Abstract
Rotator cuff tears can be associated with significant shoulder dysfunction and pain. Despite improved surgical techniques and new materials for rotator cuff reconstruction, there is no significant reduction in the re-rupture rate. Innovative approaches for enhanced tendon healing are required. The potential of biologically optimized tendon integration has probably been insufficiently explored so far. The existing practice of debridement might eliminate repair tissue and a major source of cells and blood vessels necessary for tendon healing. Biological augmentation may be an option to improve the healing process. The subacromial bursa is a highly proliferative tissue with mesenchymal stem cells capable of differentiating into various cell lines and is easily accessible during rotator cuff repair. We describe the technique of bursal augmentation in arthroscopic double-row SutureBridge repair of a posterosuperior rotator cuff tear with the aim of improving tendon-to-bone healing.
Rotator cuff tears are one of the most common musculoskeletal diseases, with an incidence rate of 30% in the older population.1 The socioeconomic impact is high, with over 300,000 surgical repairs of rotator cuff tears per year in the United States.2
Although good clinical outcomes have been shown, in the past few decades, the rate of re-ruptures after surgical repair has not decreased and is reported to be around 40% for large and massive tears.3, 4 This rate has not dropped with the introduction of arthroscopic repairs, and reviews have described even higher rates of re-rupture compared with open repairs.5
When we look at the current literature, the role of the subacromial bursa regarding the supportive influence on tendon healing of the rotator cuff has been discussed controversially. On the one hand, the bursa is attributed a high regeneration potential.6, 7 On the other hand, the frequently associated inflammation of the bursa alone is considered the primary pain generator of the shoulder.8, 9 The different cytokine expression patterns in the bursal tissue, in particular the proinflammatory cytokines, in rotator cuff ruptures compared with shoulder instability, impingement syndrome, or calcifying tendinosis suggest a connection with the underlying disease. The answer to the clinically relevant question of whether the result of an operation can be optimized by a bursectomy or, rather, the use of the bursa as a biological augmentation is currently unknown. Recent analyses of various shoulder tissues showed subacromial bursal tissue to be an important reservoir of mesenchymal stem cells (MSCs) that may contribute to the healing process at injured sites, such as the rotator cuff tendons.10, 11
The purpose of this article was to present the operative technique of bursal augmentation in arthroscopic rotator cuff repair of posterosuperior (supraspinatus with or without infraspinatus) ruptures. This technique includes a double-row SutureBridge repair (Arthrex, Naples, FL) followed by an additional bursal adaptation via PDS suture (polydioxanone monofilament thread; Ethicon, Somerville, NJ) on the reconstructed tendon for enhancing vascularization and healing of the repair (Video 1).
Surgical Technique
Preoperative Planning
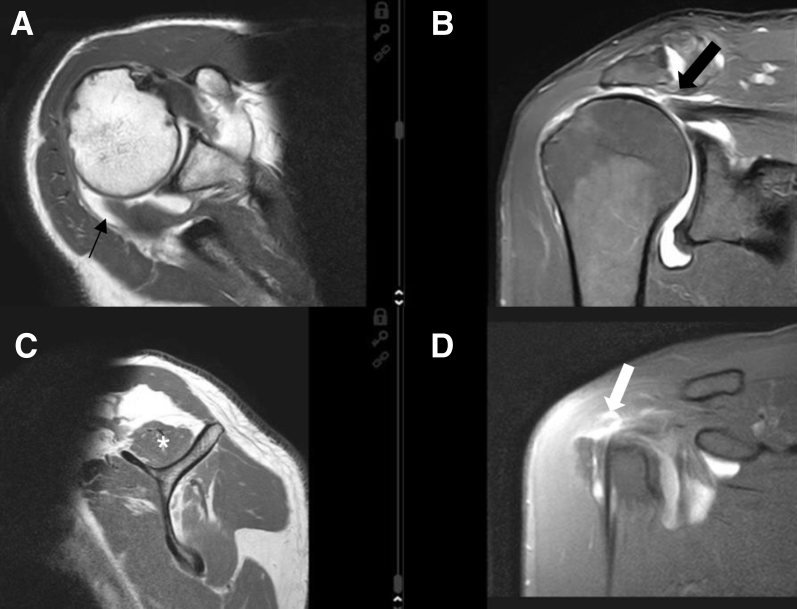
In addition to obtaining a careful patient history, performing a functional assessment, and performing a complete clinical examination to determine active and passive range of motion (ROM), strength of the single rotator cuff components, functional demand, and possible concomitant pathologies, the surgeon is required to perform a detailed radiologic assessment. Magnetic resonance imaging (Fig 1) is conducted to evaluate atrophy of the supraspinatus according to Thomazeau et al.12; tendon retraction according to Patte13; and fatty infiltration of the supraspinatus and infraspinatus according to Goutallier et al.14 and Fuchs et al.15
Fig 1.
Preoperative magnetic resonance imaging views of a right shoulder showing, in the coronal view (B), a large posterosuperior rotator cuff tear with grade II supraspinatus tendon retraction according to Patte13 (arrow) and, in the parasagittal view (C), grade II supraspinatus muscular atrophy according to Thomazeau et al.12 (asterisk). (A) In the axial view, the infraspinatus tendon displays a retracted full-thickness tear (arrow). (C) The muscle shows signs of atrophy and grade II fatty infiltration according to Goutallier et al.14 in the parasagittal view. (D) In the anterior coronal sequence, the supraspinatus tear is expanding ventrally into the interval tissue (arrow).
Patient Positioning and Diagnostic Arthroscopy
After administration of perioperative antibiotics and while under general anesthesia, the patient is placed in the beach-chair position. The affected arm is fixed in a hydraulic flexible arm holder.
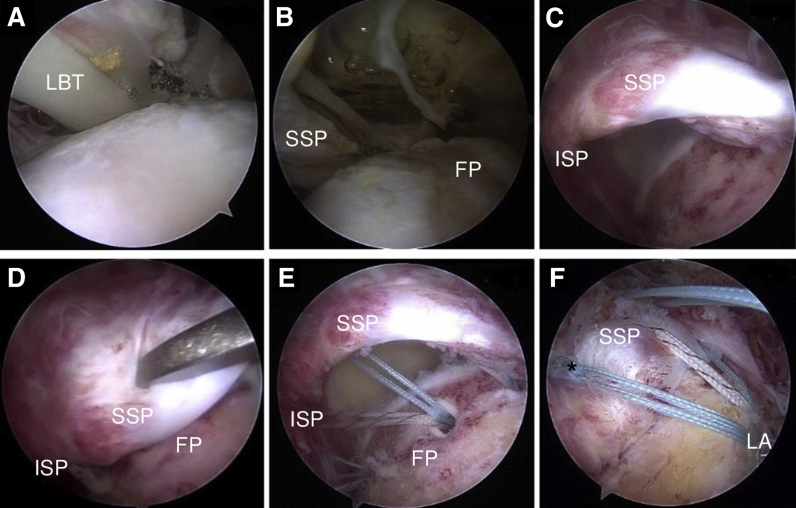
A diagnostic arthroscopy is performed through the standard posterior portal to identify the primary structural defects, as well as concomitant lesions (Fig 2 A and B). If the biceps tendon shows pathology and/or instability, a tenotomy or tenodesis is performed. For a suprapectoral arthroscopic anchor tenodesis, anteroinferior and anterolateral portals are used. Alternatively, a subpectoral mini-open tenodesis can be performed, depending on the surgeon's preference.
Fig 2.
Arthroscopy in a right shoulder with the patient in the beach-chair position. The interval region is visualized with the scope in the posterior portal, showing a torn lateral pulley due to the complete supraspinatus (SSP) lesion expanding into the interval tissue (A) and an uncovered SSP footprint (FP) area (B). (C) With the scope in the anterolateral portal, the posterior extension of the retracted full-thickness tear in the posterior SSP portion and infraspinatus (ISP) can be seen. (D-F) Arthroscopic views from lateral portal. (D) After mobilization, the tendon can be reduced with a grasper in the footprint area. In this case, a biceps (long head of the biceps tendon [LBT]) tenodesis was performed. The footprint is decorticated and medialized 0.5 mm with a bone-cutting device, the medial-row anchors (BioComposite Corkscrew FT Suture Anchors, 5.5 mm × 14.7 mm, with 4 No. 2 FiberWire sutures) are placed at the bone-cartilage junction (E), and sutures are shuttled through the tendon by a mattress technique. (F) The sutures (asterisk) are tied and, under slight tension, inserted in the lateral row of anchors (LA) (BioComposite SwiveLock SP, 4.75 mm × 24.5 mm).
Assessment of Rupture Dimension and Tendon Mobility
A lateral portal is used for visualization of the rupture (Fig 2C). Cannulas are used for suture management. Depending on the dimension and localization of the rupture, various additional lateral and posterolateral portals are used to facilitate instrumentation and suture management. After debridement of the rotator cuff margin, the mobility of the rotator cuff is assessed with a grasper (Fig 2D). Bursal resection on the tendon surface is limited only to visualize of the free margins of the rupture. The medial-to-lateral and anterior-to-posterior mobility of the tear margins is evaluated and the tear pattern classified (e.g. crescent shaped, U shaped, or L shaped). Mobilization and release of the tendon are performed. If the tear can be reduced to the lateral aspect of the bone bed, a double-row repair can be performed. In general, most small to large crescent-, U-, and L-shaped tears have sufficient mobility to allow a double-row repair. However, depending on the mobility of the tear margins, massive tears can also be repaired using a double-row technique.
Footprint Preparation, Medial Anchor Placement, and Suture Passage
The bone bed of the footprint is prepared with an arthroscopic bone shaver to decorticate the greater tuberosity and to create bleeding. Medialization of the footprint by 5 to 7 mm can be performed without changing the biomechanics of the repair (Fig 2C). Then, the medial-row anchors (BioComposite Corkscrew FT Suture Anchors, 5.5 mm × 14.7 mm, with 4 No. 2 FiberWire sutures; Arthrex) are placed just lateral to the articular surface of the humeral head (Fig 2E). Depending on the size, 1 to 3 anchors are used (for the rupture illustrated, we used 3 anchors [2 for the infraspinatus and 1 for the supraspinatus] for the medial row). We commonly use a bone punch for preparation, followed by a double-loaded anchor. By use of a suture passer, the tendon is perforated with a double-mattress technique, which has shown high primary fixation strength16 (Fig 2E). In the case of suprapectoral biceps tenodesis and supraspinatus tear expansion into the interval, a FiberWire is used from the tenodesis anchor for passage in the anterior portion of the supraspinatus.
Knot Tying, Lateral-Row Placement, and Microfracturing of Insertion Site
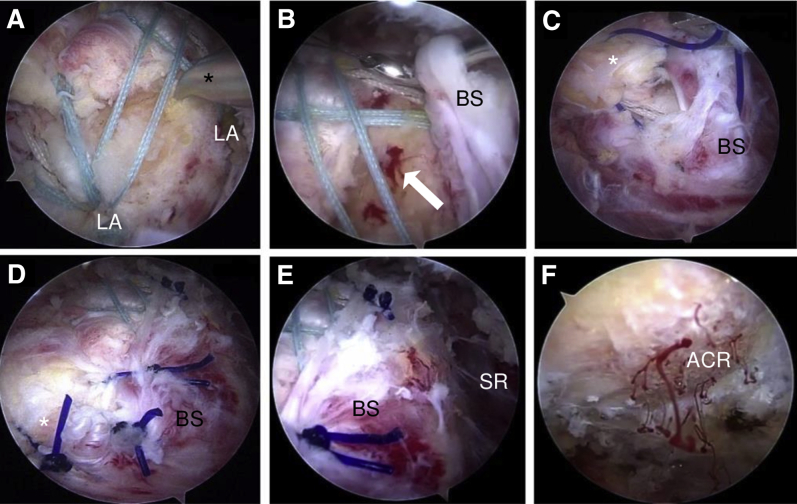
The medial mattress sutures are knotted (Fig 2F) from posterior to anterior using a knot pusher. Subsequently, the suture tails are tensioned and fixated laterally by a SutureBridge technique using 2 knotless anchors (BioComposite SwiveLock SP, 4.75 mm × 24.5 mm; Arthrex) (Fig 3A). During this procedure, visualization of the lateral aspect of the greater tuberosity can be enhanced by using a changing rod introduced from the anteroinferior or posterior portal and pressing it laterally to widen the subdeltoid recess. The arthroscopic awl is inserted to microfracture the insertion side to allow bone marrow stem cells to flow in (Fig 3 A and B).
Fig 3.
Arthroscopic visualization from the posterolateral portal in a right shoulder with the patient in the beach-chair position. (A) After the sutures have been tensioned into the laterally placed anchors (LA) (BioComposite SwiveLock SP, 4.75 mm × 24.5 mm) with a SutureBridge conformation, the awl (asterisk) is introduced from the lateral portal to microfracture the insertion bed. (B) Blood flow out of the microfractured areas (arrow) can be observed. The parietal sheet of the subacromial bursa (BS) in the subdeltoid region (SR) is perforated with a lasso introduced from the lateral portal, and a No. 2 PDS suture (polydioxanone monofilament thread) is shuttled through. (C) The same procedure is performed with the tendon tissue (asterisk) using a lasso from the anterolateral or anteroinferior portal to shuttle the same PDS wire. Tensioning both ends of the PDS wire, the bursal tissue is converging on the tendon, covering the tendon margin and the insertion area. (D, E) The procedure is repeated until the whole insertion area is covered with highly vascularized bursal tissue. (F) An acromioplasty provides additional blood support descending on the tendon insertion area. (ACR, acromion.)
Bursa Augmentation and Acromioplasty
The parietal sheet of the subacromial bursa is mobilized from the lateral subdeltoid region. This additional bursal tissue shows no signs of inflammation and is now sutured onto the tendon in a side-to-side manner. For this procedure, a suture-lasso device is introduced into each margin, and 1 No. 2 PDS suture (polydioxanone monofilament thread) is shuttled into both free margins (Fig 3 B and C). Subsequently, the PDS suture is tied using a knot pusher. This procedure is repeated further posterior in the same manner. The tendon footprint is now covered completely with a well-vascularized bursal layer (Fig 3 D and E). Soft tissue is removed from the acromion surface using an arthroscopic shaver, and an acromioplasty is performed using an acromionizer for sufficient extension of the subacromial space (Fig 3F).
Postoperative Rehabilitation
The postoperative rehabilitation protocol follows general recommendations for arthroscopic posterosuperior rotator cuff repair. The shoulder is immobilized in a shoulder orthosis in 30° of abduction and 15° of external rotation for 6 weeks. During this period, guided passive mobilization exercises should be performed. In the first 3 weeks, the limits are 60° of flexion and abduction with external and internal rotation of 30°. In weeks 4 to 6, active-assisted flexion and abduction up to 90°, as well as external and internal rotation up to 60°, are allowed. If the infraspinatus is involved, no active external rotation is allowed in the first 6 weeks and a maximum of 60° of internal rotation is possible.
Active ROM exercises are initiated 6 weeks after the operation with no limits for external and internal rotation. Muscle strengthening is commenced 12 weeks after surgery. A return to shoulder-demanding work or sports is usually possible after 6 months, depending on a patient-specific assessment.
If a tenodesis of the long head of the biceps is performed, no active flexion or supination of the elbow is allowed. After 4 weeks, active ROM of the elbow is permitted, but flexion or supination against resistance is forbidden for another 8 weeks.
Discussion
The described technique introduces the arthroscopic sewing of the mobilized parietal bursal layer onto the reinserted tendon. There is no specific technical risk in using a well-established lasso-loop shuttling technique for inserting the PDS thread in the bursal and tendon tissue. The bursal tissue is fragile, and it is mandatory to load enough tissue while pinching with the lasso loop (Fig 3B) to not pull out when using the shuttling device or while tying the knot. A potential postoperative risk is the formation of adhesions in the subdeltoid recess with possible stiffness of the glenohumeral joint. Tables 1 and 2 show pearls and pitfalls, as well as advantages and disadvantages.
Table 1.
Pearls and Pitfalls
| Pearls |
| Directly available bursal tissue |
| Technique easily performed through arthroscopic portals already installed for tendon reconstruction |
| No special devices needed |
| Pitfalls |
| Pullout of lasso-loop device or PDS suture (polydioxanone monofilament thread) due to fragile bursal tissue |
| Visualization problems because of bleeding of highly vascularized bursal tissue |
| Monofilament PDS suture knots must be properly placed to be secure |
Table 2.
Advantages and Disadvantages
| Advantages |
| Autologous tissue |
| No additional donor site |
| Economical |
| Promising proliferation and differentiation potential of bursal tissue |
| Disadvantages |
| Unknown effect of bursal inflammatory mediators |
| Longer operation time |
| Possible higher risk of subdeltoid adhesions causing glenohumeral stiffness |
The hypothesis of the healing potential of the subacromial bursa for rotator cuff repair has long been known, and a lower success rate for surgical techniques resecting the subacromial bursa radically has been described.6, 7 In contrast, subacromial bursal tissue has been considered a pain-generating tissue regarded as a major pathologic factor for shoulder discomfort, so this tissue is routinely removed during surgery. Several studies have underlined the importance of subacromial bursectomy to reduce inflammation in rotator cuff disease.8, 9
Yet, whether inflammation has a positive or negative effect on tendon healing has been discussed controversially. More information is required to understand the role of inflammatory and mediator cells in the healing process. Soon after surgical repair, it is likely that signals produced by inflammatory cells play an important role in the initiation and regulation of the healing process.17
A major effort has been put into understanding and finding strategies to enhance tendon healing. Several grafts are available for mechanical augmentation of a rotator cuff reconstruction. Synthetic grafts made of polyurethane, polytetrafluoroethylene, or nondegradable polymers are not biocompatible, providing no integration into the tendon tissue. Biological grafts (e.g. human dermis) or xenografts include the risk of rejection reactions. For xenografts, no therapeutic benefit was found.18 Growth factors have shown promising results in vivo and in animal models, but clinical evidence is yet to be determined.19, 20 Despite promising in vitro results, controlled clinical trials have shown controversial results for the use of platelet-rich-plasma.21
Another focus of research is the use of stem cells. MSCs are multipotent stromal elements able to differentiate into mature cells of mesenchymal tissues (e.g. osteocytes, chondrocytes, tenocytes, and adipocytes).19 Clinical research on MSC use in shoulder disorders is very limited.22 Ellera Gomes et al.23 injected bone marrow mononuclear cells harvested from the iliac crest to augment their repair of complete rotator cuff tears in 14 patients and reported tendon integrity in all patients by use of magnetic resonance imaging scans with a follow-up period of 12 months. No control group was examined, so the conclusions have to be evaluated in the context of an investigation with Level IV evidence.
Techniques of harvesting bone marrow MSCs from the proximal humerus, thus avoiding an additional surgical site, have been described.24 MSCs isolated from synovial tissue showed higher proliferation and differentiation potential than MSCs harvested from bone marrow or adipose tissue.25 Utsunomiya et al.11 explored the in vitro potential of MSCs derived from shoulder tissue by obtaining samples of the synovium, subacromial bursa, ruptured tendon, and enthesis. The subacromial bursa was easy to harvest arthroscopically. The yield obtained from the subacromial bursa was the highest, as were expandability and multipotency.11
The rotator cuff insertion on the humerus has a fibrocartilage column. Despite anatomic reinsertion in rotator cuff repair, the formation of the natural enthesis remains unequaled. The chondrogenic potential of MSCs harvested from the enthesis is higher than that of other shoulder tissues but inferior compared with subacromial bursal tissue in terms of sample size and expandability of MSCs.11 Song et al.2 reported that bursa-derived MSCs in vivo formed bone, fibrocartilage, and tendon-like tissue and the fibrocartilaginous tissue was built without supplementing any growth factors. These features indicate a probable reparative response of the bursal tissue that might play a significant role in tendon reconstitution and remodeling.
The additional bursal augmentation in arthroscopic rotator cuff repair has the potential to provide an important biological accessory able to take a significant step forward toward improved tendon healing. The bursa is directly accessible without the need for preparation or an additional donor site. The presented technique of augmented rotator cuff repair by bursal adaptation is easy to perform at the end of a conventional double-row SutureBridge repair.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: M.S. receives personal fees and nonfinancial support from Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
The preoperative radiographic scans of the right shoulder in this 60-year-old male patient show no significant glenohumeral arthritis. The magnetic resonance images show, in the coronal view, a large posterosuperior rotator cuff tear with grade II supraspinatus tendon retraction according to Patte.13 In the axial view, the infraspinatus tendon displays a retracted full-thickness tear with signs of atrophy. The parasagittal view shows the infraspinatus muscle with grade II fatty infiltration according to Goutallier et al.14 The supraspinatus tendon shows grade II muscular atrophy according to Thomazeau et al.12 The patient is in the beach-chair position. After diagnostic arthroscopy and biceps tenodesis, lateral and posterolateral portals are used for visualization of the rupture. The mobility of the tear margins is evaluated. After mobilization, the tendon can be reduced with a grasper, introduced from the anterolateral portal, to the footprint area. The footprint is decorticated, and a medial row of double-loaded anchors (BioComposite Corkscrew FT Suture Anchors, 5.5 mm × 14.7 mm, with 4 No. 2 FiberWire sutures) is placed at the bone-cartilage junction. The threads are shuttled through the tendons using a suture passer with a double-mattress technique. By pulling the threads, complete coverage of the footprint is possible. The sutures are tensioned and fixated laterally by a SutureBridge technique using 2 knotless anchors (BioComposite SwiveLock SP, 4.75 mm × 24.5 mm). Subsequently, an awl is introduced to microfracture the insertion bed. Blood flow out of the microfractured areas can be observed. The parietal sheet of the subacromial bursa in the subdeltoid recess is perforated with a lasso introduced from the lateral portal, and a No. 2 PDS thread is shuttled through using a nitinol wire. The same procedure is performed with the tendon tissue using a lasso from the anterolateral or anteroinferior portal to shuttle the same PDS thread. Tensioning both ends of the PDS thread, the bursal tissue is converging on the tendon, covering the tendon margin and the insertion area. This procedure is repeated posteriorly in the same manner. The PDS threads are tied using an arthroscopic knot-tying device. In this manner, the whole insertion area is covered with highly vascularized bursal tissue. Viewing from the posterior portal, the surgeon exposes the anterolateral acromial spur using an electrocautery device. An acromioplasty is performed using an acromionizer to widen the subacromial space. This provides ulterior blood support descending on the tendon insertion area. Sonographic control performed 2 days after surgery shows vascularized bursal tissue, with a grade I Doppler signal, attached to the distal footprint and overlying the insertion area. After 6 weeks, a clear Doppler signal increase to grade II is seen in the anterior part of the supraspinatus tendon and bursal area. A new grade I Doppler signal appears at the tear edge.
References
- 1.Steinert A.F., Kunz M., Prager P. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res Ther. 2015;6:114. doi: 10.1186/s13287-015-0104-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song N., Armstrong A.D., Li F., Ouyang H., Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugaya H., Maeda K., Matsuki K., Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 4.Gerber C., Fuchs B., Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000;82:505–515. doi: 10.2106/00004623-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Bishop J., Klepps S., Lo I.K., Bird J., Gladstone J.N., Flatow E.L. Cuff integrity after arthroscopic versus open rotator cuff repair: A prospective study. J Shoulder Elbow Surg. 2006;15:290–299. doi: 10.1016/j.jse.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Uhthoff H.K. Surgical repair of rotator cuff ruptures. the importance of the subacromial bursa. J Bone Joint Surg Br. 1991;73:399–401. doi: 10.1302/0301-620X.73B3.1670436. [DOI] [PubMed] [Google Scholar]
- 7.Gohlke F., Rolf O., Bohm D. Open reconstruction of the rotator cuff. Orthopade. 2007;36:834–847. doi: 10.1007/s00132-007-1135-y. [in German] [DOI] [PubMed] [Google Scholar]
- 8.Blaine T.A., Kim Y.S., Voloshin I. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Shoulder Elbow Surg. 2005;14:84S–89S. doi: 10.1016/j.jse.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 9.Voloshin I., Gelinas J., Maloney M.D., O'Keefe R.J., Bigliani L.U., Blaine T.A. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy. 2005;21:1076.e1–1076.e9. doi: 10.1016/j.arthro.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Lhee S.H., Jo Y.H., Kim B.Y. Novel supplier of mesenchymal stem cell: Subacromial bursa. Transplant Proc. 2013;45:3118–3121. doi: 10.1016/j.transproceed.2013.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 12.Thomazeau H., Rolland Y., Lucas C. Atrophy of the supraspinatus belly. Assessment by MRI in 55 patients with rotator cuff pathology. Acta Orthopaedica Scandinavica. 2009;67:264–268. doi: 10.3109/17453679608994685. [DOI] [PubMed] [Google Scholar]
- 13.Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990;254:81–86. [PubMed] [Google Scholar]
- 14.Goutallier D., Postel J.M., Bernageau J., Lavau L., Voisin M.C. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 15.Fuchs B., Weishaupt D., Zanetti M., Hodler J., Gerber C. Fatty degeneration of the muscles of the rotator cuff: Assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg. 1999;8:599–605. doi: 10.1016/s1058-2746(99)90097-6. [DOI] [PubMed] [Google Scholar]
- 16.Pauly S., Kieser B., Schill A., Gerhardt C., Scheibel M. Biomechanical comparison of 4 double-row suture-bridging rotator cuff repair techniques using different medial-row configurations. Arthroscopy. 2010;26:1281–1288. doi: 10.1016/j.arthro.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Kovacevic D., Rodeo S.A. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauly S., Scheibel M. Arthroscopic rotator cuff surgery: New and established methods. Orthopade. 2018;47:92–102. doi: 10.1007/s00132-018-3526-7. [in German] [DOI] [PubMed] [Google Scholar]
- 19.Randelli P., Randelli F., Ragone V. Regenerative medicine in rotator cuff injuries. Biomed Res Int. 2014;2014:129515. doi: 10.1155/2014/129515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorbach O., Baums M.H., Kostuj T. Advances in biology and mechanics of rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2015;23:530–541. doi: 10.1007/s00167-014-3487-2. [DOI] [PubMed] [Google Scholar]
- 21.Rha D.W., Park G.Y., Kim Y.K., Kim M.T., Lee S.C. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: A randomized controlled trial. Clin Rehabil. 2013;27:113–122. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 22.Beitzel K., Solovyova O., Cote M.P. The future role of mesenchymal stem cells in the management of shoulder disorders. Arthroscopy. 2013;29:1702–1711. doi: 10.1016/j.arthro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Ellera Gomes J.L., da Silva R.C., Silla L.M., Abreu M.R., Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg Sports Traumatol Arthrosc. 2012;20:373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beitzel K., McCarthy M.B., Cote M.P. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthroscopy. 2013;29:301–308. doi: 10.1016/j.arthro.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Jones E.A., Crawford A., English A. Synovial fluid mesenchymal stem cells in health and early osteoarthritis: Detection and functional evaluation at the single-cell level. Arthritis Rheum. 2008;58:1731–1740. doi: 10.1002/art.23485. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The preoperative radiographic scans of the right shoulder in this 60-year-old male patient show no significant glenohumeral arthritis. The magnetic resonance images show, in the coronal view, a large posterosuperior rotator cuff tear with grade II supraspinatus tendon retraction according to Patte.13 In the axial view, the infraspinatus tendon displays a retracted full-thickness tear with signs of atrophy. The parasagittal view shows the infraspinatus muscle with grade II fatty infiltration according to Goutallier et al.14 The supraspinatus tendon shows grade II muscular atrophy according to Thomazeau et al.12 The patient is in the beach-chair position. After diagnostic arthroscopy and biceps tenodesis, lateral and posterolateral portals are used for visualization of the rupture. The mobility of the tear margins is evaluated. After mobilization, the tendon can be reduced with a grasper, introduced from the anterolateral portal, to the footprint area. The footprint is decorticated, and a medial row of double-loaded anchors (BioComposite Corkscrew FT Suture Anchors, 5.5 mm × 14.7 mm, with 4 No. 2 FiberWire sutures) is placed at the bone-cartilage junction. The threads are shuttled through the tendons using a suture passer with a double-mattress technique. By pulling the threads, complete coverage of the footprint is possible. The sutures are tensioned and fixated laterally by a SutureBridge technique using 2 knotless anchors (BioComposite SwiveLock SP, 4.75 mm × 24.5 mm). Subsequently, an awl is introduced to microfracture the insertion bed. Blood flow out of the microfractured areas can be observed. The parietal sheet of the subacromial bursa in the subdeltoid recess is perforated with a lasso introduced from the lateral portal, and a No. 2 PDS thread is shuttled through using a nitinol wire. The same procedure is performed with the tendon tissue using a lasso from the anterolateral or anteroinferior portal to shuttle the same PDS thread. Tensioning both ends of the PDS thread, the bursal tissue is converging on the tendon, covering the tendon margin and the insertion area. This procedure is repeated posteriorly in the same manner. The PDS threads are tied using an arthroscopic knot-tying device. In this manner, the whole insertion area is covered with highly vascularized bursal tissue. Viewing from the posterior portal, the surgeon exposes the anterolateral acromial spur using an electrocautery device. An acromioplasty is performed using an acromionizer to widen the subacromial space. This provides ulterior blood support descending on the tendon insertion area. Sonographic control performed 2 days after surgery shows vascularized bursal tissue, with a grade I Doppler signal, attached to the distal footprint and overlying the insertion area. After 6 weeks, a clear Doppler signal increase to grade II is seen in the anterior part of the supraspinatus tendon and bursal area. A new grade I Doppler signal appears at the tear edge.