Abstract
Background
THK5351 and flortaucipir tau ligands have high affinity for paired helical filament tau, yet diverse off-target bindings have been reported. Recent data support the hypothesis that THK5351 binds to monoamine oxidase B (MAO-B) expressed from reactive astrocytes and that flortaucipir has an affinity toward MAO-A and B; however, pathological evidence is lacking. We performed a head-to-head comparison of the two tau ligands in a sporadic Creutzfeldt-Jakob disease (CJD) patient and performed an imaging-pathological correlation study.
Case presentation
A 67-year-old man visited our clinic a history of 6 months of rapidly progressive dementia, visual disturbance, and akinetic mutism. Diffusion-weighted imaging showed cortical diffusion restrictions in the left temporo-parieto-occipital regions. 18F-THK5351 PET, but not 18F-flortaucipir PET showed high uptake in the left temporo-parieto-occipital regions, largely overlapping with the diffusion restricted areas. Cerebrospinal fluid analysis was weakly positive for 14–3-3 protein and pathogenic prion protein was found. The patient showed rapid cognitive decline along with myoclonic seizures and died 13 months after his first visit. A post-mortem study revealed immunoreactivity for PrPsc, no evidence of neurofibrillary tangles, and abundant astrocytosis which was reactive for MAO-B antibody.
Conclusions
Our findings add pathological evidence that increased THK5351 uptake in sporadic CJD patients might be caused by an off-target binding driven by its high affinity for MAO-B.
Electronic supplementary material
The online version of this article (10.1186/s12883-019-1434-z) contains supplementary material, which is available to authorized users.
Keywords: THK5351 PET, Flortaucipir PET, Monoamine oxidase B, Creutzfeldt-Jakob disease
Background
THK5351 and flortaucipir tau ligands were developed with the anticipation that they would have specific high affinity for paired helical filament (PHF) type tau, the building blocks of neurofibrillary tangles (NFT) in Alzheimer’s disease. Yet, diverse off-target binding has been reported on positron emission tomography (PET) imaging for each ligand [1]. Accumulating data support the hypothesis that THK5351, a quinolone-derivative agent, binds to monoamine oxidase B (MAO-B) expressed from reactive astrocytes [2]. Flortaucipir has also shown in vitro affinity toward MAO-A and B [3]. In addition, a recent head-to-head comparison of two tau ligands raised the possibility that THK5351 and flortaucipir have distinct characteristics [4].
Sporadic Creutzfeldt-Jakob disease (sCJD), caused by prion protein, is a rapidly progressive neurodegenerative disorder that can have increased MAO-B activity as well as NFT. Previous imaging and pathology studies of CJD showed increased MAO-B expression by activated astrocytes and microglia [5, 6]. Meanwhile, various neuronal and glial tau pathologies exist in sCJD patients, including NFT, and the co-existence of Alzheimer’s disease occurs in 10% of patients with sCJD [7, 8]. Cerebrospinal fluid (CSF) analysis of CJD patients shows highly elevated total tau and slightly elevated phosphorylated tau [9].
In the present study, we performed a head-to-head comparison of two tau (THK5351 and flortaucipir) PET images in a patient with sCJD to find out whether the patient’s brain showed distinct uptake patterns. We further performed an imaging-pathological correlation study to determine whether increased uptake of tau ligands in this case represented increased PHF-type tau burden or MAO-B activity.
Case presentation
A 67-year-old right-handed man with a history of hypertension visited the Memory Clinic at Samsung Medical Center for rapidly progressive dementia, visual disturbance, and akinetic mutism, which started six months prior to his first visit. On neurologic examination, he showed bilateral bradykinesia, parkinsonian gait, and postural instability. On neuropsychological tests, his Mini-Mental State Examination score was 21 (6 years of formal education). He showed poor performance on language (confrontational naming, comprehension, and repetition tests), visuospatial, memory, and frontal/executive function.
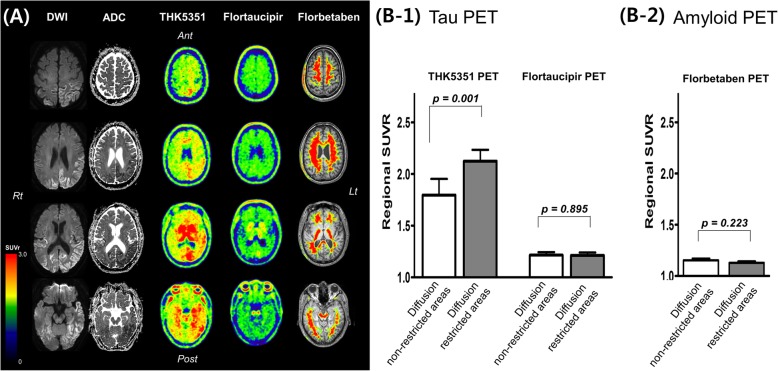
CSF analysis showed that white blood cell count, red blood cell count, protein and glucose levels were all normal. However, 14–3-3 protein was weakly positive, total tau was highly elevated (1081.9 pg/ml, normal range 116–370 pg/ml), and phosphorylated tau was mildly elevated (87.0 pg/ml, normal range 35.84–66.26 pg/ml). Amyloid-ß was within the normal range (910.0 pg/ml, normal range 562–1018 pg/ml). Pathogenic prion protein (PrPSc) was found using a RT-QuIC assay; however, the PRNP mutation was not found. Diffusion-weighted imaging (DWI) showed cortical diffusion restrictions in the left temporo-parieto-occipital regions (Fig. 1a). Based on his clinical symptoms and laboratory tests, the patient was diagnosed with probable sCJD.
Fig. 1.
a Diffusion weighted images (DWI), apparent diffusion coefficient (ADC), tau (THK5351 and flortaucipir, and amyloid (florbetaben) PET images in a patient with sporadic Creutzfeldt-Jakob disease. b Regional standardized uptake value ratio (SUVR) of THK5351, flortaucipir, and florbetaben in diffusion non-restricted and restricted areas
The patient further underwent molecular PET imaging using ligands that bind to amyloid (18F-florbetaben) and PHF tau (18F-Flortaucipir and 18F-THK5351). 18F-florbetaben PET revealed amyloid negative. 18F-Flortaucipir PET showed focal uptake only in the left occipital white matter region. However, 18F-THK5351 PET showed diffuse high uptake on the left temporo-parieto-occipital regions, which largely overlapped with the diffusion restricted areas (Fig. 1a). To quantitatively measure tau uptake, we determined the voxel-wise ROI of diffusion restricted regions by hand-drawing using MRIcro (https://www.mccauslandcenter.sc.edu/crnl/). We calculated the standardized uptake value ratio (SUVR) of each THK5351, flortaucipir, and florbetaben uptake in diffusion restricted and unrestricted voxels. Then, to analyze whether the diffusion restricted area showed higher tau uptake, we parcellated the whole cerebral cortex into 84 regions based on an AAL template. We manually classified each parcellated region as a diffusion restricted area when more than 50% of the region showed diffusion restriction or as a diffusion non-restricted area when less than 50% of the region showed diffusion restriction. We then compared the regional SUVR of THK5351, flortaucipir, and florbetaben between diffusion restricted and non-restricted areas. We found that the mean THK5351 SUVR of diffusion restricted voxels was 2.17 whereas the mean THK5351 SUVR of diffusion non-restricted voxels was 1.79. The mean flortaucipir SUVR of diffusion restricted voxels was 1.16 whereas the mean flortaucipir SUVR of diffusion non-restricted voxels was 1.20. The mean forbetaben SUVR of diffusion restricted voxels was 1.07 whereas the mean florbetaben SUVR of diffusion non-restricted voxels was 1.16. Quantitative analyses showed that THK5351 standardized uptake value ratio (SUVR) in diffusion restricted areas was higher compared to diffusion non-restricted areas, while flortaucipir SUVR and florbetaben SUVR did not show any difference (Fig. 1b) (Additional file 1).
The patient died 13 months after his first visit and underwent brain autopsy. The time interval between imaging scans and autopsy was approximately 13 months (399 days for MRI, 396 days for florbetaben PET, 388 days for flortaucipir PET, and 378 days for THK5351 PET). Neuropathological analysis was performed at Chuncheon Sacred Heart Hospital, Chuncheon, Korea. Autopsies were performed according to the standard protocols of National Neuropathology Reference and Diagnostic Laboratories for Dementia (NRD) supported by Korea National Institute of Health [10, 11]. Neuropathological diagnostic analysis was performed on sections, including the frontal, occipital, and basal ganglia of the right and left hemisphere. For each immunohistochemical stain, the degree of pathology was graded as none, mild (< 10%), moderate (10–30%), or severe (> 30%).
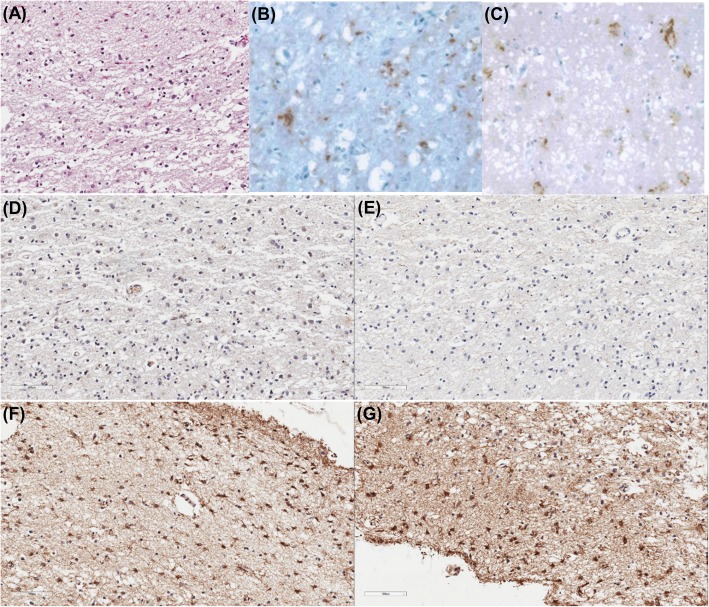
A post-mortem study confirmed the diagnosis of CJD, as we found neuronal loss and micro-vacuolar degeneration on H&E (Fig. 2a) and immunoreactive for PrPsc (3F4 and 1C5 antibody) (Fig. 2b-c). There was no evidence of neuritic plaques (Fig. 2d) or NFT (Fig. 2e) in the bilateral frontal, occipital cortices, and basal ganglia. However, we found mild diffuse amyloid plaques and mild neuropil threads. Glial fibrillary acidic protein (GFAP) stain showed the following results: moderate reactivity in the bilateral frontal and left occipital cortices; mild reactivity in the right occipital cortex and left basal ganglia; and non-reactivity in the right basal ganglia (Fig. 2f). MAO-B stain showed severe reactivity in the left frontal and bilateral occipital cortices and moderate reactivity in the right frontal cortex and bilateral basal ganglia (Fig. 2g).
Fig. 2.
Pathological findings in the left occipital cortex of a patient with sporadic Creutzfeldt-Jakob disease. H&E staining (a) showed neuronal loss and vacuolation. Immunohistochemistry showed reactivity for PrPsc, b 3F4 antibody and c 1C5 antibody. Immunohistochemistry against amyloid-ß (d) and phosphorylated tau (e) showed no amyloid plaques and no neurofibrillary tangles, respectively. GFAP staining (f) showed active astrocytosis and MAO-B staining (g) showed increased MAO-B activity
Discussion and conclusions
We report the PET findings of two tau ligands (flortaucipir and THK5351) and the autopsy results in a patient with sCJD. Our novel finding was that THK5351 uptake was increased in regions similar to diffusion restricted cortical areas, while flortaucipir uptake was not. The post-mortem study revealed no NFT but severe astrocytosis which was reactive for MAO-B staining. Therefore, our findings add pathological evidence that flortaucipir is more specific to PHF tau, and increased THK5351 uptake in sCJD might be an off-target binding driven by its high affinities to MAO-B.
These two tau ligands showed different uptake patterns in our patient with CJD. THK5351 uptake was increased in regions similar to diffusion restricted cortical areas, while flortaucipir uptake was not. Our finding is consistent with a recent study showing that CJD patients did not have any increased uptakes of flortaucipir [12]. Our imaging-pathological correlation study showed that there were mild neurophil threads but no NFT, suggesting that increased uptakes of THK5351 might represent off-target bindings. Indeed, previous studies showed that increased CSF tau in CJD is related to increased burdens of dystrophic neurites due to rapid destruction of neurons rather than development of PHF-type tau [7]. Our results are in line with our previous head to head comparison of the two ligands showing that flortaucipir is more sensitive and specific to PHF-type tau than THK5351 [4].
The underlying pathological substrate for THK5351 uptake in CJD might be related to increased MAO-B activity. We observed that GFAP staining of the region with diffusion restriction and high THK5351 uptake showed severe astrocytosis which was reactive for MAO-B staining. Our results are in line with previous reports showing sCJD patients have increased reactive astrocytes and MAO-B activity in the brain [5]. Previous studies suggested that THK5351 has high affinity for MAO-B, as ingestion of MAO-B inhibitor (selegiline) reduced THK5351 uptake [2], whereas MAO-B inhibitor did not block flortaucipir uptake in human brains [13]. Our findings, therefore, suggested that increased THK5351 uptake in this case might represent increased MAO-B activity within increased reactive astrocytosis.
Although THK5351 uptake regions largely overlapped with diffusion restricted areas, left frontal region showed discrepancy. The discrepancy between negative DWI and positive THK5351 uptake in the left frontal region might be explained by the difference in the underlying pathological substrate. Previous pathological studies showed that diffusion restriction on DWI correlated best with spongiform changes and PrP deposition, followed by reactive astrocytic gliosis [14, 15]. On the other hand, THK5351 uptake reflects increased MAO-B activity and increased reactive astrocytosis. Although autopsy findings in the left frontal region showed advanced features of sCJD (neuronal loss, micro-vacuolar degeneration, PrPsc immunoreactivity, moderate reactivity on GFAP staining, and severe reactivity on MAO-B staining), we assume that at the time the patient underwent brain imaging, this region might have had astrocytosis with increased MAO-B activity but not spongiform changes or PrP deposition.
The limitation of this study is the 13-month delay between imaging scans and autopsy. As histopathological data were obtained at a more advanced stage than imaging data, vacuolation, PrPsc deposition, and reactive astrocytic gliosis with increased MAO-B activity were likely less severe at the time the patient underwent imaging. Therefore, DWI and THK5351 might not fully reflect the pathological findings. However, as there was no evidence of neuritic plaques or NFT even at the advanced stage, we conclude that THK5351 uptake in this sCJD patient represents off-target binding.
In conclusion, our imaging-pathological correlation study of THK5351 and flortaucipir suggested that flortaucipir is more specific to PHF tau, and increased THK5351 uptake in sCJD might be an off-target binding driven by its high affinities to MAO-B.
Additional file
Supplementary Methods. Detailed methods for PET acquisition and analysis. (DOCX 15 kb)
Acknowledgements
Not applicable.
Abbreviations
- CSF
Cerebrospinal fluid
- DWI
Diffusion-weighted imaging
- GFAP
Glial fibrillary acidic protein
- MAO-B
Monoamine oxidase B
- NFT
Neurofibrillary tangles
- PET
Positron emission tomography
- PHF
Paired helical filament
- PrPSc
Pathogenic prion protein
- sCJD
Sporadic Creutzfeldt-Jakob Disease
- SUVR
standardized uptake value ratio
Authors’ contributions
HJK, KCC and SWS contributed to the conception and design of the study. CH, SP, HJ, YHR, JYC, SHM, SJO, MO, DLN, CHL, EJK, WWS, JSK and KCC contributed to acquisition and analysis of data. HJK, CHL, and SWS contributed to drafting a significant portion of the manuscript or figures. All authors have read and approved the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C2768, HI18C0335, and HI18C1629); Research of Korea Centers for Disease Control and Prevention (2018-ER6202–01); and the Brain Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1913844). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The collection of the data was conducted as set forth in the Declaration of Helsinki. This study was approved by the Institutional Review Board of Samsung Medical Center and we obtained informed consent from the participant and his next of kin (son).
Consent for publication
Written informed consent was obtained from the patient’s next of kin (son) for publication of this report.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kyung Chan Choi and Sang Won Seo contributed equally to this work.
Contributor Information
Hee Jin Kim, Email: evekhj@gmail.com.
Hanna Cho, Email: iguhanna@naver.com.
Seongbeom Park, Email: ayl1815@gmail.com.
Hyemin Jang, Email: hmjang57@gmail.com.
Young Hoon Ryu, Email: ryuyh@yuhs.ac.
Jae Yong Choi, Email: smhany@kirams.re.kr.
Seung Hwan Moon, Email: seunghwan.moons.moon@samsung.com.
Seung Jun Oh, Email: sjoh@amc.seoul.kr.
Minyoung Oh, Email: my@amc.seoul.kr.
Duk L. Na, Email: dukna@naver.com
Chul Hyoung Lyoo, Email: lyoochel@yuhs.ac.
Eun-Joo Kim, Email: cadasil@hanmail.net.
William W. Seeley, Email: wseeley@memory.ucsf.edu
Jae Seung Kim, Email: jaeskim@amc.seoul.kr.
Kyung Chan Choi, Phone: +82-10-3483-1324, Email: kcchoi@hallym.ac.kr.
Sang Won Seo, Phone: +82-2-3410-1233, Email: sw72.seo@samsung.com.
References
- 1.Lemoine L, Leuzy A, Chiotis K, Rodriguez-Vieitez E, Nordberg A. Tau positron emission tomography imaging in tauopathies: the added hurdle of off-target binding. Alzheimers Dement (Amsterdam, Netherlands) 2018;10:232–236. doi: 10.1016/j.dadm.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng KP, Pascoal TA, Mathotaarachchi S, Therriault J, Kang MS, Shin M, Guiot MC, Guo Q, Harada R, Comley RA, et al. Monoamine oxidase B inhibitor, selegiline, reduces (18) F-THK5351 uptake in the human brain. Alzheimers Res Ther. 2017;9(1):25. doi: 10.1186/s13195-017-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeiren C, Motte P, Viot D, Mairet-Coello G, Courade JP, Citron M, Mercier J, Hannestad J, Gillard M. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov Disord. 2018;33(2):273–281. doi: 10.1002/mds.27271. [DOI] [PubMed] [Google Scholar]
- 4.Jang YK, Lyoo CH, Park S, Oh SJ, Cho H, Oh M, Ryu YH, Choi JY, Rabinovici GD, Kim HJ, et al. Head to head comparison of [(18) F] AV-1451 and [(18) F] THK5351 for tau imaging in Alzheimer's disease and frontotemporal dementia. Eur J Nucl Med Mol Imaging. 2018;45(3):432–442. doi: 10.1007/s00259-017-3876-0. [DOI] [PubMed] [Google Scholar]
- 5.Engler H, Lundberg PO, Ekbom K, Nennesmo I, Nilsson A, Bergstrom M, Tsukada H, Hartvig P, Langstrom B. Multitracer study with positron emission tomography in Creutzfeldt-Jakob disease. Eur J Nucl Med Mol Imaging. 2003;30(1):85–95. doi: 10.1007/s00259-002-1008-x. [DOI] [PubMed] [Google Scholar]
- 6.Engler H, Nennesmo I, Kumlien E, Gambini JP, Lundberg P, Savitcheva I, Langstrom B. Imaging astrocytosis with PET in Creutzfeldt-Jakob disease: case report with histopathological findings. Int J Clin Exp Med. 2012;5(2):201–207. [PMC free article] [PubMed] [Google Scholar]
- 7.Kovacs Gabor G., Rahimi Jasmin, Ströbel Thomas, Lutz Mirjam I., Regelsberger Günther, Streichenberger Nathalie, Perret-Liaudet Armand, Höftberger Romana, Liberski Pawel P., Budka Herbert, Sikorska Beata. Tau pathology in Creutzfeldt-Jakob disease revisited. Brain Pathology. 2016;27(3):332–344. doi: 10.1111/bpa.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang HD, Ho D-H, Yi M-J, Seol W, Kim SY. Misfolded proteins in neurodegenerative dementias: molecular mechanisms. Dement Neurocogn Disord. 2012;11(2):38–52. doi: 10.12779/dnd.2012.11.2.38. [DOI] [Google Scholar]
- 9.Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Heinemann U, Breithaupt M, Varges D, Meissner B, Ladogana A, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain. 2009;132(Pt 10):2659–2668. doi: 10.1093/brain/awp191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KH, Seo SW, Lim TS, Kim EJ, Kim BC, Kim Y, Lee HW, Jeon JP, Shim SM, Na DL, et al. Proposal guidelines for standardized operating procedures of brain autopsy: brain bank in South Korea. Yonsei Med J. 2017;58(5):1055–1060. doi: 10.3349/ymj.2017.58.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim Y, Suh YL, Kim SJ, Bae MH, Kim JB, Kim Y, Choi KC, Huh GY, Kim EJ, Lee JS, et al. The brain donation program in South Korea. Yonsei Med J. 2018;59(10):1197–1204. doi: 10.3349/ymj.2018.59.10.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Day GS, Gordon BA, Perrin RJ, Cairns NJ, Beaumont H, Schwetye K, Ferguson C, Sinha N, Bucelli R, Musiek ES, et al. In vivo [(18) F]-AV-1451 tau-PET imaging in sporadic Creutzfeldt-Jakob disease. Neurology. 2018;90(10):e896–e906. doi: 10.1212/WNL.0000000000005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen AK, Brooks DJ, Borghammer P. MAO-B inhibitors do not block in vivo Flortaucipir([F-18]-AV-1451) binding. Mol Imaging Biol. 2018;20(3):356–360. doi: 10.1007/s11307-017-1143-1. [DOI] [PubMed] [Google Scholar]
- 14.Iwasaki Y. Creutzfeldt-Jakob disease. Neuropathology. 2017;37(2):174–188. doi: 10.1111/neup.12355. [DOI] [PubMed] [Google Scholar]
- 15.Geschwind MD, Potter CA, Sattavat M, Garcia PA, Rosen HJ, Miller BL, DeArmond SJ. Correlating DWI MRI with pathologic and other features of Jakob-Creutzfeldt disease. Alzheimer Dis Assoc Disord. 2009;23(1):82–87. doi: 10.1097/WAD.0b013e31818323ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Detailed methods for PET acquisition and analysis. (DOCX 15 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.