), [2H3C]-dG-C8-MeIQx, N-(2′-deoxyguanosin-8-yl)-PhIP (dG-C8-PhIP), and [13C10]-dG-C8-PhIP were synthesized as described (Bessette et al. 2009; Guo et al. 2018). N-(2′-Deoxyguanosin-8-yl)-2-NA (dG-C8–2-NA), 1-(2′-deoxyguanosin-N2-yl)-2-NA (dG-N2-2-NA), and 1-(2′-deoxyadenosin-N6-yl)-2-NA dA-N6-2-NA) were synthesized as reported (Guo et al. 2018).
Cell culture and treatment
RT4 cell (ATCC, Manassas, VA) were cultured in McCoy’s 5A medium (ATCC, Manassas, VA) containing 10% fetal calf serum (Sigma Aldrich, St. Louis, MO, USA), penicillin (100 IU/ml) (Gibco, Life Technologies, Carlsbad, CA, USA), and streptomycin (100 μg/ml) (Gibco, Life Technologies, Carlsbad, CA, USA) at 37 °C in a humidified atmosphere of 5% CO2 in air. For DNA adduct experiments, 1.5 × 106 cells were seeded in 6-well plates. At 80–90% confluence, the cells were washed with pre-warmed phosphate-buffered saline (PBS), and fresh media containing AAs, HAAs, HONH-AAs, or HONH-HAAs (1 μM or 10 μM) or DMSO (0.1% v/v) as a solvent control were added. For CYPs enzyme inhibition experiments, cells were pre-treated with either α-NF, 8-MOP, or DMSO (0.1% v/v) for 1 h, followed by 4-ABP, AαC or DMSO (0.1%) treatment. After 24 h, the cells were washed three times with cold PBS and scraped into 1 ml of cold PBS. After centrifugation at 200 x g for 10 min at 4 °C, the PBS was removed, and the cell pellets were stored at −80 °C until further analysis. For all the cell experiments, no cytotoxicity was observed at the used concentrations of chemicals assayed
DNA isolation and enzymatic digestion
Cell pellets (1.5 – 2 × 106) were homogenized in TE buffer (300 μL, 50 mM Tris-HCl containing 10 mM EDTA, and 10 mM BME, pH 8.0) and DNA was purified with the Gentra Puregene kit (Qiagen, Valencia, CA) (Cai et al. 2017). The DNA was washed with 70%, ethanol and reconstituted in LC/MS grade water. The concentration of DNA was determined using an Agilent 8453 UV/vis spectrophotometer (Agilent Technologies, Santa Clara, CA). Ten μg of DNA were spiked with isotopically labeled internal standards ([13C10]-dG-C8–4-ABP, [13C10]-dG-C8-PhIP, [13C10]-dG-C8-AαC, [2H3C]-dG-C8-MeIQx, each at a level of 1 adducts per 107 nucleotides) and were digested with a cocktail of enzymes in 5 mM Bis-Tris-HCl buffer (pH 7.1) as previously described (Goodenough et al. 2007; Nauwelaers et al. 2011). Isotopically labeled 2-NA DNA adducts were not available, and [13C10]-dG-C8–4-ABP was used as a surrogate internal standard assuming similar ionization efficiencies for all adducts.
Ultraperformance liquid chromatography-electrospray ionization multistage scan mass spectrometry (UPLC-ESI/MS3) of DNA adducts
The DNA adducts were measured by UPLC-ESI/MS3 employing a Dionex Ultimate 3000 LC (Thermo Fisher, San Jose, CA) equipped with a Thermo Acclaim PepMap trap cartridge RP C18 (0.3mm × 5 mm, 5 μm particle size, 100 Å), a Michrom Magic C18 AQ column (0.3 mm×150 mm, 3 μm particle size), and a Michrom Captive Spray source (Auburn, CA) interfaced with a linear ion trap mass spectrometer Velos Pro (Thermo Scientific, San Jose, CA). The chromatography conditions, MS parameters and the MS3 transitions used to monitor the DNA adducts in the positive ionization mode are reported in supporting information (Nauwelaers et al. 2011). External calibration curves were constructed for quantification (Goodenough et al. 2007).
CYP activities
Ethoxyresorufin O-deethylase (EROD) and methoxyresorufin O-demethylase (MROD) activity associated with CYP1 and CYP1A2, respectively (Burke and Mayer 1983) were measured in RT4 cells. Intact cells were incubated in phosphate-buffered saline, pH 7.4, containing 5 μM ethoxyresorufin and 1.5 mM salicyclamide as an inhibitor UGTs and SULTs enzymes. Resorufin formation was monitored by fluorescence employing a Synergy H1 plate reader (Biotek, Winooski, VT) with excitation at 530 nm and emission at 585 nm, over 5 min intervals for 30 minutes of reaction. Coumarin 7-hydroxylation associated with CYP2A activity was measured in RT4 cells cytosols. The reaction mixture consisted of 50 mM Tris buffer, pH 7.4 containing cytosolic proteins (1 mg/ml), NADPH (1 mM) and coumarin (50 μM). After incubation for 30 min at 37 °C, the samples were diluted (1:2) in 0.1 M Tris, pH 9.0, and 7-hydroxycoumarin formation was quantified by fluorimetry using the Synergy H1 plate reader, with an excitation at 355 nm and emission at 460 nm. The reaction rates were linear over time and proportional to protein concentration.
4-ABP and AαC bioactivation by CYP2A6 and CYP2A13 and DNA binding assays
CYP2A6 and CYP2A13 (10 pmol) were reconstituted with NADPH-P450 oxidoreductase (20 pmol) for 5 min on ice, and then mixed with DLPC (2 μg), and incubated on ice for 45 min. Therafter, 50 mM Tris buffer, pH 7.4 containing; 1 mM NADPH and 10 μM of 4-ABP, or AαC were added to attain a final volume of 100 μl and incubated at 37 °C for 15 min. The reactive HONH-4-ABP and HONH-AαC intermediates were trapped with CT DNA (1 mg/ml) in argon-purged 50 mM citric acid buffer (pH 5.0) in a final volume of 200 μl and incubated at 37 °C for 30 min. The acidic pH was used to maximize DNA binding (Tang et al. 2012). The reactions were terminated and DNA was precipitated as discribed (Bellamri et al. 2017). DNA adducts were measured (vide supra).
Statistics
Statistics were performed with Prism 5.03 (GraphPad Software, La Jolla, CA). The statistical significance was determined by the Student’s t-test. The data are presented as the mean ± SD (* P< 0.05; **P < 0.01, ***P < 0.005 versus control). All experiments were conducted at least with three independent experiments on different days, and three different passages of the cell line.
Results
AAs and HAA-DNA adduct formation in RT4 cells
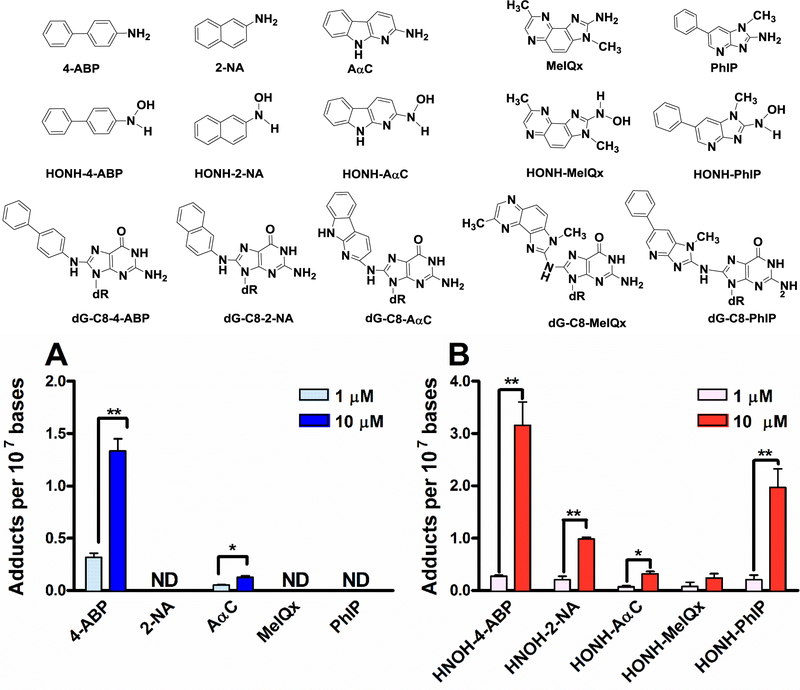
DNA adducts formed in RT4 cells by 4-ABP, 2-NA, PhIP, MeIQx, and AαC, or their N-hydroxy metabolites were measured after 24 h of cell treatment with 1 or 10 μM of each compound. As shown in Fig. 1a, only 4-ABP, and AαC underwent appreciable bioactivation and formed DNA adducts. The levels of both 4-ABP and AαC DNA adducts increased in a concentration-dependent manner; however; the levels of 4-ABP adducts were 10-fold higher. In contrast to the parent compounds, all the N-hydroxy AAs and N-hydroxy HAAs metabolites formed DNA adducts (Fig. 1b). While the levels of DNA adducts formed by, HONH-MeIQx, and HONH-AαC were comparable; those formed by HONH-4-ABP, HONH-2-NA and HONH-PhIP were significantly higher. UPLC-ESI/MS3 chromatograms of DNA adducts and their MS3 product ion spectra are shown in Supporting Information (Supplemental Fig. 1 and 2).
Fig. 1: AAs, HONH-AAs, HAAs, HONH-HAAs DNA adducts formation in RT4 cells.
(a) 4-ABP, 2-NA, PhIP, MeIQx, and AαC, and (b) HONH-4-ABP, HONH-2-NA, HONH-PhIP, HONH-MeIQx, and HONH-AαC. DNA adducts were measured after 24 h of carcinogen treatment by UPLC-ESI/MS3. (Three independent experiments,*P <0.05; **P <0.01, ***P < 0.005). ND: not detected.
4-ABP DNA adduct formation in RT4 cells
We previously identified dG-C8–4-ABP in bladder DNA of BC patients but also detected its proposed isomer, the dG-N2-N4-4-ABP, and the dA-C8–4-ABP adduct (Guo et al. 2018). The extracted ion chromatograms of the dG-N2-N4-4-ABP and dA-C8–4-ABP adducts are shown in Supplemental Fig. 3 with the product ion spectra of the adducts at the MS3 scan stage (Guo et al. 2018). The DNA adduct data of 4-ABP generated in RT4 cells recapitulate our DNA adduct biomarker data in the genome of BC patients and serve to validate the usage of RT4 cells as an in vitro cell culture model for characterization of 4-ABP bioactivation in human bladder.
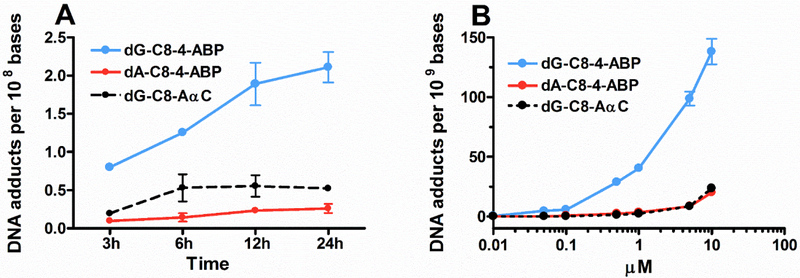
Time and concentration-dependent formation of 4-ABP and AαC adducts in RT4 cells
The kinetics of 4-ABP and AαC DNA adduct formation was characterized in RT4 cells after treatment with 4-ABP (1 μM) or AαC (10 μM). As shown in Fig. 2a, 4-ABP and AαC DNA adducts were formed within 3 h of treatment and reached their maximum levels at 24 h and 6 h respectively. DNA adduct formation was also determined as a function of 4-ABP and AαC concentration (Fig. 2b), showing DNA adduct formation occurred in a concentration-dependent manner.
Figure 2: Kinetic and dose effect formation of 4-ABP and AαC DNA adducts in RT4 cells.
(a) After 3, 6, 12, and 24 h of treatment with 1 μM of 4-ABP or 10 μM AαC DNA adducts were measured by UPLC-ESI/MS3, and (b) After 24 h of treatment with 4-ABP or AαC (10 nM – 10 μM), DNA adducts were measured by UPLC-ESI/MS3. (Three independent experiments,*P <0.05; **P <0.01, ***P <0.005).
Role of CYP1 enzyme in 4-ABP and AαC bioactivation in RT4 cells
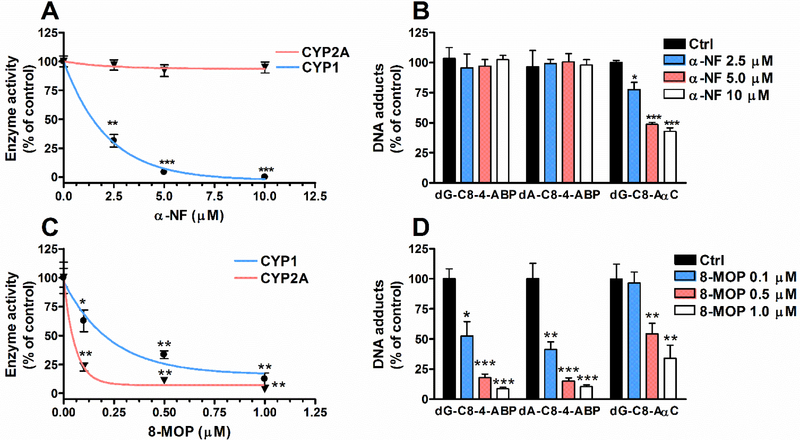
We characterized the activities of CYP1A1, CYP1A2 and CYP1B1 enzymes that are involved in the bioactivation of 4-ABP and AαC in humans (Turesky and Le Marchand 2011). EROD activity, attributed to CYP1A1 and CYP1B1, was detected at an average of 2.46 ± 0.22 pmol/min/mg protein, while CYP1A2 activity was not detected (<0.2 pmol/min/mg protein). α-NF a well-known CYP1 inhibitor (Tassaneeyakul et al. 1993), led to a significant concentration-dependent decrease in EROD activity (Fig. 3a), and a substantial reduction in the levels of AαC adducts. Thus, CYP1A1 and CYP1B1 are involved in the bioactivation of AαC in RT4 cells. In contrast, α-NF did not affect the levels of 4-ABP adducts, signifying that either other CYPs or non-CYP oxidases bioactivate 4-ABP (Fig. 3b).
Fig. 3: α-NF and 8-MOP inhibition of CYP1, CYP2A, and 4-ABP and AαC DNA adducts formation in RT4 cells.
(a) Following pretreatment with α-NF (1 – 10 μM), CYP1 and CYP2A activity, and (b) DNA adducts formed by 4-ABP (1 μM) and AαC (10μM) RT4 cells were measured. (c) Following pretreatment with 8-MOP (0.1 – 1 μM), CYP1 and CYP2A activity, and (d) DNA adducts formed by 4-ABP (1 μM) and AαC (10 μM) in RT4 cells were measured (Three independent experiments,*P <0.05; **P <0.01, ***P <0.005 versus Ctrl).
Role of CYP2 enzyme in 4-ABP and AαC bioactivation in RT4 cells
Nakajima reported that human recombinant CYP2A13, a CYP expressed in human bladder, can bioactivate 4-ABP (Nakajima et al. 2006). The human CYP2A gene subfamily consists of two functional genes, CYP2A6 and CYP2A13 (Fernandez-Salguero et al. 1995) Coumarin was used as a selective substrate for CYP2A isoform activities in RT4 cells. Based on the formation of 7-hydroxycoumarin, our data showed a functional expression of CYP2A activity at levels reaching 5.28 ± 0.5 pmol/min/mg protein. 8-MOP, a potent inhibitor of CYP2A family (Maenpaa et al. 1993), resulted in a complete inhibition of CYP2A activity (Fig. 3c) and a decrease of 4-ABP adducts (Fig. 3d). Thus, the CYP2A family bioactivates 4-ABP. 8-MOP also resulted in a significant decrease of AαC adducts (Fig 3d), which is attributed to the inhibition of CYP1A1 and CYP1B1 activity by 8-MOP (Fig. 3c).
Bioactivation of 4-ABP by human recombinant CYP2A6 and CYP2A13
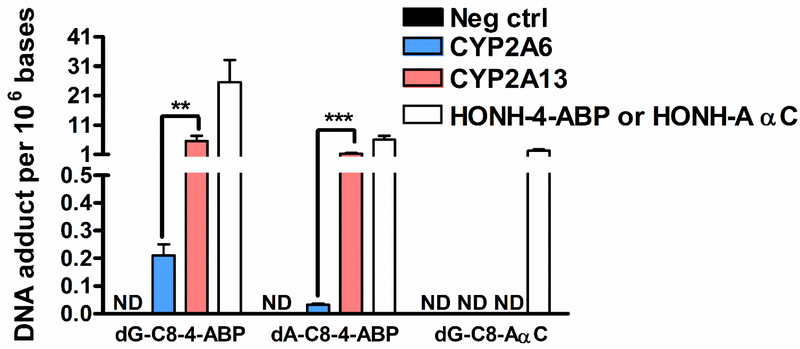
We examined the capacities of human recombinant CYP2A6 and CYP2A13 to catalyze 4-ABP and AαC bioactivation. As shown in Fig. 4, the rate of CYP2A13 catalyzed bioactivation of 4-ABP, as measured by 4-ABP adducts, was significantly higher than for CYP2A6. In contrast, AαC did not undergo bioactivation by either CYP2A6 and CYP2A13.
Fig. 4: 4-ABP and AαC bioactivation by CYP2A6 and CYP2A13.
4-ABP and AαC (10 μM) were incubated with human recombinant CYP2A6 and CYP2A13 for 30 min at 37 °C. The formed HONH-4-ABP and HONH-AαC or the synthetic HONH-4-ABP and HONH-AαC were then incubated with CT-DNA at pH 5, for 30 min. 4-ABP and AαC DNA adducts were measured by UPLC-ESI/MS3. (Three independent experiments,*P <0.05; **P <0.01, ***P <0.005). ND: not detected.
Discussion
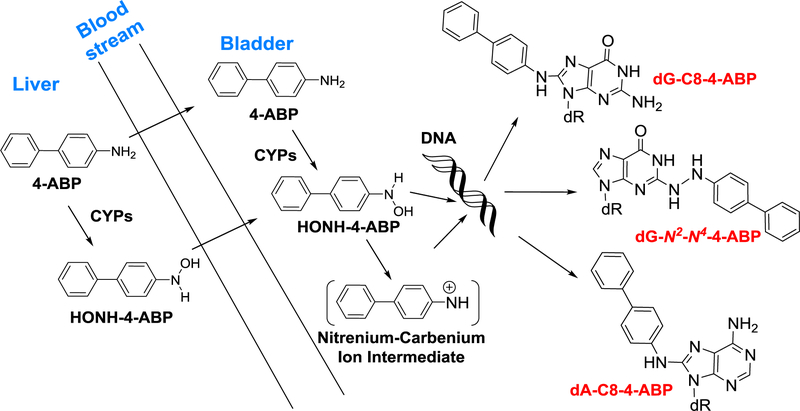
4-ABP arises in tobacco smoke at several ng per cigarette (Hoffmann et al. 2001). In a commentary, Poirier and Beland suggested that these low levels of 4-ABP may be insufficient to contribute to BC etiology significantly (Poirier and Beland 1997). Nevertheless, we detected 4-ABP DNA adducts in the bladder genome of some BC patients by high-resolution mass spectrometry (Guo et al. 2018). An earlier study employing triple quadrupole mass spectrometry also reported the occurrence of 4-ABP DNA adducts in the bladder of some BC patients (Zayas et al. 2007). These biomarker data provide support for the paradigm of 4-ABP as an etiological agent in human BC. However, there are many other carcinogenic AAs and HAAs excreted in the urine of smokers and omnivores (DeMarini 2004; Peluso et al. 1991; Peters et al. 2003; Smith et al. 1996; Turesky and Le Marchand 2011). A portion of these compounds may undergo bioactivation by CYPs expressed in the liver or bladder epithelium to form genotoxicants capable of forming DNA adducts which contribute to BC etiology (Scheme 1).
Scheme 1: Bioactivation of 4-ABP leading to DNA adduct formation in bladder.
We characterized the capacity of human RT4 bladder cells to bioactivate 4-ABP, 2-NA, and the prototypical HAAs, PhIP, MeIQx, and AαC, by the measurement of their DNA adducts. Among all, only 4-ABP and AαC underwent bioactivation in RT4 cells to form DNA adducts. All of the N-hydroxylated AAs and HAAs formed DNA adducts in RT4 cells, signifying that CYPs expressed in RT4 cells do not mediate the N-oxidation of 2-NA, PhIP, and MeIQx.
The N-oxidation of many AAs and HAAs is efficiently catalyzed by CYP1A2 expressed in the liver but also by CYP1A1 and CYP1B1 in extra-hepatic tissues (Turesky and Le Marchand 2011). CYP1A1 and CYP1B1 activities, but not CYP1A2 activity, were detected in RT4 cells. These data are in agreement with previous reports on CYP1A1 and CYP1B1 protein (Plottner et al. 2016), and enzyme activity (Baker et al. 2018; Reshetnikova et al. 2016) in RT4 cells. The complete inhibition of CYP1A1 and CYP1B1 by α-NF resulted in a 50% decrease in the levels of AαC DNA adducts in RT4 cells, signifying that these isoforms prominently carry out the bioactivation of AαC in RT4 cells. Previous studies also reported AαC bioactivation by CYP1A1, CYP1B1, and CYP1A2 (Bellamri et al. 2016; Raza et al. 1996). Other CYPs or non-CYP oxidases, such as peroxidases, are likely to contribute to bioactivation of AαC in RT4 cells (Danon et al. 1986; Turesky et al. 2015). In contrast, α-NF did not diminish the levels of 4-ABP adducts, signifying that other CYPs or phase I enzymes are involved in the bioactivation of 4-ABP in RT4 cells.
There are two functional genes in the human CYP2A enzyme family (Fernandez-Salguero et al. 1995). CYP2A6 prominently expressed in the liver (Yun et al. 1991) and CYP2A13 expressed in extrahepatic tissues including the lung and bladder (Nakajima et al. 2006; Su et al. 2000). CYP2A6 and CYP2A13 mRNA transcripts have been detected in RT4 cells (Kanemoto et al. 2016). We showed that RT4 cells express CYP2A by the 7-hydroxylation of the coumarin, a selective substrate for CYP2A (Pelkonen et al. 2000; Von Weymarn and Murphy 2003). 8-MOP, a potent inhibitor of CYP2A (Maenpaa et al. 1993), resulted in complete inhibition of CYP2A activity correlated to a decrease in the level of 4-ABP adducts. Thus, the CYP2A family enzymes appear to catalyze the bioactivation of 4-ABP in RT4 cells prominently. However, it is possible that CYP isoform(s) other than CYP2A13, which can be inhibited by 8-MOP, may also contribute to ABP N-oxidation in RT4 cells. 1-Hydroxypyrene and 1-acetylpyrene are selective and potent inhibitors of bacterial membrane expressed CYP2A6 and CYP2A13, respectively (Shimada et al. 2013). However, the pretreatment of RT4 cells with these inhibitors did not diminish CYP2A activity nor 4-ABP DNA adduct formation (data not shown), perhaps due to their metabolism in RT4 cells. Therefore, human recombinant CYP2A6 and CYP2A13 enzymes were employed to assess their potential roles in the bioactivation of 4-APB. Both isoforms catalyzed 4-ABP DNA adduct formation. However, CYP2A13-catalyzed bioactivation of 4-ABP was 20-fold higher than the level catalyzed by CYP2A6. These findings are consistent with the previous report on the capacity of CYP2A13 to N-oxidize 4-ABP (Nakajima et al. 2006), and show for the first time the ability of CYP2A6 to bioactivate 4-ABP.
The relative contributions of CYP enzymes expressed in the liver versus bladder to induce DNA damage in the bladder by environmental carcinogens requires further study (Radomski and Brill 1970) (Scheme 1). Although AαC occurs at up 100-fold higher levels than 4-ABP in tobacco smoke (Hoffmann et al. 2001), AαC DNA adducts were below the limit of quantification (3 adducts per 109 bases) (Guo et al. 2018). AαC is far more efficiently bioactivated by CYP1A2 expressed in primary human hepatocytes than in bladder cells (Nauwelaers et al. 2011); however, the ability of the reactive HONH-AαC metabolite to reach the bladder through the blood stream is not known
2-NA is classified as a human bladder carcinogen (IARC. 2004). However, 2-NA did not undergo bioactivation by RT4 cells (Fig. 2a), and we did not detect DNA adducts of 2-NA in the bladder DNA of BC patients (Guo et al. 2018). Our study with RT4 cells suggests that CYP activity expressed in bladder cells may be a critical factor in the bioactivation, and induction of bladder DNA damage by aromatic amine-carcinogens.
In summary, our findings show that CYP2A13 expressed in the bladder efficiently bioactivates 4-ABP to form DNA adducts. These data are consistent with human data showing the occurrence of 4-ABP DNA adducts, but not DNA adducts of 2-NA or HAAs, in the bladder. Further studies are required to better characterize other Phase I and II enzymes involved in the bioactivation of 4-ABP, other AAs, and HAAs in human bladder. The employment of biomarkers to assess recent exposures to tobacco smoke and cooked meat in conjunction with DNA adduct screening of the bladder is required to identify AAs, HAAs, and other environmental genotoxicants that may contribute to DNA damage and the risk of BC.
Supplementary Material
Funding
This work is supported by R01ES019564 and R01ES030559 (R.J.T.) from the National Institute of Environmental Health Sciences and by R01CA220367 (R.J.T.) from the National Cancer Institute, National Institutes of Health. Mass spectrometry was carried out in the Analytical Biochemistry Share Resources of the Masonic Cancer Center, the University of Minnesota, funded in part by Cancer Center Support Grant CA-077598.
Footnotes
Conflict of interest:
The authors declare that they have no conflict of interest
References
- Baker SC, Arlt VM, Indra R, et al. (2018) Differentiation-associated urothelial cytochrome P450 oxidoreductase predicates the xenobiotic-metabolizing activity of “luminal” muscle-invasive bladder cancers. Mol Carcinog 57(5):606–618 doi: 10.1002/mc.22784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi JC, Larrinaga MT, De Stefani E, et al. (2001) Foods and risk of bladder cancer: a case-control study in Uruguay. Eur J Cancer Prev 10(5):453–8 [DOI] [PubMed] [Google Scholar]
- Bellamri M, Le Hegarat L, Turesky RJ, Langouet S (2017) Metabolism of the Tobacco Carcinogen 2-Amino-9H-pyrido[2,3-b]indole (AalphaC) in Primary Human Hepatocytes. Chem Res Toxicol 30(2):657–668 doi: 10.1021/acs.chemrestox.6b00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamri M, Le Hegarat L, Vernhet L, Baffet G, Turesky RJ, Langouet S (2016) Human T lymphocytes bioactivate heterocyclic aromatic amines by forming DNA adducts. Environ Mol Mutagen 57(9):656–667 doi: 10.1002/em.22059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessette EE, Goodenough AK, Langouet S, et al. (2009) Screening for DNA adducts by data-dependent constant neutral loss-triple stage mass spectrometry with a linear quadrupole ion trap mass spectrometer. Anal Chem 81(2):809–19 doi: 10.1021/ac802096p [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger M, Catto JW, Dalbagni G, et al. (2013) Epidemiology and risk factors of urothelial bladder cancer. Eur Urol 63(2):234–41 doi: 10.1016/j.eururo.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Burke MD, Mayer RT (1983) Differential effects of phenobarbitone and 3-methylcholanthrene induction on the hepatic microsomal metabolism and cytochrome P-450-binding of phenoxazone and a homologous series of its n-alkyl ethers (alkoxyresorufins). Chem Biol Interact 45(2):243–58 [DOI] [PubMed] [Google Scholar]
- Cai T, Bellamri M, Ming X, Koh WP, Yu MC, Turesky RJ (2017) Quantification of Hemoglobin and White Blood Cell DNA Adducts of the Tobacco Carcinogens 2-Amino-9H-pyrido[2,3-b]indole and 4-Aminobiphenyl Formed in Humans by Nanoflow Liquid Chromatography/Ion Trap Multistage Mass Spectrometry. Chem Res Toxicol 30(6):1333–1343 doi: 10.1021/acs.chemrestox.7b00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Zenser TV, Thomasson DL, Davis BB (1986) Eicosanoid synthesis by cultured human urothelial cells: potential role in bladder cancer. Cancer Res 46(11):5676–81 [PubMed] [Google Scholar]
- DeMarini DM (2004) Genotoxicity of tobacco smoke and tobacco smoke condensate: a review. Mutat Res 567(2–3):447–74 doi: 10.1016/j.mrrev.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Hoffman SM, Cholerton S, et al. (1995) A genetic polymorphism in coumarin 7-hydroxylation: sequence of the human CYP2A genes and identification of variant CYP2A6 alleles. Am J Hum Genet 57(3):651–60 [PMC free article] [PubMed] [Google Scholar]
- Ferrucci LM, Sinha R, Ward MH, et al. (2010) Meat and components of meat and the risk of bladder cancer in the NIH-AARP Diet and Health Study. Cancer 116(18):4345–53 doi: 10.1002/cncr.25463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Zhao G, Wang S, et al. (2014) Simultaneous determination of fifteen heterocyclic aromatic amines in the urine of smokers and nonsmokers using ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1333:45–53 doi: 10.1016/j.chroma.2014.01.057 [DOI] [PubMed] [Google Scholar]
- Goodenough AK, Schut HA, Turesky RJ (2007) Novel LC-ESI/MS/MS(n) method for the characterization and quantification of 2’-deoxyguanosine adducts of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by 2-D linear quadrupole ion trap mass spectrometry. Chem Res Toxicol 20(2):263–76 doi: 10.1021/tx0601713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Villalta PW, Weight CJ, et al. (2018) Targeted and Untargeted Detection of DNA Adducts of Aromatic Amine Carcinogens in Human Bladder by Ultra-Performance Liquid Chromatography-High-Resolution Mass Spectrometry. Chem Res Toxicol 31(12):1382–1397 doi: 10.1021/acs.chemrestox.8b00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I, El-Bayoumy K (2001) The less harmful cigarette: a controversial issue. a tribute to Ernst L. Wynder. Chem Res Toxicol 14(7):767–90 doi: 10.1021/tx000260u [DOI] [PubMed] [Google Scholar]
- IARC. (2004) Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 83:1–1438 [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90 doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- Kaderlik KR, Minchin RF, Mulder GJ, et al. (1994) Metabolic activation pathway for the formation of DNA adducts of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat extrahepatic tissues. Carcinogenesis 15(8):1703–9 [DOI] [PubMed] [Google Scholar]
- Kanemoto K, Fukuta K, Kawai N, et al. (2016) Genomic Landscape of Experimental Bladder Cancer in Rodents and Its Application to Human Bladder Cancer: Gene Amplification and Potential Overexpression of Cyp2a5/CYP2A6 Are Associated with the Invasive Phenotype. PLoS One 11(11):e0167374 doi: 10.1371/journal.pone.0167374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Forman MR, Wang J, et al. (2012) Intake of red meat and heterocyclic amines, metabolic pathway genes and bladder cancer risk. Int J Cancer 131(8):1892–903 doi: 10.1002/ijc.27437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbreras B, Garte S, Overvad K, et al. (2008) Meat intake and bladder cancer in a prospective study: a role for heterocyclic aromatic amines? Cancer Causes Control 19(6):649–56 doi: 10.1007/s10552-008-9121-1 [DOI] [PubMed] [Google Scholar]
- Maenpaa J, Sigusch H, Raunio H, et al. (1993) Differential inhibition of coumarin 7-hydroxylase activity in mouse and human liver microsomes. Biochem Pharmacol 45(5):1035–42 doi: 10.1007/BF03190960. [DOI] [PubMed] [Google Scholar]
- Michaud DS, Holick CN, Giovannucci E, Stampfer MJ (2006) Meat intake and bladder cancer risk in 2 prospective cohort studies. Am J Clin Nutr 84(5):1177–83 doi: 10.1093/ajcn/84.5.1177 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Itoh M, Sakai H, et al. (2006) CYP2A13 expressed in human bladder metabolically activates 4-aminobiphenyl. Int J Cancer 119(11):2520–6 doi: 10.1002/ijc.22136 [DOI] [PubMed] [Google Scholar]
- Nauwelaers G, Bessette EE, Gu D, et al. (2011) DNA adduct formation of 4-aminobiphenyl and heterocyclic aromatic amines in human hepatocytes. Chem Res Toxicol 24(6):913–25 doi: 10.1021/tx200091y [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole CM, Povey S, Hepburn P, Franks LM (1983) Identity of some human bladder cancer cell lines. Nature 301(5899):429–30 [DOI] [PubMed] [Google Scholar]
- Pathak KV, Chiu TL, Amin EA, Turesky RJ (2016) Methemoglobin Formation and Characterization of Hemoglobin Adducts of Carcinogenic Aromatic Amines and Heterocyclic Aromatic Amines. Chem Res Toxicol 29(3):255–69 doi: 10.1021/acs.chemrestox.5b00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen O, Rautio A, Raunio H, Pasanen M (2000) CYP2A6: a human coumarin 7-hydroxylase. Toxicology 144(1–3):139–47 [DOI] [PubMed] [Google Scholar]
- Peluso M, Castegnaro M, Malaveille C, et al. (1991) 32P Postlabelling analysis of urinary mutagens from smokers of black tobacco implicates 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) as a major DNA-damaging agent. Carcinogenesis 12(4):713–7 [DOI] [PubMed] [Google Scholar]
- Peters U, DeMarini DM, Sinha R, et al. (2003) Urinary mutagenicity and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 12(11 Pt 1):1253–6 [PubMed] [Google Scholar]
- Plottner S, Bastian LA, Kafferlein HU, Bruning T (2016) Effects of benzo[a]pyrene, aromatic amines, and a combination of both on CYP1A1 activities in RT-4 human bladder papilloma cells. J Toxicol Environ Health A 79(22–23):1106–1117 doi: 10.1080/15287394.2016.1219598 [DOI] [PubMed] [Google Scholar]
- Poirier MC, Beland FA (1997) Aromatic amine DNA adduct formation in chronically-exposed mice: considerations for human comparison. Mutat Res 376(1–2):177–84 [DOI] [PubMed] [Google Scholar]
- Radomski JL, Brill E (1970) Bladder cancer induction by aromatic amines: role of N-hydroxy metabolites. Science 167(3920):992–3 [DOI] [PubMed] [Google Scholar]
- Raza H, King RS, Squires RB, et al. (1996) Metabolism of 2-amino-alpha-carboline. A food-borne heterocyclic amine mutagen and carcinogen by human and rodent liver microsomes and by human cytochrome P4501A2. Drug Metab Dispos 24(4):395–400 [PubMed] [Google Scholar]
- Reshetnikova G, Sidorenko VS, Whyard T, et al. (2016) Genotoxic and cytotoxic effects of the environmental pollutant 3-nitrobenzanthrone on bladder cancer cells. Exp Cell Res 349(1):101–108 doi: 10.1016/j.yexcr.2016.10.003 [DOI] [PubMed] [Google Scholar]
- Riedel K, Scherer G, Engl J, Hagedorn HW, Tricker AR (2006) Determination of three carcinogenic aromatic amines in urine of smokers and nonsmokers. J Anal Toxicol 30(3):187–95 [DOI] [PubMed] [Google Scholar]
- Shimada T, Kim D, Murayama N, et al. (2013) Binding of diverse environmental chemicals with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition. Chem Res Toxicol 26(4):517–28 doi: 10.1021/tx300492j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67(1):7–30 doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- Smith CJ, McKarns SC, Davis RA, et al. (1996) Human urine mutagenicity study comparing cigarettes which burn or primarily heat tobacco. Mutat Res 361(1):1–9 [DOI] [PubMed] [Google Scholar]
- Su T, Bao Z, Zhang QY, Smith TJ, Hong JY, Ding X (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res 60(18):5074–9 [PubMed] [Google Scholar]
- Sugimura T, Wakabayashi K, Nakagama H, Nagao M (2004) Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci 95(4):290–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, LeMaster DM, Nauwelaers G, Gu D, Langouet S, Turesky RJ (2012) UDP-glucuronosyltransferase-mediated metabolic activation of the tobacco carcinogen 2-amino-9H-pyrido[2,3-b]indole. J Biol Chem 287(18):14960–72 doi: 10.1074/jbc.M111.320093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassaneeyakul W, Birkett DJ, Veronese ME, et al. (1993) Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther 265(1):401–7 [PubMed] [Google Scholar]
- Turesky RJ, Konorev D, Fan X, et al. (2015) Effect of cytochrome P450 reductase deficiency on 2-amino-9H-pyrido[2,3-b]indole metabolism and DNA adduct formation in liver and extrahepatic tissues of mice. Chem Res Toxicol 28(12):2400–10 doi: 10.1021/acs.chemrestox.5b00405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ, Le Marchand L (2011) Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol 24(8):1169–214 doi: 10.1021/tx200135s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky RJ, Yuan JM, Wang R, Peterson S, Yu MC (2007) Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol Biomarkers Prev 16(8):1554–60 doi: 10.1158/1055-9965.EPI-07-0132 [DOI] [PubMed] [Google Scholar]
- Von Weymarn LB, Murphy SE (2003) CYP2A13-catalysed coumarin metabolism: comparison with CYP2A5 and CYP2A6. Xenobiotica 33(1):73–81 doi:Doi 10.1080/0049825021000022302 [DOI] [PubMed] [Google Scholar]
- Yun CH, Shimada T, Guengerich FP (1991) Purification and characterization of human liver microsomal cytochrome P-450 2A6. Mol Pharmacol 40(5):679–85 [PubMed] [Google Scholar]
- Zayas B, Stillwell SW, Wishnok JS, et al. (2007) Detection and quantification of 4-ABP adducts in DNA from bladder cancer patients. Carcinogenesis 28(2):342–9 doi: 10.1093/carcin/bgl142 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.