Abstract
Immune checkpoint blockade holds great promise in the treatment of solid tumors but has not yet been approved for use in advanced prostate cancer. This is largely due to the relatively modest response in clinical trials in unselected patients and the lack of available biomarkers to predict clinical benefit. Germline and somatic mismatch repair (MMR) gene deficiencies are more prevalent than previously thought, especially in the metastatic setting, in patients with high-grade Gleason scores and in patients with variant histologies. An early signal suggests that patients with deficiency in MMR may respond well to immunotherapy. Both germline and somatic genetic testing are recommended, yet questions remain on the best modality for testing given lack of standardization and false-negative results in patients with complex genomic structural rearrangements. Expanded panels, such as next generation sequencing may increase the sensitivity without compromising specificity. Future studies are still needed to explore the relationships of hypermutation, tumor mutational burden, tumor-infiltrating lymphocytes and microsatellite instability-H status as predictors of response to immunotherapy. The drivers of variable response is largely unknown, and a more mature understanding of the mechanisms of resistance in deficiencies in MMR tumors may help to more precisely inform use of immunotherapy in prostate cancer.
Keywords: : germline mutations, immunotherapy, mismatch repair defects, prostate cancer, somatic mutations
Nearly one in six men are diagnosed with prostate cancer over the course of their lifetime. It is the second most deadly cancer in men and is responsible for over 10% of all cancer-related deaths [1]. While many men are diagnosed with limited-stage disease and are treated definitively with surgery or radiation therapy (RT) [2], many patients experience recurrence and progression of their disease. Metastatic prostate cancer is an incurable entity. Standard therapy for metastatic disease generally involves androgen deprivation, via surgical or chemical castration, together with additional systemic therapies. Although effective for some time, androgen deprivation has significant effects on health-related quality of life. Eventually, the disease will progress and patients will develop metastatic castration-resistant prostate cancer (mCRPC). At this time, additional systemic therapies are needed, with cumulative toxicities. Thus, an unmet therapeutic need remains for patients with prostate cancer. The unprecedented success of immune checkpoint inhibitors in various cancer types has provided evidence that the immune system can be modulated to combat cancer. Given the toxicities of currently available prostate cancer treatments, the use of immune-based therapies is very attractive.
Improvements in the molecular understanding of prostate cancer demonstrate a substantial incidence of both germline (inherited) and somatic (acquired) alterations in genes involved in DNA damage repair. DNA is constantly exposed to a variety of insults that result in mutations. Failure to repair this DNA damage may lead to tumorigenesis or accelerated tumor growth. Although cells have evolved molecular processes aimed to maintain genomic integrity, cancer arises due to alterations in either pro-survival and/or antiapoptotic pathways. Thus, defective DNA damage repair pathways (especially if occurring in the germline DNA) have been associated with several cancer syndromes [3–7].
The DNA mismatch repair (MMR) pathway maintains the integrity of postreplicative repair. Tumors with deficient MMR pathways have nearly a 1000-fold greater rate of mutations than tumors with intact pathways. Deficiencies in mismatch repair (dMMR) lead to the development of unstable microsatellites or short tandem repetitive DNA sequences [8]. Microsatellites are particularly vulnerable to replication errors over a repetitive sequence due to DNA strand slippage. Without a means to repair mistakes in DNA replication and recombination, the number of microsatellites then increase or decrease, leading to microsatellite instability (MSI). MSI refers to the hypermutated phenotype secondary to frequent alterations. MSI secondary to germline mutation in DNA MMR genes is the molecular hallmark of Lynch syndrome (LS), while epigenetic inactivation of these genes is more commonly found in sporadic tumors. MLH1, MSH2, MSH6 and PMS2 genes modify MMR activity and defects in these genes have been associated with MSI-high tumors across cancer types. MSI occurs at varied frequencies in different malignancies. However, the original methods to assess MSI or MMR deficiency were developed mostly in LS-associated cancers. Tumors with dMMR also exhibit high neo-antigen burden and are extremely susceptible to immune checkpoint inhibitors [9].
Although checkpoint inhibitors represent one of the greatest advances of modern clinical oncology, with demonstrated efficacy in many cancers, their activity is limited to a certain percentage of patients, ranging from 10 to 40% depending on the cancer type [10,11]. In unselected patients with metastatic castration-resistant prostate cancer, the response rate to immune checkpoint blockade is a modest 10% (range: 5–15%). Therefore, the question remains: which prostate cancer patients should be considered for immunotherapy?
A small portion of prostate cancers are thought to harbor deficiencies in MMR. Thus, deeper understanding of this subset of prostate cancers is extremely important given the recent tumor-agnostic approval for pembrolizumab by the US FDA in patients with MMR gene mutations or MSI [9,11,12].
Why is this Review clinically important?
Alterations in the DNA repair pathways are estimated to occur in nearly 20% of mCRPC and in a smaller, yet significant, number of men with localized prostate cancer harboring either somatic or germline mutations [3,13]. Given the high prevalence of prostate cancer, the cost of treatment and the effects of chemotherapy on patient-reported quality of life, it is important to define best treatment for a biologically distinct subset of prostate cancers.
The development of immune checkpoint blockade has demonstrated clinical benefit in cancers deficient in MMR. While the frequency of MMR deficiency is well defined in colorectal, uterine, and other LS-associated cancers, it is poorly defined in prostate cancer. Favorable responses to immunotherapies have also been described in advanced tumors with MMR gene inactivation and hypermutation [9,11,14]. This Review will aim to summarize recent advances in the understanding of prostate cancer with special attention to MMR deficiency. We will Review DNA repair biology, assays for detecting MMR deficiency, prognostic implications, response to treatments, mechanisms of resistance and future opportunities for research.
DNA damage, MMR & prostate cancer: lessons learned from Lynch syndrome
The ability to repair DNA with high fidelity is necessary to prevent malignant transformation. Germline inherited deficiencies in DNA repair genes increase genomic stress over time and are associated with increased accumulation of mutations, and possible development of subsequent cancer [15]. The MMR pathway corrects mistakes made during DNA replication and recombination, often due to base–base mismatch and insertion–deletion loops. Tumors with mutations in MMR genes are most commonly found in colorectal and other gastrointestinal malignancies, endometrial and ovarian cancer [16]. A small but potentially important fraction of prostate cancers are thought to harbor MMR mutations.
The role of MMR defects in the development of cancer was first established when mutation in MSH2, a canonical MMR gene, was linked to hereditary nonpolyposis colorectal cancer, also known as LS [17]. Over time, the classical MMR genes, MLH1, MSH2, MSH6 and PMS2, were linked to an autosomal dominant, hereditary predisposition to colon cancer. These cancers were hallmarked by germline loss of function alterations. The LS has since been strongly linked to colorectal, endometrial, ovarian, urothelial and other malignancies [16,18,19]. Though the published familial data are not compelling to group prostate as a Lynch-associated cancer, a focus on somatic mutations leading to the dMMR prostate cancer phenotype has potential for advancing the field.
In the era of immunotherapy, great interest has emerged in identifying patients with dMMR. The close association of MMR deficiency and MSI was first shown in yeast and later identified in patients with LS [20,21]. The MSI has since been used as a surrogate marker for MMR deficiency and is the basis of enrollment in many clinical trials in oncology. In fact, MSI-high status has been observed at modest levels across at least 24 different tumor types [9,11]. Importantly, as we will further discuss in a later section of the Review, MSI and MMR defects can be found not only in germline cancer syndromes but can also be acquired sporadically through somatic mutation or epigenetic alterations leading to loss of expression of the corresponding proteins. At the time of this Review, they have already been identified and sequenced in colorectal, endometrial, stomach, non-small-cell lung cancer, esophageal, pancreatic, gastric and ovarian cancers [22].
Among the MMR genes, defects within the MSH2 and MSH6 gene have been the most frequently reported in patients with PC [23–26]. MSH2, in particular, has been the MMR gene most commonly expressed in both primary and advance prostate cancer [27,28]. It is also the MMR gene most frequently implicated in LS patients who develop microsatellite-unstable prostate cancer [23,26,29,30]. Over time, with advances in molecular sequencing, several investigators have correlated deficiencies in MMR with poorly differentiated, aggressive phenotype and late stage (nodal involvement and distant metastatic) prostate cancer [31–34]. Continued focus in epidemiology and outcomes of prostate cancers deficient in MMR is important, as roughly half are refractory to immunotherapy, and our understanding remains rudimentary given lack of standardization in genomic testing.
What is the prevalence of germline MMR mutations in prostate cancer patients?
Though prostate cancer cannot clearly be defined as a Lynch-associated malignancy, it is clear that a subset of patients harbor germline MMR gene mutations. Given the recent US FDA-approval of the PD-1 inhibitor pembrolizumab to treat metastatic tumors of all tissue types with MMR deficiency or MSI-high status, it is important to ask: how common are germline mutations in the canonical MMR genes (MSH2, MSH6, MLH1 and PMS2) in patients with prostate cancer?
The estimated overall prevalence of MMR gene mutations in the germline DNA of advanced prostate cancer patients is likely around 1% (range: 0.5–1.5%), and mostly involving the MSH2 and MSH6 loci [27,35,36]. The first comprehensive germline DNA sequencing effort investigating this question was published by Pritchard and colleagues. They explored 692 men with metastatic prostate cancer and found 11.8% of patients harbored an underlying inherited germline mutation in a DNA repair gene. MMR gene alternations were rare and noted in only four patients (0.6% collectively) [37]. Germline DNA repair gene mutations were less common in localized prostate cancer (4.6%), suggesting enrichment in those who present with or develop metastatic spread. Subsequently, other investigators have noted that certain patients populations may be enriched for MMR mutations (Box 1), including patients with unusual sites of metastases, such as de novo pulmonary disease, aggressive histologic subtypes (Gleason grade group 5 and primary Gleason pattern 5) or variant histologies such as intraductal prostate cancer and small-cell prostate carcinoma [14,27,35,36,38–40].
Box 1. Prostate cancers that may respond to immunotherapy.
Histologic features: Gleason pattern 5, ductal and intraductal variants, small-cell prostate cancer
Genetic features: mismatch repair deficient, mutations in CDK12 gene, mutations in POLE and POLD1 genes, deletion in 3′-untranslated region of CD274 locus, cancers with high mutational burden (>20 mutations/Mb)
Clinical features: strong family history, metastatic disease with short duration to development of castration resistance, de novo presentation with pulmonary metastases
In the study by Pritchard, 8.8% of patients with metastatic disease treated had germline mutations compared with 18.5% of those treated at Memorial Sloan Kettering as reported by Nicolosi and colleagues. A recent publication included 3607 men with a personal history of prostate cancer, mean age: 67, who underwent germline genetic testing and were unselected for family history, stage of disease or age at diagnosis. Total 620 (17.2%) had positive germline variants; 30.7% were variants in BRCA1/2. DNA MMR variants were detected in 1.74% of patients [41]. While the study highlighted the potential implications of a new distinct subtype of prostate cancer, with dMMR, the issue of germline testing is fraught with controversy. There is debate and uncertainty on who to test, how to test, what sequence of testing and timing. The NCCN guidelines recently updated their recommendations to include germline genetic testing for all men with high-risk, very high-risk, regional or metastatic prostate cancer [42]. It is important to note that virtually no next-generation sequencing (NGS) tests are designed for germline assessment; in addition, over-testing and over-interpretation of germline findings remains an area of concern [43].
Overall, studies using recommended assays suggest the rate of MSI-high status and MMR deficiency in advanced prostate cancer is enough to warrant sequencing [28,39,44]. Patients found to harbor dMMR should be referred to a genetics clinic as somatic sequencing has never been validated for germline testing.
What is the prevalence of somatic mutations in MMR genes in prostate cancer?
The role of somatic cancer mutations in priming the immune system is becoming more evident as the experience with immunotherapy matures. The management of many cancers now incorporates genomic analysis to guide decision-making. Defining the prevalence of MMR mutations and response to checkpoint blockade in prostate cancer is difficult, given the inconsistencies and lack of standardization in studies thus far. Somatic mutation testing is not routine in clinical practice in prostate cancer, though perhaps, as we hope to portray, it should be. Given the implications on treatment decision and potential toxicities incurred by patients, it is also important to ask: how common are somatic mutations in the canonical MMR genes (MSH2, MSH6, MLH1 and PMS2) in prostate cancer?
Similar to our comments on germline mutations, it is difficult to precisely answer the question, but the prevalence of somatic MMR gene mutations or MSI-high status in metastatic prostate cancer is probably in the order of 5% (range: 3–8%) and all four genes can be affected [13,35,45]. In one study, the frequency was reported to be 12%, though this was likely an overestimate derived from an autopsy series enriched for lethal prostate cancer cases [27]. What is clear, is that the majority of MMR mutations in prostate cancer (about 75%) occur at the somatic level and are not inherited. Thus, the relevance of whether prostate cancer is a Lynch-associated cancer and the importance of germline inheritance are frequently overstated. A focus on the somatic mutations leading to dMMR prostate cancer subtype are more relevant for a common and lethal cancer. Therefore, within this context, somatic testing for MMR deficiency should be prioritized for certain men with prostate cancer, with considering of germline testing when appropriate.
Several large DNA sequencing efforts have explored the underlying genetic makeup observed in prostate cancer. The Cancer Genome Atlas Research Network sequenced 333 primary localized prostate cancers, without regard to subsequent recurrence or metastasis [28]. The authors also did not tease out somatic from germline mutations but did note DNA-repair pathway gene alterations in 19% of cases, though most involved the homologous recombination repair pathway. Mutations in MMR genes were very rare (<1%) in these early-stage localized tumors. A second study of 477 intermediate-risk localized prostate cancers similarly noted MMR mutations in very few patients (<1%) [46].
Pritchard and colleagues further explored hypermutation in patients with advanced prostate cancer, detecting mutations with predicted loss of function in MSH2, MSH6, or both genes in four of five rapid autopsy patients. In all patients, hypermutation status and MMR mutations were concordant at metastatic sites. Of note, none of the MMR mutations were inherited in the germline [27]. This study further suggests that somatic mutations are more common than germline mutations in advanced prostate cancer. A larger study of 1133 primary prostate adenocarcinomas and 43 prostatic small-cell carcinomas reported findings using immunohistochemistry (IHC) with confirmation by next-generation sequencing. The authors found a small percentage (1.2%) had MSH2 loss. Of the 12 patients confirmed to have loss-of-function alterations, only three patients had germline mutations. MSH2 inactivation was enriched in patients with primary Gleason pattern 5 cancers (8%) and small-cell prostate cancers (5%). Tumors with MSH2 loss had a higher density of infiltrating CD8+ lymphocytes compared with grade-matched controls without MSH2 loss. CD8+ density correlated with mutation burden [39].
Very few studies have isolated their reporting of germline and somatic mutations. An analysis of 150 metastatic site biopsies of castrate-resistant patients for whole exome, matched germline, copy number and transcriptome sequencing found DNA repair pathway defects in 22.7% of cases. Though the majority of cases had mutations in BRCA1, BRCA2 and ATM, a significant number of MMR defects (MLH1 at 4.7%, MSH2 at 0.7%) were noted [13]. An updated analysis from the same authors, with expanded genomic data from 335 mCRPC samples, reported a prevalence of somatic pathogenic DNA repair alterations upward of 20% [47].
All in all, current estimates suggest that MMR mutations in advanced prostate cancer are likely two- to threefold higher than what is observed in localized disease. Patients with a Gleason score of ≥8 are more likely to harbor dMMR. In the metastatic setting, given the much higher prevalence of DNA damage repair mutations, we would support the use of somatic mutational testing in the castration-resistant setting. Given the unknown frequency of somatic pathogenic DNA repair alterations and the potential for targeted therapy, it may be advisable to sequence tumor DNA.
Unknown at this time are the prognostic and therapeutic impact of germline versus somatic DNA mutations. It is not yet clear if enrichment in metastatic sites and castration-resistant disease is due to tumor evolution in response to therapy exposure or whether it serves as a surrogate marker of more aggressive prostate cancer. Only time and more frequent DNA sequencing will tell.
What are the pitfalls in clinical testing for deficiencies in MMR/MSI status?
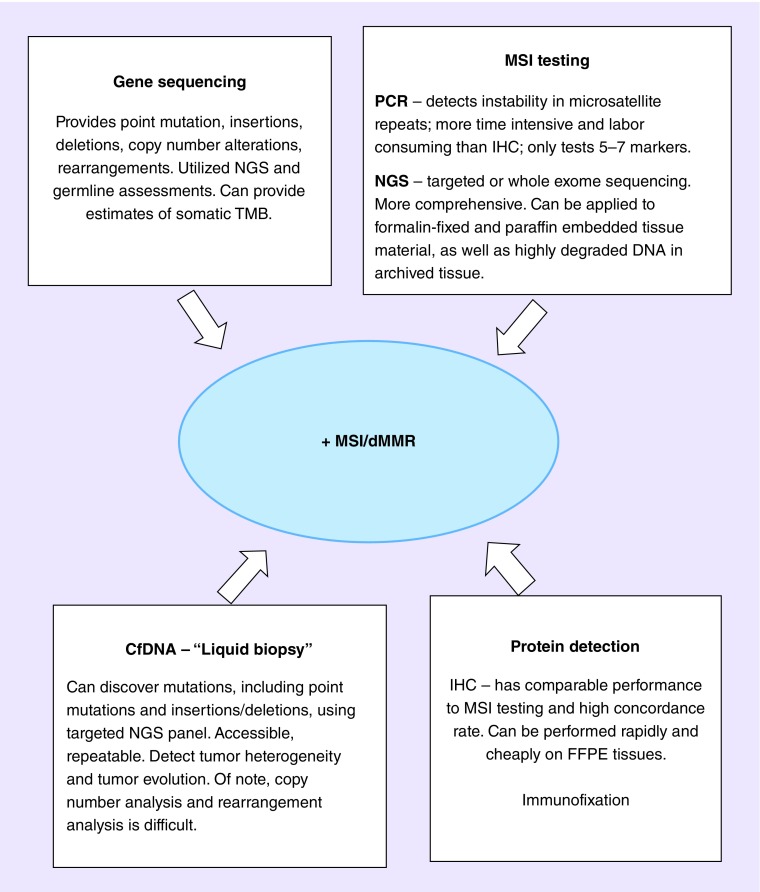
Elucidating the incidence of germline MMR-deficient prostate cancer been challenged by several limitations. Most studies investigating the prevalence of MSI in prostate cancer were performed more than a decade ago, and report a wide range of MSI frequency [32,33,48]. Different assays and definitions used to determine MSI status yield inconsistent results. Older studies used multiplex PCR testing, based on the NIH panel validated only in colorectal cancers [5,16,18]. Use of these five microsatellites might be less relevant in dMMR prostate cancers than in colorectal cancers. In a recent case series of 13 MMR-mutated advanced prostate cancers, 27% of these patients had no MSI marker shifted, 36% had 1–2 markers shifted, 36% had 3–4 markers shifted and none had all five markers shifted [35]. In fact, the original report suggested the Bethesda reference panel be used for MSI characterization in colorectal cancer only [49]. While subsequent studies validated the test for MSI in cancer types other than colon cancer, the instability of microsatellite loci continues to vary greatly across tumor type, with wide ranges from 2–65% [50,51].
Testing using IHC detection of MMR proteins also has its diagnostic limitations [52,53]. Most clinical-grade somatic assays do not report loss of heterozygosity of tumor suppressor genes; therefore, bi-allelic inactivation cannot be determined. Thus, functional MMR deficiency, via complex genomic rearrangements may be missed. False-negative results also occur in standard exon-only sequencing which miss the vast majority of genomic structural arrangements, especially those occurring within noncoding DNA regions. Alternative approaches to determine MSI from NGS is available and more appropriate (Figure 1) [54]. Expanded NGS assays improve identification of tumors with complex structural genomic rearrangements of MMR genes, not always detectable by clinical-grade targeted exon sequencing, but are not readily available in current clinical practice [39,54]. In a recent study using NGS to interrogate MSH2, MSH6, MLH1 and PMS2, the authors found 5/50 mCRPC patients (10%) harbored complex genomic rearrangements involving one or more of the MMR genes, primarily in MSH2 and MSH6. Most of these cases resulted in MSI-high tumors with multiple unstable satellite regions accompanied by hypermutation [55]. Interestingly, the complex structural genomic rearrangements of MMR genes in prostate cancer, differs from the inactivating mutations, loss of heterozygosity and epigenetic silencing typical of colorectal cancers in LS, further highlighting the need to consider NGS testing in this patient population [14,27,39,56].
Figure 1. . Testing for microsatellite instability/mismatch repair deficiency.
IHC: Immunohistochemistry; MSI: Microsatellite instability; NGS: Next-generation sequencing; TMB: Tumor mutational burden.
NGS offers unique opportunities to identify tumor-specific DNA and key molecular changes of interest to novel drug development. This includes quantifying tumor mutational burden (TMB) and exploring the clonality of T-cells in tumor microenvironments. TMB has emerged as a promising potential biomarker for immunotherapy. Several observations have been made, especially in the early trials of melanoma and lung cancer, with high TMB being linked to mutagenic exposure and thus enhanced immunogenicity. The mechanism for the enhanced immunogenicity of high TMB cancers is believed to be through the production of so-called mutation-associated neoantigens, which are more strongly immunogenic than their nonmutant protein counterparts. It remains controversial whether the sheer quantity of neoantigens or the presence of certain high-quality neoantigens is needed to generate an antitumor immune response.
As of this time, most cases of MMR deficient cancers result in MSI-H tumors with multiple unstable satellite regions and hypermutation [3,14,27,37]. Historically, tumors with prominent dMMR mutational signatures have had higher inferred immune cell infiltration. However, not all dMMR prostate cancers demonstrate hypermutation or dense CD8+ T-cell infiltrates as expected in other MMR deficient cancers, adding to the diagnostic challenge [14,39]. In a study at our institution of 13 dMMR prostate cancers, [35] only 63% of patients with MSI-H had TMB of >20 mutations/Mb. In another study [39], only 61% of MSH2-deficient prostate cancers demonstrated MSI, while 83% showed hypermutation. Interestingly, only half of MSH2-deficient cases demonstrated a high CD8+ tumor-infiltrating lymphocyte density.
At this time, there is wide recognition that dMMR genes have implications on the immune response through complex mechanisms, some of which remain unknown. Kelderman and colleagues have shown that dMMR may trigger changes in the tumor-secreted cytokines and chemokines, which could potentially influence the antitumor immune response [57]. Other labs have added to our understanding of immune activation, and the depth of presentation at the interface of dMMR and checkpoint inhibition. Though these mechanisms are beyond the scope of this Review, they highlight the complexity of predicting host responses to immunotherapy, particularly in prostate cancer. More work is necessary to investigate the molecular factors and biomarkers that predict responses and the levels of genomic instability may be fruitful.
These studies highlight the issues in predicting response to immunotherapy. Though Abida and colleagues elegantly used NGS and the MSI sensor algorithm to determine a quantitative MSI sensor score, it is not a standardized or universal method. It remains unclear which test’s positive result is most associated with a response and whether it can be practical for widespread use in the community. What their study does highlight is the need for standardization and the important of testing MSI in metastatic samples, though cell-free DNA may one day obviate the need for testing metastatic sites [58]. The ideal test should provide information about somatic and germline mutations, be low cost and easy to obtain.
Identifying tumor-specific mutations that occur across all genes in the tumor genome remains an important area of scientific exploration. In order to better understand tumor-specific mutations, comparison of the tumor genome with the germline genome may be of interest if available, and if not, burdensome to patient related costs. A major limitation of many molecular studies to date is the under-representation of African–American, Asian, Hispanic and other minority groups and adds to the complexity of predicting responses to therapy for all patients.
What are the pathologic characteristics & clinical outcomes in patients with prostate cancer deficiencies in MMR?
Owing to the low prevalence of dMMR in prostate cancer, data exploring the clinical and pathologic characteristics and clinical outcomes are slow to mature. At this time, little is known regarding the natural history and sensitivity of dMMR prostate cancer to standard therapies. A retrospective study at Johns Hopkins was recently published and highlights results from a database of somatic mutations in prostate cancer cases (n = 236) with pathogenic loss-of-function MMR mutations and a germline genetic database (n = 348) for similar inherited MMR mutations. Both databases include recurrent and/or metastatic prostate cancer cases and evaluate MMR genes. Pathogenic mutations included protein-truncating mutations, genomic deletions or structural rearrangements. Somatic next-generation tumor DNA sequencing and germline genetic testing were performed; where available, IHC testing for the detection of the four MMR proteins was also conducted.
In this study, 13 metastatic prostate cancer patients with pathogenic MMR gene mutations were identified: ten from somatic sequencing results (4.2%) and three from germline sequencing results (0.9%). Median age was 64 years, 75% had Gleason sum 9 or 10, 23% had intraductal histology. Nearly half had metastases at initial diagnosis; 31% had visceral involvement. All patients received standard androgen deprivation therapy (ADT), 46% received abiraterone or enzalutamide, 15% received docetaxel and 31% received PD-1 blockade. Similar to previously published literature, MSH6 (46%; 6/13) or MSH2 mutations (23%; 3/13) were the most common gene mutations. Median tumor mutation burden was 18 mutations/Mb. Of those with adequate tissue available for sequencing, 73% (8/11) demonstrated MSI. Median TMB were 21 mutations/Mb for MSI-H and six mutations/Mb for MSS patients.
The MMR-deficient patients demonstrated high sensitivity to hormonal therapies. All 13 patients received standard ADT as initial systemic therapy for metastatic disease and 85% achieved a >90% PSA response rate (11/13), with a median PSA progression-free survival (PFS) of 55 months and median PFS of 66 months. Median PFS for ADT among 114 MMR-proficient men was 27 months. Sensitivity to first-line abiraterone or enzalutamide was also high among the six MMR-deficient patients evaluable for this outcome, of whom 83% achieved a >50% PSA response, with a median PFS of 26 months. By comparison, median PFS to first-line abiraterone/enzalutamide among 75 MMR-proficient men was 12 months. Median PFS for MMR-deficient patients receiving docetaxel was 7 months. Four patients received anti-PD-1 treatment as fourth- to sixth-line therapy: two using nivolumab and two using pembrolizumab. Two of four patients achieved a >50% response with a median PFS of 9 months; three of these four patients also achieved an objective soft-tissue response lasting 3–9 months [35].
In addition to these data, a recent analysis of the prevalence of MSI in prostate cancer and response to immune checkpoint blockade was published by Abida and colleagues from Memorial Sloan Kettering Cancer Center. In this case series, the investigators sequenced 1551 tumors from 1346 patients with prostate cancer prospectively using a targeted sequencing assay over a 3-year period. Among the 1033 patients who had adequate tumor quality for study, 3.1% had MSI-H/dMMR prostate cancers. As an aside, this estimate is remarkably similar to that of another recent study using circulating cell-free tumor DNA, which also estimated an MSI-high prevalence of 3.8% in men with mCRPC [59]. Seven of these 32 patients in the Abida study (21.9%) had a pathogenic germline mutation in a LS-associated gene, including five in MSH2, one in MSH6 and one in PMS2. Six patients had more than one tumor analyzed, two of whom displayed an acquired MSI-H phenotype later in their disease course. The clinical characteristics of these 32 patients included median age of 64 years (range: 39–85 years). One patient had small-cell histologic findings. For the patients with adenocarcinoma, median time to castration resistance was 8.6 months and the median duration of treatment with first-line abiraterone acetate or enzalutamide for MCRPC was 9.9 months. Total 11 patients with MSI-H/dMMR castration-resistant prostate cancer received anti-PD-1/PD-L1 therapy. Six of these (54.5%) had a greater than 50% decline in PSA levels, four of whom also had radiographic responses [60]. Their data confirm that, though the molecular phenotype of dMMR is uncommon, it may be therapeutically meaningful in prostate cancer and can be somatically acquired during disease evolution. Given the potential for durable responses to anti-PD-1/PD-L1 therapy, these findings further support the use of prospective tumor sequencing to screen all patients with metastatic prostate cancer for deficiencies in MMR. As the authors suggest, more research is needed to explore mechanisms of resistance, which we will briefly describe later in the Review.
What is the responsiveness to PD-1/PD-L1 inhibitors in prostate cancer patients with dMMR?
Although MMR-deficient tumors are rare, their detection has important therapeutic implications [9,14,35]. As previously described, loss of MMR is often associated with an increased mutational load [61], an increase in tumor neoantigens [10,11] and a potential to respond well to checkpoint inhibition. Data from a pivotal Phase II trial of pembrolizumab for the treatment of tumors with and without MMR deficiency supported the hypothesis that tumors dMMR are more responsive to PD-1 blockade when compared with tumors without MMR deficiency (40 vs 0%) [11]. Significant differences were also seen with regards to progression-free and overall survival. This, and other studies, led to the US FDA approval of the anti-PD-1 agent pembrolizumab for the treatment of patients with unresectable or metastatic MSI-high or MMR-deficient solid tumors, the first FDA approved cancer therapy irrespective of tissue origin. Whether similar success can be seen in patients with MSI-high prostate cancer is clearly of interest.
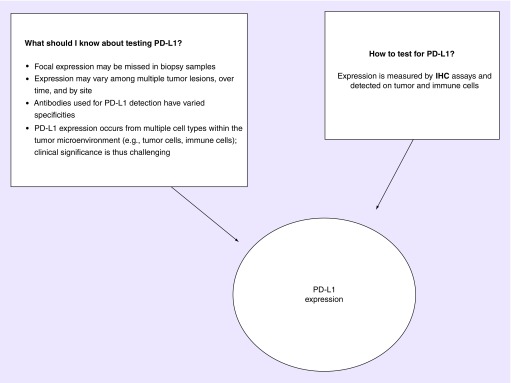
Tumor cell PD-1 expression has been demonstrated to be a valuable prognostic biomarker for immunotherapy sensitivity in some, but not all, cancers. However, the relationship is still premature, and significance varies among the different types of solid tumors. The different methods of evaluating PD-L1 expression and the varying cut-offs have also not been standardized in several trials thus far (Figure 2). The PD-L1 expression in prostate cancer ultimately depends on the assay cut-off, technique and the antibody clone used in evaluation. It is important to note that most prostate cancer studies have evaluated primary prostate specimens only and limited data exist about PD-L1 expression on metastatic lesions [62]. One study, by Fankhauser and colleagues, explored PD-L1 expression in prostate cancer [63]. This comprehensive study measured PD-L1 expression with two distinct antibodies and showed that mCRPC is correlated with higher PD-L1 expression compared with primary prostate cancer.
Figure 2. . Considerations for testing PD-L1.
What is PD-L1? It is a ligand for the immune checkpoint receptor PD-1 expressed on the surface of cytotoxic T-cells. Tumors cells within a sample can express PD-L1 ranging from 0 to 100% expression.
IHC: Immunohistochemistry.
Prostate cancer was the first solid tumor to have an immunotherapy that prolonged survival, following the approval of sipuleucel-T [64]. Thus, prostate cancer was initially an exciting candidate for immune checkpoint blockade with anti-PD-1, PD-L1 and anti-CTLA-4 therapy. A high number of mutations occur in cells with unstable DNA repair pathways and in theory, these patients are primed to respond to immune checkpoint inhibitors. Yet, early studies using immune checkpoint inhibitors have been disappointing.
A Phase II study evaluated the activity of pembrolizumab, a PD-1 blocking antibody, in patients with docetaxel-refractory mCRPC [65], a first monotherapy evaluation in mCRPC. Anti-PD-1 monotherapy resulted in minor antitumor effects, with RECIST response rate of 3–5%. This was modest within the world of genitourinary tumors. In comparison, in kidney cancer, response rates to monotherapy is 40% and in bladder cancer it is 25% [66]. In patients with docetaxel-refractory mCRPC, pembrolizumab showed antitumor activity in roughly 10% of patients with PD-L1+, and PD-L1- disease, and responses were also noted in patients with nonmeasurable, bone-predominant disease. Response rates were numerically higher in patients with somatic BRCA1, BRCA2 and ATM mutations [67]. In the KEYNOTE-028 study, pembrolizumab resulted in durable objective response rates in a subset of patients (17%) with heavily pretreated, advanced PD-L1 positive prostate cancer [68]. Anti-CTLA-4 agent ipilimumab was tested in two separate Phase III studies in unselected mCRPC. One of these trials enrolled 799 patients with chemotherapy-resistant disease. Patients were assigned to treatment with ipilimumab and low-dose RT or RT plus placebo. There was no difference in overall survival between groups (11.2 vs 10.0 months, HR: 0.85; p = 0.053) [69]. A subgroup analysis suggested patients without poor-prognostic features benefited more from ipilimumab. The second Phase III trial enrolled 602 chemotherapy-naive, castration-resistant patients, who were minimally symptomatic and without visceral metastases. Patients were randomized to ipilimumab or placebo; this trial did not include RT. The median OS of patients receiving ipilimumab was 28.7 months, compared with 29.7 months in the placebo group (HR: 1.11; p = 0.36) [70]. Despite the low overall response rates in both studies, PSA and objective RECIST-responses were noted in some individuals. The community concluded that immune-based therapy may not be a practical approach for an unselected population of prostate cancer patients, but targeted therapy in a select group, at an appropriate time, may be useful. Of the more widely used immune-based therapies, nivolumab, an anti-PD-1 therapy, remains the least studied and only one trial was performed with poor results. Only 17 patients with mCRPC were included in a Phase I study and no objective responses were seen [71].
An ASCO 2018 presentation by Smits and colleagues evaluated correlates of response to anti-PD-1 immune checkpoint blockade in MMR proficient and deficient patients with metastatic castration resistant prostate cancer. Pretreatment and postprogression biopsies were optional and collected for whole-genome sequencing and multiplex IHC. The MMR status was evaluated in subsets using nested amplification PCR. Their cohort included 13 CRPC patients with a median follow-up of 5.3 months. Total 10/13 patients had PD-L1 expression of >1%, 6/13 patients were MMR deficient. Range of mutation burden was 2–8 mutations per megabase in proficient patients and 25–74 mutations per megabase in deficient patients. Objective response were only seen in MMR deficient patients with PSA >50% declines seen in 75% of these patients. They also reported improved PFS (3.7 vs 7.8 months; p = 0.007), and significant differences in CD4+PD-1+cells circulating T-cell subsets, between responders and nonresponders (p = 0.03) [72].
Details in early trial design are likely contributory to the disappointing results of therapy thus far. In part, the results can be explained by the low mutational load observed in prostate cancer as a whole [73]. However, this is only part of the story. Early trials were not exclusionary and included broad populations of patients with prostate cancer. Refined study design is needed to selectively choose patients more likely to respond to therapy. There is also interest in combining other novel agents that stimulate immunotherapy responses in patients without MMR deficiency, perhaps including PARP inhibitors. PARP inhibitors are thought to trigger genomic instability and are agents primed for combination with immune-based regimens. Yet, for all potential advancements in drug design and drug combinations, a deeper understanding in mechanisms for resistance to immunotherapy is needed.
What are the potential mechanisms of resistance to immunotherapy?
The data from Le and colleagues demonstrated that in some malignancies, high mutational load can increase the likelihood of clinical response to immune checkpoint-blocking therapies. And though our understanding of the interactions between the immune system and tumors has increased, most cancer patients remain unresponsive to immunotherapy, especially in prostate cancer. This can be due to intrinsic or acquired tumor resistance, or changes in the host immune system (Table 1).
Table 1. . Potential mechanisms of PD-1 inhibitor resistance in somatic mismatch repair cancers.
| Resistance pathway | Hypothesized mechanism of action | Ref. |
|---|---|---|
| Tumor mutational burden and neoantigen load | Low mutational load results in lack of antigenic proteins and increased subclonal mutation/neoantigens loads are associated with poor response. Adaptive resistance may occur with variation of neoantigen repertoire | [9,74,75] |
| PTEN loss | Loss of PTEN causes oncogenic expression of PI3K pathway, which reduces tumor infiltrating lymphocytes | [76] |
| Upregulation of compensatory checkpoints (LAG3) | Increased expression is accompanied by decreased T-cell effector function; as an immune inhibitory molecule, synergistically interacts with PD-1 to regulate T-cell function to promote tumoral immune escape | [77,78] |
| JAK1 and JAK2 mutations | Inactivation of JAK1 or JAK2 allows cancer cells to escape immune recognition via receptor-level signaling bottlenecks – selectively blocks IFN-γ signaling that leads to cell-growth inhibition | [79] |
| CTNNB1/Wnt/β-catenin mutations | Gain of function mutation potentially mediates acquired resistance by excluding T cells from immune activation. Tumors in which this pathway is active are relatively ‘cold’ and less likely to respond to PD-1/PD-L1 | [80,81] |
| Expression of MHC antigens | MHC is significantly downregulated in anti-PD-1 resistant tumors. Multiple routes to loss of expression. Whereas β-2-microglobulin mutations predominate in Lynch syndrome, the inactivation of various antigen-presenting machinery components differs in sporadic cancers. Different expression levels affect the ability of natural killer T-cells to engage tumor cells | [82,83] |
| β-2-microglobulin mutations | Loss of function mutations alter antigen presentation genes can result in tumor evasion of immune response. β-2-microglobulin is responsible for proper MHC class I folding and transport to cell surface required for CD8+ T-cell recognition | [84,85] |
| Overexpression of TGFβ | Expression of TGFβ enhances the function of T-regulators, limiting the infiltration of T cells. TGFβ also downregulates the activity of cytotoxic lymphocytes and natural killer cells | [86,87] |
Primary resistance to checkpoint inhibitors are well described and are likely due to lack of recognition by T cells due to absence of tumor antigens. Of more interest are the mechanisms developed, either from alterations in the antigen presenting machinery or expression of certain genes that prevent immune infiltrate within the microenvironment. These may exist at the time of initial presentation following use of immunotherapy (primary resistance) or may evolve later (adaptive resistance). Multiple tumor-intrinsic or host-specific mechanisms have been identified and are included in Table 1.
DNA repair-deficient tumors could potentially have changes in signaling leading an inflammatory tumor micro-environment, through altered cytokine or chemokine expression [57]. Others have suggested that high levels of DNA damage in MMR-deficient tumors are associated with high levels of cellular stress that are sensed by the host immune system.
Other important mechanisms of resistance, of likely importance in prostate cancer include loss of MHC class I expression, PTEN loss, and LAG3, JAK1/2, Wnt, B2M and TGF β as resistance mechanisms (Table 1). Although they are beyond the scope of this Review, loss of MHC class I expression is a common immune evasion mechanism documented in metastatic prostate cell lines [88] and PTEN loss is frequent in prostate cancer and carries a poor prognosis [89]. Findings from melanoma literature correlate PTEN loss with decreased tumor infiltration, decreased immunogenicity and poorer outcomes to immunotherapy. These data have not yet been validated in prostate cancer, though is intriguing given the frequency of PTEN loss in prostate cancer.
Varied host and tumor characteristics contribute to tumor resistance. Research into mechanisms of resistance will elucidate the complex interactions between prostate cancer and the immune system. Additional patient populations of interest for study are early responders who relapse over time despite continued therapy and exceptional responders who maintain therapeutic benefit. Continued study investigating loss of T-cell function, loss of T-cell recognition and development of escape mutation variants remain of great interest.
Where do we go from here? Implications for care & future research questions
Despite the generally disappointing results in clinical trials thus far, immune-based therapy remains promising in prostate cancer. Trials should incorporate genomic sequencing results to better define patient selection and responses. Though DNA sequencing is gaining traction in patients with mCRPC, it is not yet considered standard and is not readily available in the community. Many questions remain regarding optimal tissue for study of MMR deficiency and/or MSI. Fresh biopsies are often the requested sample and are reasonable as they account for the evolutionary history of the tumor. However, given the frequency of bone-only disease in prostate cancer, and poor yield from these metastatic sites, alternate methods of detection are needed. For this purpose, circulating tumor DNA (ctDNA) remains promising and may eliminate the need for tissue biopsy or archival tissue altogether [59].
A recent study evaluated a combination of targeted and low-pass whole genome sequencing on plasma cell-free DNA and matched germline DNA in 217 metastatic prostate cancer patients. ctDNA was detected in 85.9% of baseline samples. Comprehensive profiling of the androgen receptor revealed continuous evolution of the receptor during the course of disease. The blood samples were able to detect alterations in DNA repair genes and MSI. This study demonstrated for the first time, that MSI phenotype may be detected directly from cell-free DNA (MSI-high status was found in 3.8% of evaluable samples). CtDNA analysis may enable acceleration into clinical trials, though it should be in combination with synchronous profiling of whole genome sequencing for accuracy. The technology adds an additional benefit in the ability to capture genomic events over the life cycle of cancer.
In addition to improving detection at metastatic sites, a better understanding of disease biology and phenotype is needed. Why some tumors become enriched in MMR mutations is not fully understood. Future studies examining samples from primary and metastatic sites across the disease spectrum should be considered. Genomic sequencing and sampling of tumors both before and after therapy will aid our understanding of the biology of prostate cancer, mechanisms of resistance, and in whom immune-based therapy is appropriate. As of this time, there remains no signature suggesting who will definitively respond to anti-PD-1 therapy, even among those with MMR-deficiency and MSI-high status. Correlation of PD-1/PD-L1 expression is conflicting and our understanding of disease biology premature [90,91]. A summary of some of the documented or presumed mechanisms of primary or acquired immunotherapy resistance among patients with dMMR cancers is shown in Table 1.
One hypothesis is that PD-L1 expression may be dynamic in prostate cancer, though prospective comparisons of metastatic sites during progression are needed to validate this theory. One IHC study of PD-L1 expression in prostate cancer demonstrated 7.7% of primary tumors and 32.1% of mCRPC express PD-L1 [62]. In a parallel study, resistance to enzalutamide, an antiandrogen commonly used in the treatment of mCRPC, was associated with increased PD-L1 expression [92]. In this study, patients who progressed while on enzalutamide had a significantly increased number of PD-L1 positive dendritic cells in their blood compared with treatment-naive patients. Thus, by blocking PD-L1, pembrolizumab may enhance the immune response to resistant cells that emerge from treatment such as enzalutamide. The pattern of expression of PD-L1 in mCRPC suggests therapy might have a role in appropriately selected cases of prostate cancer and improved trial design is needed to identify an appropriate cohort of patients.
Tumors with high somatic mutational load have proven to have greater and more sustained responses from immune checkpoint inhibitors across many tumor types. However, it is also known that the prevalence of somatic mutations is highly variable between and within cancer types, with prostate cancer having a low mutational burden in general. Further studies examining the role of varied histologies, hypermutated phenotype, tumor-infiltrating lymphocyte density, or TMB would clarify which biomarkers signal extreme sensitivity to immunotherapy approaches, as demonstrated in various other disease types. The role of other therapies for prostate cancer, such as chemotherapy, radiation therapy, or antiandrogens and their synergy with or antagonism to immune-based therapy remains unclear. Optimal timing and sequence of treatments also requires exploration. We speculate that immunotherapy should be studied early in the treatment sequence rather than reserving its use for heavily pretreated tumors, given the selective pressures and resistance acquired over multiple lines of therapy.
To aid future patients, it is important to identify predictive biomarkers and generate information that will better guide immune-based therapies. Biospecimen collection via blood components, tumor material and cellular components, and other circulating molecules will assist in our understanding of disease. Such research will evaluate whether genetic variation within clinical trial populations correlate with response to treatments under evaluation. Moving forward, MSI will continue to be evaluated as it remains an important biomarker for some cancers. Tumor and blood analyses should be performed to define gene signatures that correlate to clinical responses with immune-based treatment. Additional tumor or blood-derived proteins are likely to develop and improve our understanding of response to immunotherapy. This novel development may be a major advance, especially in prostate cancer, given the difficulties of today’s reliance on assessing tumor biomarkers.
Conclusion
In the last few years, our understanding of the molecular alterations that define prostate cancer have improved, though not enough to truly personalize patient care. Precision oncology can only advance with improvements in tumor sample acquisition. Technical limitations likely underestimate MMR mutations in prostate cancer and therefore, therapeutic benefits of targeted therapy in select patients remains premature. While clinically validated assays for dMMR via IHC, PCR or next-generation sequencing exist, accurate assessment has proved complex. Targeted sequencing requires thorough understanding of the relative contribution of involved proteins in the pathway and may miss altered expression through genomic rearrangements, epigenetic alterations or promoter mutations. Basket trials focused on patients with MMR-defective cancers may enhance our understanding of response to immunotherapy. Due to the variable prevalence reported in different prostate cancer studies, next-generation sequencing should become standard and ctDNA approaches remain promising. Of note, the National Comprehensive Cancer Network guidelines for prostate cancer were recently amended to include consideration of MSI-H/dMMR testing and pembrolizumab treatment for MSI-H/dMMR mCRPC in the second-line setting or beyond [42]. The guidelines further stated that clinicians should consider genetic testing for all patients with metastatic, regional, very high-risk disease or high-risk disease regardless of family history. For those with lower risk disease, the guidelines also recommend consideration of testing when a strong family history exists. Interestingly, in the recent JAMA Oncology publication, 229 individuals (37%) with positive variants in their cohort would not have been approved for genetic testing using the NCCN genetic/familial breast and ovarian guidelines for patients with prostate cancer [41].
As patients are living longer and accruing more comorbidity, clinicians are more likely to encounter frail patients. As PD-1/PD-L1 inhibitors (when used as monotherapies) tend to be generally well tolerated, these therapies remain an attractive approach, though finding the right setting remains important. The use of these medications for advanced prostate cancer should be encouraged in the setting of clinical trials. Investigators will hopefully design these assessments in such a way that allows development of a biomarker panel that predicts benefit and response of these and other checkpoint inhibitors.
Future perspective
What will prostate cancer immunotherapy look like 5 years from now? We believe that PD-1/PD-L1 inhibitors will be approved to treatment some proportion of advance prostate cancer patients; perhaps those with germline and/or somatic DNA repair defects or other molecular markers of immunotherapy sensitivity. We also anticipate a better understanding and stronger data to support the use of these medications in men with dMMR. Some pathologic and histologic factors may potentially help identify these subgroups, including Gleason pattern 5 disease, ductal/intraductal histology and perhaps AR-V7–positive prostate cancers. This field will mature following well-designed studies preselecting patients with a high chance of deriving benefit from PD-1/PD-L1 blockade. In addition, the combination of PARP inhibitors, a class of drug which blocks another important DNA-repair pathway, may act synergistically and remains an intriguing combinatorial strategy.
Further study is also needed to better understand the role of the androgen receptor in the development of MSH2 and MSH6 structural alterations. Clinical trials examining clinical course, patient responsiveness and sequencing of treatments, especially with relation to immune-based therapies are needed. Genomic profiling of tumors will influence treatment selection of advanced prostate cancer treatment in future years. Linking genomics to immunologic features will remain a major interest in the field of immuno-oncology. The prognostic and therapeutic importance of germline versus somatic DNA repair alterations, and the evolution of tumor genomics over time are important areas for research development.
A revised molecular taxonomy will one day better predict immunotherapy responses in prostate cancer. In addition to MMR deficiency, this may include inactivating mutations in the CDK12 gene [93,94], exonuclease domain mutations in the DNA polymerase genes POLE and POLD1 [95,96], deletion of the 3′-untrasnslated region of the CD274 (PD-L1) locus [97], and perhaps inactivation of homologous recombination DNA repair genes [98]. Further studies exploring mechanisms of resistance, tumor antigen-presenting machinery and tumor-extrinsic factors remain challenges in our future. Any serious conversation regarding expansion of germline and somatic testing requires thoughtful consideration of the systems and financial issues such an expansion would create. Yet, if targeted therapies hold promise, the ends may justify the means, even from a financial point of view. With these paradigm shifts occurring in the prostate cancer landscape, we are hopeful to find the right treatment, for the right patient, at the right time.
Executive summary.
Prostate cancers with deficiencies in mismatch repair (MMR) are characterized by sequence alterations in microsatellites and can accumulate thousands of mutations. This high mutational burden renders tumors immunogenic and sensitive to immunotherapy. Treatment with PD-1 inhibitors are warranted for patients with MMR alterations and is approved by the US FDA.
The presence of both germline and somatic mutations is present in advanced prostate cancer; who to test and how to test is subject to considerable debate and in need of standardization.
The prevalence of men with metastatic prostate cancer carrying pathologic variants of MMR deficiency is enough to consider testing. Germline and somatic pathogenic alterations may have therapeutic implications.
Whether prostate cancer is a Lynch-associated malignacy can be debated, although we believe that there is adequate evidence implicating germline MSH2 and MSH6 mutations in prostate cancer predisposition. Patients found to harbor tumor MMR gene mutations should be referred for genetic counseling and germline genetic testing.
Though metastatic biopsies may pose difficulty with specimen acquisition, they remain the gold standard. Tumor sampling techniques and circulating tumor cell sequencing methods are not yet agreed upon in the research realm or as standard of care.
Assays are largely tissue based and prostate metastatic tissue is hard to obtain, there is a high need for developing assays that are blood based.
Patients with a Gleason score of ≥8, with unusual sites of metastases, and variant histologies are more likely to harbor deficiencies in MMR. In the metastatic castration-resistant setting, we support somatic mutational testing.
Unknown are the prognostic and therapeutic impact of germline versus somatic DNA mutations. It is not yet clear if enrichment in metastatic sites and castration-resistant disease is due to tumor evolution in response to therapy exposure or whether it serves as a surrogate marker of more aggressive prostate cancer. Prospective studies looking at whether this molecular classification results in clinically relevant stratification for prognosis and treatment response are needed.
Footnotes
Financial & competing interests disclosure
ES Antonarakis: Janssen, Astellas, Sanofi, Dendreon, Medivation, ESSA, AstraZeneca, Amgen, Clovis, Merck (consulting/advisory), Janssen, Johnson & Johnson, Sanofi, Dendreon, Genentech, Novartis, Tokai, Bristol-Myers Squibb, AstraZeneca, Celgene, Clovis, Merck (research funding), Qiagen (intellectual property). Consulting/advisory relationship; research funding; employment; expert testimony; honoraria received; ownership interests; intellectual property rights/inventor/patent holder; scientific advisory board. This work was partially supported by NIH Cancer Center Support Grant P30 CA006973, and Department of Defense grant W81XWH-16-PCRP-CCRSA. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J. Clin. 67(1), 7–30 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N. Engl. J. Med. 357(26), 2696–2705 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N. Engl. J. Med. 375(18), 1804–1805 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 144(5), 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 138(6), 2073–2087.e2073 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackay J, Szecsei CM. Genetic counselling for hereditary predisposition to ovarian and breast cancer. Ann. Oncol. 21(Suppl. 7), vii334–vii338 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Brown JS, O'Carrigan B, Jackson SP, Yap TA. Targeting DNA repair in cancer: beyond PARP inhibitors. Cancer Discov. 7(1), 20–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene 27(49), 6313–6321 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Le DT, Durham JN, Smith KN. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 357(6349), 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• With objective response rates in 53% of patients, this study highlighted the efficacy of PD-1 blockade in patients with advanced mismatch repair-deficient cancers.
- 10.Diaz LA, Le DT. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 373(20), 1979 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Le DT, Uram JN, Wang H. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372(26), 2509–2520 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US FDA. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. (2017). www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm560040.htm
- 13.Robinson D, Van Allen EM, Wu YM. et al. Integrative clinical genomics of advanced prostate cancer. Cell 162(2), 454 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Schweizer MT, Cheng HH, Tretiakova MS. et al. Mismatch repair deficiency may be common in ductal adenocarcinoma of the prostate. Oncotarget 7(50), 82504–82510 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature 411(6835), 366–374 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 15(3), 181–194 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Leach FS, Nicolaides NC, Papadopoulos N. et al. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 75(6), 1215–1225 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N. Engl. J. Med. 348(10), 919–932 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Palomaki GE, McClain MR, Melillo S, Hampel HL, Thibodeau SN. EGAPP supplementary evidence review: DNA testing strategies aimed at reducing morbidity and mortality from Lynch syndrome. Genet. Med. 11(1), 42–65 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand M, Prolla TA, Liskay RM, Petes TD. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature 365(6443), 274–276 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Peltomäki P, Lothe RA, Aaltonen LA. et al. Microsatellite instability is associated with tumors that characterize the hereditary non-polyposis colorectal carcinoma syndrome. Cancer Res. 53(24), 5853–5855 (1993). [PubMed] [Google Scholar]
- 22.Lawes DA, SenGupta S, Boulos PB. The clinical importance and prognostic implications of microsatellite instability in sporadic cancer. Eur. J. Surg. Oncol. 29(3), 201–212 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Bauer CM, Ray AM, Halstead-Nussloch BA. et al. Hereditary prostate cancer as a feature of Lynch syndrome. Fam. Cancer 10(1), 37–42 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raymond VM, Mukherjee B, Wang F. et al. Elevated risk of prostate cancer among men with Lynch syndrome. J. Clin. Oncol. 31(14), 1713–1718 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bratt O. What should a urologist know about hereditary predisposition to prostate cancer? BJU Int. 99(4), 743–747 discussion 747–748 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Win AK, Lindor NM, Young JP. et al. Risks of primary extracolonic cancers following colorectal cancer in lynch syndrome. J. Natl Cancer Inst. 104(18), 1363–1372 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard CC, Morrissey C, Kumar A. et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat. Commun. 5, 4988 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identified parallels and differences in mechanisms of hypermutation in prostate cancer compared with other microsatellite instability-associated cancers.
- 28. Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163(4), 1011–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Win AK, Young JP, Lindor NM. et al. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J. Clin. Oncol. 30(9), 958–964 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haraldsdottir S, Hampel H, Wei L. et al. Prostate cancer incidence in males with Lynch syndrome. Genet. Med. 16(7), 553–557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grindedal EM, Møller P, Eeles R. et al. Germ-line mutations in mismatch repair genes associated with prostate cancer. Cancer Epidemiol. Biomarkers Prev. 18(9), 2460–2467 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Wang J, Fraig MM. et al. Defects of DNA mismatch repair in human prostate cancer. Cancer Res. 61(10), 4112–4121 (2001). [PubMed] [Google Scholar]
- 33.Egawa S, Uchida T, Suyama K. et al. Genomic instability of microsatellite repeats in prostate cancer: relationship to clinicopathological variables. Cancer Res. 55(11), 2418–2421 (1995). [PubMed] [Google Scholar]
- 34.Uchida T, Wada C, Wang C. et al. Microsatellite instability in prostate cancer. Oncogene 10(5), 1019–1022 (1995). [PubMed] [Google Scholar]
- 35.Antonarakis ES, Shaukat F, Isaacsson Velho P. et al. Clinical features and therapeutic outcomes in men with advanced prostate cancer and DNA mismatch repair gene mutations. Eur. Urol. 75(3), 378–382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Complement to the Abida paper, highlights outcomes of men with advanced prostate cancer and DNA mismatch repair mutations.
- 36.Isaacsson Velho P, Silberstein JL, Markowski MC. et al. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. Prostate 78(5), 401–407 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pritchard CC, Mateo J, Walsh MF. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375(5), 443–453 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This multicenter study highlighted the incidence of germline mutations in genes mediating DNA-repair processes in men with metastatic prostate cancer, as compared with localized prostate cancer.
- 38.Robinson D, Van Allen EM, Wu YM. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161(5), 1215–1228 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guedes LB, Antonarakis ES, Schweizer MT. et al. MSH2 loss in primary prostate cancer. Clin. Cancer Res. 23(22), 6863–6874 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenderov EIP, Velho PI, Awan A. et al. Genomic characterization of pulmonary-metastatic prostate cancer: a unique molecular subtype. J. Clin. Oncol. 37, 210–211 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicolosi P, Ledet E, Yang S. et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 5(4), 523–528 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Prostate Cancer Version 2.2018 www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- 43.Mygatt JG, Osborn DJ. DNA-repair gene mutations in metastatic prostate cancer. N. Engl. J. Med. 375(18), 1803–1804 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Azzouzi AR, Catto JW, Rehman I. et al. Clinically localised prostate cancer is microsatellite stable. BJU Int. 99(5), 1031–1035 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Rodrigues DN, Rescigno P, Liu D. et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Invest. 128(11), 5185 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fraser M, Sabelnykova VY, Yamaguchi TN. et al. Genomic hallmarks of localized, non-indolent prostate cancer. Nature 541(7637), 359–364 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Armenia J, Wankowicz SAM, Liu D. et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50(5), 645–651 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X, Wu N, Grignon D. et al. High frequency of mutator phenotype in human prostatic adenocarcinoma. Oncogene 9(10), 2999–3003 (1994). [PubMed] [Google Scholar]
- 49.Antonarakis ES. A new molecular taxonomy to predict immune checkpoint inhibitor sensitivity in prostate cancer. Oncologist 24(4), 430–432 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Highlights advances in our understanding of PD-1 inhibition in prostate cancer.
- 50.Goodfellow PJ, Billingsley CC, Lankes HA. et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. 33(36), 4301–4308 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 22(11), 1342–1350 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Bacher JW, Flanagan LA, Smalley RL. et al. Development of a fluorescent multiplex assay for detection of MSI-High tumors. Dis. Markers 20(4–5), 237–250 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palmieri G, Colombino M, Cossu A, Marchetti A, Botti G, Ascierto PA. Genetic instability and increased mutational load: which diagnostic tool best direct patients with cancer to immunotherapy? J. Transl. Med. 15(1), 17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hempelmann JA, Scroggins SM, Pritchard CC, Salipante SJ. MSI plus for integrated colorectal cancer molecular testing by next-generation sequencing. J. Mol. Diagn. 17(6), 705–714 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Teply BA, Antonarakis ES. Treatment strategies for DNA repair-deficient prostate cancer. Expert Rev. Clin. Pharmacol. 10(8), 889–898 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pritchard CC, Morrissey C, Kumar A. et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat. Commun. 5, 1–6 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • MSI-H prostate cancer relatively common in this dataset, with description of unique comp rearrangements as underlying mechanism.
- 57.Kelderman S, Schumacher TN, Kvistborg P. Mismatch repair-deficient cancers are targets for anti-PD-1 therapy. Cancer Cell 28(1), 11–13 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Abida W, Cheng ML, Armenia J. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA 5(4), 471–478 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Largest study to date evaluating the prevalence of microsatellite instability in prostate cancer and its association with response to immune checkpoint blockade.
- 59.Mayrhofer M, De Laere B, Whitington T. et al. Cell-free DNA profiling of metastatic prostate cancer reveals microsatellite instability, structural rearrangements and clonal hematopoiesis. Genome Med. 10(1), 85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abida W, Gopalan A, Armenia J. et al. Identifying mismatch repair-deficient prostate cancer for immune checkpoint therapy. J. Clin. Oncol. 36(15 Suppl.), 5020–5021 (2018). [Google Scholar]
- 61.Alexandrov LB, Nik-Zainal S, Wedge DC. et al. Signatures of mutational processes in human cancer. Nature 500(7463), 415–421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haffner MC, Guner G, Taheri D. et al. Comprehensive evaluation of programmed death-ligand 1 expression in primary and metastatic prostate cancer. Am. J. Pathol. 188(6), 1478–1485 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fankhauser CD, Schüffler PJ, Gillessen S. et al. Comprehensive immunohistochemical analysis of PD-L1 shows scarce expression in castration-resistant prostate cancer. Oncotarget 9(12), 10284–10293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kantoff PW, Higano CS, Shore ND. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363(5), 411–422 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Rexer H, Graefen M, Merseburger A. [Phase II study of pembrolizumab (MK-3475) in patients with metastatic castration-resistant prostate cancer (KEYNOTE-199)-study AP 93/16 of the AUO]. Urologe A 56(11), 1471–1472 (2017). [DOI] [PubMed] [Google Scholar]
- 66.Attalla K, Sfakianos JP, Galsky MD. Current role of checkpoint inhibitors in urologic cancers. Cancer Treat Res. 175, 241–258 (2018). [DOI] [PubMed] [Google Scholar]
- 67.De Bono JS, Goh JC, Ojamaa K. et al. KEYNOTE-199: pembrolizumab (pembro) for docetaxel-refractory metastatic castration-resistant prostate cancer. J. Clin. Oncol. 36(15), 5007–5008 (2018). [Google Scholar]
- 68.Hansen AR, Massard C, Ott PA. et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann. Oncol. 29(8), 1807–1813 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Kwon ED, Drake CG, Scher HI. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, Phase III trial. Lancet Oncol. 15(7), 700–712 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Beer TM, Kwon ED, Drake CG. et al. Randomized, double-blind, Phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J. Clin. Oncol. 35(1), 40–47 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Topalian SL, Hodi FS, Brahmer JR. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366(26), 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smits M, Westdorp H, Gorris M. et al. Correlates of response to anti-PD-1 immune checkpoint blockade in mismatch repair proficient and deficient patients with metastatic castration resistant prostate cancer. ASCO Meeting Abstracts 5036, (2018). [Google Scholar]
- 73.Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA, Kinzler KW. Cancer genome landscapes. Science 339(6127), 1546–1558 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rizvi NA, Hellmann MD, Snyder A. et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348(6230), 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miao D, Margolis CA, Vokes NI. et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 50(9), 1271–1281 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millis SZ, Ikeda S, Reddy S, Gatalica Z, Kurzrock R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol. 2(12), 1565–1573 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Grosso JF, Goldberg MV, Getnet D. et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J. Immunol. 182(11), 6659–6669 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woo SR, Turnis ME, Goldberg MV. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72(4), 917–927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shin DS, Zaretsky JM, Escuin-Ordinas H. et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7(2), 188–201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klempner SS, Schrock A, Ali S. et al. Acquired CTNNB1 mutation drives immune checkpoint inhibitor-acquired resistance in a microsatellite instability-high gastroesophageal adenocarcinoma with brain metastases. JCO Precis. Oncol. 1–8 (2019). [DOI] [PubMed] [Google Scholar]
- 81.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dierssen JW, de Miranda NF, Ferrone S. et al. HNPCC versus sporadic microsatellite-unstable colon cancers follow different routes toward loss of HLA class I expression. BMC Cancer 7, 33 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaretsky JM, Garcia-Diaz A, Shin DS. et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 375(9), 819–829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sade-Feldman M, Jiao YJ, Chen JH. et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 8(1), 1136 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X, Schoenhals JE, Li A. et al. Suppression of type I IFN signaling in tumors mediates resistance to anti-PD-1 treatment that can be overcome by radiotherapy. Cancer Res. 77(4), 839–850 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8(5), 369–380 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Baas M, Besançon A, Goncalves T. et al. TGFβ-dependent expression of PD-1 and PD-L1 controls CD8(+) T cell anergy in transplant tolerance. Elife 5, e08133 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Found that five marker microsatellite instability-PCR panel had inferior sensitivity when applied to prostate cancer and that next-generation sequencing testing with expanded panel of markers performs well.
- 88.Kaur HB, Guedes LB, Lu J. et al. Association of tumor-infiltrating T-cell density with molecular subtype, racial ancestry and clinical outcomes in prostate cancer. Mod. Pathol. 31(10), 1539–1552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheng F, Eng C. PTEN mutations trigger resistance to immunotherapy. Trends Mol. Med. 25(6), 461–463 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Massari F, Ciccarese C, Caliò A. et al. Magnitude of PD-1, PD-L1 and T lymphocyte expression on tissue from castration-resistant prostate adenocarcinoma: an exploratory analysis. Target Oncol. 11(3), 345–351 (2016). [DOI] [PubMed] [Google Scholar]
- 91.Gevensleben H, Dietrich D, Golletz C. et al. The immune checkpoint regulator PD-L1 is highly expressed in aggressive primary prostate cancer. Clin. Cancer Res. 22(8), 1969–1977 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Bishop JL, Sio A, Angeles A. et al. PD-L1 is highly expressed in enzalutamide resistant prostate cancer. Oncotarget 6(1), 234–242 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu YM, Cieślik M, Lonigro RJ. et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173(7), 1770–1782.e1714 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Antonarakis ES. Cyclin-dependent kinase 12, immunity, and prostate cancer. N. Engl. J. Med. 379(11), 1087–1089 (2018). [DOI] [PubMed] [Google Scholar]
- 95.Campbell BB, Light N, Fabrizio D. et al. Comprehensive analysis of hypermutation in human cancer. Cell 171(5), 1042–1056.e1010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee L, Ali S, Genega E. et al. Aggressive-variant microsatellite-stable POLE mutant prostate cancer with high mutation burden and durable response to immune checkpoint inhibitor therapy. JCO Precis. Oncol. 1–10 (2018). [DOI] [PubMed] [Google Scholar]
- 97.Kataoka K, Shiraishi Y, Takeda Y. et al. Aberrant PD-L1 expression through 3′-UTR disruption in multiple cancers. Nature 534(7607), 402–406 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Boudadi K, Suzman DL, Anagnostou V. et al. Ipilimumab plus nivolumab and DNA-repair defects in AR-V7-expressing metastatic prostate cancer. Oncotarget 9(47), 28561–28571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]