Abstract
Aim:
This study evaluated the overall survival (OS) of older patients (≥60 years) with acute myeloid leukemia based on the intensity of treatment.
Methods:
This single center, retrospective study included 211 patients diagnosed between 2000 and 2016, who received 10-day decitabine, low-intensity therapy or high-intensity therapy. Cox regression examined the impact of therapy on OS.
Results:
Younger patients were more likely to receive high-intensity therapy. Patients who received low-intensity therapy had worse OS compared with high-intensity therapy (median OS: 1.2 vs 8.5 months; p < 0.01). OS was similar with 10-day decitabine (median OS of 6.3 months) compared with either low-intensity therapy or high-intensity therapy.
Conclusion:
Ten-day decitabine is an effective alternative in older patients with newly diagnosed acute myeloid leukemia.
Keywords: : 10-day decitabine, acute myeloid leukemia, frail patients, hypomethylating agents, older adults, overall survival
The management of acute myeloid leukemia (AML) is complex in older patients because of associated comorbidities, intolerance to high-dose chemotherapy and high-risk tumor biology. For example, in clinical practice, over half of the patients aged 60 years and older do not receive initial chemotherapy for AML. Consequent to such complexities of AML in older patients and current practice patterns, only 10–20% of patients are alive at 3–5 years [1–6]. Despite decades of research, overall survival (OS) has not improved significantly in last few decades.
In older patients with AML, practical and rational therapy selection is crucial to receive treatment that is most likely to benefit an individual patient [2]. Select patients can tolerate intensive therapy and subsequently achieve high rates of complete remission and long-term OS [7,8]. However, the identification of these patients can be difficult. Many older patients have significant comorbidities requiring multiple medications, have baseline cognitive impairment, are malnourished and may be physically debilitated [7–10]. The use of intensive chemotherapy in such patients may result in significant toxicities, poor quality of life, deterioration in physical and neurocognitive status and high early mortality [10]. Discriminating patients that may benefit from intensive chemotherapy for newly diagnosed AML can be difficult.
The use of hypomethylating agents such as decitabine therapy for a 5-day or a 7-day course of azacitidine has been extensively studied in older, unfit patients with AML [11–13]. Even in these studies, hypomethylating agents have achieved OS comparable to that achieved with intensive chemotherapy, despite a lower probability of complete remission with hypomethylating agents [11–13]. Recent studies have demonstrated relatively high rates of complete remission when the duration of decitabine is extended to 10 days [14–16]. The use of 10-day decitabine therapy, compared with intensive chemotherapy, has emerged as an option that may result in lower risk of toxicities and functional decline while achieving good complete remission rates. In a few studies, the use of 10-day decitabine has resulted in up to 40–50% complete response rate in newly diagnosed high-risk AML [14–16]. In the current report, we evaluated treatment practices and the effect of treatment intensity on OS in older patients newly diagnosed with AML. Our study demonstrates 10-day decitabine as a useful alternative to high-intensity chemotherapy in older patients.
Methods
A total of 211 patients older than 60 years of age with a diagnosis of AML diagnosed between the years of 2000 and 2016 were identified via a query of the electronic health record at the University of Nebraska Medical Center. Patients were divided into three groups based on the intensity of therapy received. Low-intensity regimens included 5 days of decitabine, 5- or 7-day azacitadine or low-dose cytarabine. High-intensity regimens included cytarabine plus an anthracycline (7 + 3) or fludarabine plus cytarabine, with or without gemtuzumab ozogamicin. The third group included patients that received 10 days of decitabine alone. Descriptive statistics (means, standard deviations, medians, interquartile ranges, frequencies and percentages) were used to summarize demographic and clinical characteristics. Fisher’s exact test was used to look at the association of type of therapy with other patient characteristics. OS was determined by the Kaplan–Meier method and comparisons of survival curves was done using the log-rank test. OS was defined as the time from diagnosis of AML to death from any cause. Cox regression was used to look at the impact of therapy on OS adjusting for covariates that were statistically significantly associated with type of therapy on univariate analysis. p < 0.05 was considered statistically significant. The project was reviewed and approved by the University of Nebraska Medical Center institutional review board.
Results
Patient characteristics
Most patients were aged 60–69 years old (52.6%), were male (52.1%) and Caucasian (97.2%; Table 1). Most patients had intermediate (55%, n = 104) or adverse (40.2%, n = 76) cytogenetics. Most patients had a Karnofsky Performance Score (KPS) of 90–100 (55.9%, n = 109) and of the 198 patients with a hematopoietic cell transplant comorbidity index (HCT CI) available, 90% had a score of 4 or less (n = 178).
Table 1. . Baseline patient characteristics and factors associated with the use of varying intensity of chemotherapy.
| Variable (%) | Therapy | p-value | ||
|---|---|---|---|---|
| 10-day decitabine | Low intensity | High intensity | ||
| Age: | ||||
| – 60–69 (52.6%) | 13 (41%) | 10 (21%) | 88 (67%) | <0.0001 |
| – 70+ (47.4%) | 19 (59%) | 37 (79%) | 44 (33%) | |
| Sex: | ||||
| – Female (47.9%) | 23 (72%) | 22 (46.8%) | 56 (42%) | 0.0099 |
| – Male (52.1%) | 9 (28%) | 25 (53.2%) | 76 (58%) | |
| Race: | ||||
| – White (97.2%) | 30 (94%) | 47 (100%) | 129 (99%) | 0.0957 |
| – Non-white (2.8%) | 2 (6%) | 0 (0%) | 1 (<1%) | |
| Cytogenetic risk categories: | ||||
| – Adverse (40.2%) | 10 (35%) | 24 (57%) | 42 (36%) | 0.1270 |
| – Favorable (4.8%) | 1 (3%) | 2 (5%) | 6 (5%) | |
| – Intermediate (55.0%) | 18 (62%) | 16 (38%) | 70 (59%) | |
| Hematopoietic transplant comorbidity index: | ||||
| – 0 (31.3%) | 10 (33%) | 11 (24%) | 41 (33%) | 0.1996 |
| – 1–2 (28.3%) | 11 (37%) | 14 (31%) | 31 (25%) | |
| – 3–4 (30.3%) | 8 (27%) | 11 (24%) | 41 (33%) | |
| – 5+ (10%) | 1 (3%) | 9 (21%) | 10 (8%) | |
| Karnofsky score: | ||||
| – <60 (8.2%) | 1 (3.4%) | 5 (12.2%) | 10 (8%) | 0.507 |
| – 70–80 (35.9%) | 14 (48.3%) | 14 (34.2%) | 42 (33.6%) | |
| – 90–100 (55.9%) | 14 (48.3%) | 22 (53.6%) | 73 (58.4%) | |
| Diagnosis year: | ||||
| – 2000–2007 (36.2%) | 1 (3%) | 14 (30%) | 61 (46%) | <0.0001 |
| – 2008–2016 (63.8%) | 31 (97%) | 33 (70%) | 71 (54%) | |
Intensity of therapy
One hundred and thirty-two patients (62.5%) received high-intensity therapy, 47 patients (22.3%) received low-intensity therapy and 32 patients (15.2%) received 10 days of decitabine.
Based on multivariate analyses, age at the time of diagnosis was a strong predictor of the receipt of a particular intensity of therapy with younger patients more likely to have received high-intensity therapy (p < 0.0001) and older patients more likely to receive 10-day decitabine (p < 0.05) and low-intensity therapy (Supplementary Figure 1, panel A, p < 0.05). Males were more likely to have received high-intensity therapy while females were more likely to have received 10 days of decitabine (Table 1, p = 0.01). In the years 2008 or later, more patients received low-intensity therapy or 10 days of decitabine versus high-intensity therapy. Nearly all of the patients receiving 10 days of decitabine did so starting in 2008 (p < 0.0001).
Patients who received high-intensity therapy were taking significantly fewer medications than patients who received low-intensity therapy (Supplementary Figure 1, panel B, p < 0.05). Baseline renal function was also significantly different between the low-intensity and the 10-day decitabine groups (Supplementary Figure 1, panel C, p < 0.05). No significant difference was observed in multivariate analyses comparing intensity of therapy to cytogenetic risk category (p = 0.1), race (p = 0.1), HCT CI (p = 0.2) or KPS (p = 0.5; Table 1). Fewer patients who received 10 days decitabine and low intensity therapy proceeded to allogeneic transplantation when compared with those who received high intensity therapy (p < 0.01; Supplementary Table 1).
Survival outcomes
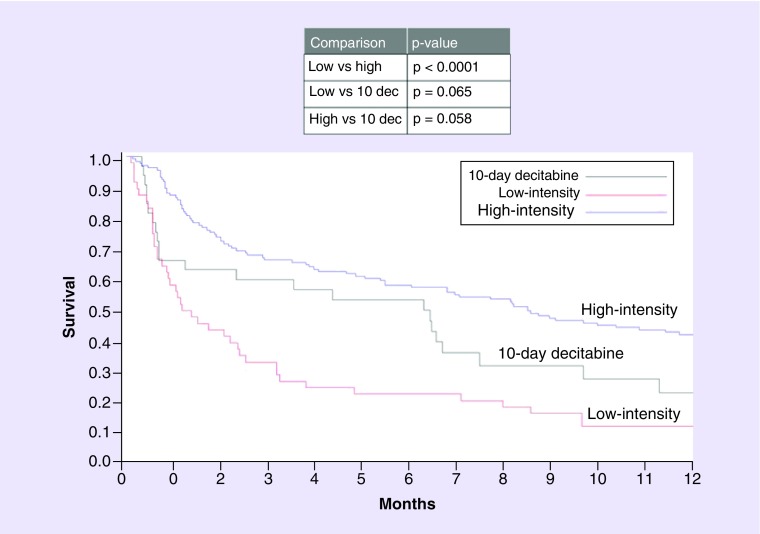
OS for the entire cohort was 70, 52 and 27% at 30 days, 90 days and 1-year post diagnosis, respectively. There was a significant difference in OS in patients who received low-intensity therapy versus high-intensity therapy (Table 2). In a univariate analysis, patients older than 70 years, those with adverse cytogenetics, a KPS score of 60 or less and an HCT CI of 3 or more fared worse (Table 3). In a multivariate analysis, the only patient characteristic that was significantly associated with OS was intermediate cytogenetics risk compared with adverse cytogenetic risk (Table 4). The effect of intensity of therapy on OS was analyzed via the Kaplan–Meier model. There was a significant difference in OS in patients who received low-intensity therapy compared with high-intensity therapy (Figure 1). There was no statistical difference in OS with 10-day decitabine compared with either low-intensity therapy or high-intensity therapy.
Table 2. . Survival estimates with 95% CIs at specific time points.
| Group | 30-day OS, 95% CI | 90-day OS, 95% CI | 1-year OS, 95% CI |
|---|---|---|---|
| 10-day decitabine | 66% (47–79%) | 59% (40–74%) | 22% (9–39%) |
| Low-intensity treatment | 57% (42–70%) | 32% (19–45%) | 11% (4–21%) |
| High-intensity treatment | 87% (80–92%) | 66% (57–73%) | 41% (32–49%) |
| All patients | 70% (64–76%) | 52% (45–58%) | 27% (22–33%) |
| All patients >2011 | 70% (60–78%) | 59% (48–68%) | 32% (23–42%) |
Overall, there was a statistically significant difference between the three groups (p < 0.001); specifically the groups that were different include low versus high-intensity treatment groups (p < 0.001).
OS: Overall survival.
Table 3. . Univariate analysis of overall survival.
| Univariate analysis of survival | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| High-intensity vs 10-day decitabine | 0.640 | 0.404–1.014 | 0.0576 |
| Low-intensity vs 10-day decitabine | 1.610 | 0.971–2.669 | 0.0646 |
| Favorable cyto risk vs adverse | 0.377 | 0.152– 0.935 | 0.0352 |
| Intermediate cyto risk vs adverse | 0.647 | 0.481– 0.869 | 0.0039 |
| 70+ years vs <70 years | 1.704 | 1.296–2.240 | 0.0001 |
| Karnofsky score ≤60 vs 90–100 | 2.530 | 1.615– 3.965 | <0.0001 |
| Co-morbidity index 3+ vs 0–2 | 1.393 | 1.055– 1.840 | 0.0195 |
Table 4. . Multivariate analysis of overall survival.
| Parameter | Hazard ratio | 95% hazard ratio confidence limits | p-value |
|---|---|---|---|
| High-intensity vs 10-day decitabine | 0.725 | 0.417–1.259 | 0.2538 |
| Low-intensity vs 10-day decitabine | 1.113 | 0.626–2.049 | 0.6803 |
| Favorable cyto risk vs adverse | 0.334 | 0.102–1.102 | 0.0716 |
| Intermediate cyto risk vs adverse | 0.608 | 0.429–0.862 | 0.0052 |
| 70 + years vs <70 years | 1.360 | 0.925–1.999 | 0.1182 |
| Karnofsky score ≤60 vs 90–100 | 1.708 | 0.942–3.096 | 0.0777 |
| Co-morbidity index 3+ vs 0–2 | 1.305 | 0.930–1.832 | 0.1233 |
Figure 1. . Kaplan–Meier’s curve demonstrating overall survival based on treatment.
dec: Decitabine.
Discussion
In this single-center retrospective study, most patients received high-intensity induction until the year 2008, when hypomethylating agents started to become popular. Age 70 years or older was the single most important predictor of using therapies other than high-intensity therapy. The absolute number of chronic medications at diagnosis and kidney function were both predictive of the intensity of therapy the patient received. While this study did not show any statistically significant association between intensity of therapy and comorbidities, KPS or cytogenetic risk categories, in the more recent years, cytogenetic risk status, comorbidities and functional status are increasingly being used to determine therapy selection. At our center, we currently use a pre-treatment geriatric assessment and cytogenetic risk categories to select between various therapies (low-intensity vs intensive chemotherapy options), and we are examining the feasibility and impact of such therapy selection strategies (NCT03226418).
Cytogenetic risk categories, age, KPS and HCT CI influenced OS in a univariate analysis. Adverse cytogenetics was the determinant of OS in a multivariate analysis. A Kaplan–Meier analysis demonstrated that patients who received low intensity therapies had worse OS than those treated with high-intensity therapies. Although the study only demonstrated older age as a predictor of receiving low-intensity therapies, other unmeasured selection biases are likely, as suggested by higher 90-day mortality risk in patients receiving 10-day decitabine. Furthermore, low-intensity therapy not only included hypomethylating agents but also other therapies such as low-dose cytarabine. The improved OS with intensive chemotherapy is consistent with the results of the Swedish Acute Leukemia Registry [17]. However, the results of a randomized clinical trial AZA-AML-001 demonstrated similarity between those treated with azacitidine versus conventional care regimen that included intensive chemotherapy [13]. In our study, no significant difference in OS was determined between 10-day decitabine and high-intensity therapy.
The mechanism of action of decitabine and azacitidine requires completion of the cell cycle to facilitate incorporation into DNA in order to lead to reduced methylation in progeny cells [18–20]. As a result, responses are theorized to take longer when compared with traditional cytotoxic chemotherapy. This concept is supported clinically with multiple cycles required to see maximal clinical response when hypomethylating agents are employed. For example, in patients who received decitabine, day 21 bone marrow biopsies revealed persistent disease in patients who eventually went on to a complete response with continued therapy [16]. In a study using 5 days of decitabine, the median time to complete response was approximately 4 months [21].
Others have demonstrated desirable outcomes utilizing the 10-day decitabine schedule with some demonstrating high rates of complete remission [14–16]. Importantly, hypomethylating agents such as decitabine therapy results in comparable remission rate within different risk categories of AML, thus indicating a possibility that such an approach may overcome the impact of high-risk cytogenetics [11,13–16,22]. The use of 10-day decitabine therapy has emerged as an option that may result in lower risk of toxicities and functional decline while achieving complete remission rate comparable to intensive chemotherapy in high-risk patients [23]. Consistent with the results of this study, a prior retrospective analysis also indicated a clinical equivalence of 10-day decitabine compared with high-intensity chemotherapy in older adults [24]. A Phase II study testing a novel HDAC inhibitor in addition to azacitidine produced a hematologic normalization rate twice the pre-defined historical comparison dataset (CALBG9221) in the 10-day azacitidine alone group [25], thus indicating a potential benefit of extending azacitidine to 10 days. In contrast, Short et al. demonstrated that in an older, unfit population, there was no difference in OS at 1 year in patients who received either 5 days of decitabine or 10 days in a randomized trial [26]. An ongoing Phase III randomized controlled trial is comparing 10-day decitabine versus 7 + 3 (NCT02172872) that is expected to further define the optimal therapy in older adults.
Our study provides additional support to the observation that 10-day decitabine may be a desirable therapy in older patients with newly diagnosed AML, particularly those with high-risk cytogenetics or mutation and suboptimal functional status. However, the data presented also needs to be interpreted with caution given a single-center retrospective design of the study and modest number of patients included. We analyzed all patients aged older than 60 years with AML newly diagnosed after the year 2000. Decitabine was approved by the US FDA in May of 2006. This contributed to the relatively modest number of patients that received 10-day decitabine in our study, thus affecting the power of the study. Among patients who went on to receive transplant, a significant minority were treated with decitabine compared with high-intensity therapy (3 vs 31 pts), hence the effect of transplant on survival could not be analyzed. In addition, nearly all of the patients included in the study were Caucasian, limiting the generalizability of this data to ethnic minorities with newly diagnosed AML.
Conclusion
In conclusion, age influenced selection of therapy intensity in our study population. We present data suggesting that 10-day decitabine is an effective alternative in older patients with newly diagnosed AML. AML in older adults frequently confers a poor prognosis, hence further studies are necessary to improve outcomes. Recently completed and ongoing studies will continue to define appropriate intensity of initial therapy for older adults, which may change with development of more effective low-intensity therapy.
Summary points.
The best therapy for older patients with newly diagnosed acute myeloid leukemia is not clearly defined.
Hypomethylating agents are effective in acute myeloid leukemia.
Methods
We performed a single center, retrospective study of 211 patients diagnosed between 2000 and 2016. Patients received 10-day decitabine, low-intensity therapy or high-intensity therapy.
We utilized cox regression to determine the effect of therapy on overall survival.
Results
Age significantly factored into the selection of the intensity of therapy in our cohort.
The use of 10-day decitabine was associated with overall survival that was not different than overall survival associated with high-intensity induction chemotherapy.
Discussion & conclusion
Within the limitations of this study, ten-day decitabine offers an effective alternative for newly diagnosed acute myeloid leukemia in older patients, particularly those who may not be able to tolerate intensive chemotherapy.
Future studies focused on development of novel therapies, trials comparing various drugs and studies aimed at developing therapy selection strategies will continue to define appropriate initial therapy for various subsets of older adults based on their functional status and disease characteristics.
Supplementary Material
Footnotes
Supplementary data
To view the supplementary data that accompany this paper please visit the journal website at: www.futuremedicine.com/doi/suppl/10.2217/fon-2019-0001
Financial & competing interests disclosure
VR Bhatt reports receiving consulting fees from Pfizer, CSL Behring, Agios, Incyte, Partner Therapeutics and Abbvie, and research funding from Incyte, Tolero Pharmaceuticals, Inc, and National Marrow Donor Program.The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Bhatt VR, Shostrom V, Gundabolu K, Armitage JO. Utilization of initial chemotherapy for newly diagnosed acute myeloid leukemia in the United States. Blood Adv. 2(11), 1277–1282 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatt VR, Gundabolu K, Koll T, Maness LJ. Initial therapy for acute myeloid leukemia in older patients: principles of care. Leuk. Lymphoma 59(1), 29–41 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Bhatt VR, Shostrom V, Giri S. et al. Early mortality and overall survival of acute myeloid leukemia based on facility type. Am. J. Hematol. 92(8), 764–771 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Upadhyay S, Dahal S, Khanal N, Bhatt VJ, Silberstein PT. Chemotherapy in elderly patients with acute myeloid leukemia (AML): analysis of socioeconomic factors using National Cancer Data Base (NCDB). Blood 124(21), 5267 (2014). [Google Scholar]
- 5.Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl. Health Econ. Health Policy 11(3), 275–286 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Alibhai SM, Leach M, Minden MD, Brandwein J. Outcomes and quality of care in acute myeloid leukemia over 40 years. Cancer 115(13), 2903–2911 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Muffly LS, Kocherginsky M, Stock W. et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 99(8), 1373–1379 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tawfik B, Pardee TS, Isom S. et al. Comorbidity, age, and mortality among adults treated intensively for acute myeloid leukemia (AML). J. Geriatr. Oncol. 7(1), 24–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepin HD, Geiger AM, Tooze JA. et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121(21), 4287–4294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum FR, Gundacker H, Head DR. et al. Age and acute myeloid leukemia. Blood 107(9), 3481–3485 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubbert M, Ruter BH, Claus R. et al. A multicenter Phase II trial of decitabine as first-line treatment for older patients with acute myeloid leukemia judged unfit for induction chemotherapy. Haematologica 97(3), 393–401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Ravandi F, Liu-Dumlao T. et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 120(24), 4840–4845 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dombret H, Seymour JF, Butrym A. et al. International Phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 126(3), 291–299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This large, randomized controlled trial demonstrates 5-azacitidine is as good as conventional care regimens in older patients with newly diagnosed AML.
- 14.Blum W, Garzon R, Klisovic RB. et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc. Natl Acad. Sci. USA 107(16), 7473–7478 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This important, prospective study highlights the role of 10-day decitabine in the treatment of older patients with AML.
- 15.Blum W, Klisovic RB, Hackanson B. et al. Phase I study of decitabine alone or in combination with valproic acid in acute myeloid leukemia. J. Clin. Oncol. 25(25), 3884–3891 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ritchie EK, Feldman EJ, Christos PJ. et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk. Lymphoma 54(9), 2003–2007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juliusson G, Antunovic P, Derolf A. et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 113(18), 4179–4187 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Wolff F, Leisch M, Greil R, Risch A, Pleyer L. The double-edged sword of (re)expression of genes by hypomethylating agents: from viral mimicry to exploitation as priming agents for targeted immune checkpoint modulation. Cell Commun. Signal 15(1), 13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Issa JP, Kantarjian HM. Targeting DNA methylation. Clin. Cancer Res. 15(12), 3938–3946 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Salihi M, Yu M, Burnett DM. et al. The depletion of DNA methyltransferase-1 and the epigenetic effects of 5-aza-2’deoxycytidine (decitabine) are differentially regulated by cell cycle progression. Epigenetics 6(8), 1021–1028 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, Phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J. Clin. Oncol. 28(4), 556–561 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Welch JS, Petti AA, Miller CA. et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N. Engl. J. Med. 375(21), 2023–2036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrates the role of 10-day decitabine in TP53 mutated patients.
- 23.Bhatnagar B, Duong VH, Gourdin TS. et al. Ten-day decitabine as initial therapy for newly diagnosed patients with acute myeloid leukemia unfit for intensive chemotherapy. Leuk. Lymphoma 55(7), 1533–1537 (2014). [DOI] [PubMed] [Google Scholar]
- 24.Gupta N, Miller A, Gandhi S. et al. Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients >/=60 years old. Am. J. Hematol. 90(7), 639–646 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prebet T, Sun Z, Figueroa ME. et al. Prolonged administration of azacitidine with or without entinostat for myelodysplastic syndrome and acute myeloid leukemia with myelodysplasia-related changes: results of the US Leukemia Intergroup trial E1905. J. Clin. Oncol. 32(12), 1242–1248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Short NJ, Kantarjian HM, Loghavi S. et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised Phase II trial. Lancet Haematol. 6(1), e29–e37 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an important, randomized Phase II trial that demonstrates no difference in outcomes in patients receiving a 10-day course of decitabine, compared with patients receiving a 5-day course of decitabine.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.