Abstract
Lead is known as an environmental contaminant with neurotoxic properties. In addition, people experience different types of chronic stress, especially in developing countries. It has been established that lead or stress causes structural and physiological damages to the neural pathway like dopaminergic connections. Nevertheless, the effect of lead and restraint stress on movement behaviors when are experienced together has not been studied yet. In this study, male albino mice were randomly divided into different groups (n = 6). Lead acetate was daily injected at 15 mg/kg intraperitoneally for 2, 4, or 6 weeks. Restraint stress (6 h in a day) was applied alone or in combination with lead acetate for 2, 4, or 6 weeks. The catalepsy, akinesia, and the balance of animals were measured by bar test, elevated beam device, and rotarod to evaluate the movement disorders. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a known neurotoxin causes movement disorders, was used as positive control group. The results showed that exposure to the lead or stress or their combination for 6 weeks caused catalepsy, akinesia, and imbalance in the animals, while exposure for 2 or 4 weeks didn’t affect the movement items indices. The combination of lead and stress did not show any significant difference compared to the exposure to each of them individually. From the findings, Lead, stress, and their combination caused movement disorders in a time dependent manner. Short time exposure did not change movement behavior. The co-exposure to the lead and stress did not show additive or synergistic effects.
Keywords: Lead, Mice, Movement disorders, Restraint stress
INTRODUCTION
Lead is known as a common environmental pollutant which can infect human in the developing societies. The people are exposed via different routes including oral, pulmonary and, dermal from food, water, air and etc (1). There is a large body of studies demonstrating that lead causes toxic effects in body tissues especially central nervous system (CNS). It is documented that lead exposure results in the biochemical, morphological, and physiological negative changes on brain and causes impaired behavioral activity (2). Dopaminergic system is one of the targets of environmental neurotoxic agents. It is reported that lead can deteriorate the brain dopaminergic pathways like nigra-striatal which regulate the movement in human and animals (3,4).
In addition, a wide range of people who infected by lead, experience various psychological and physical stresses. Similar to lead, it is well documented that chronic stress and stress-related hormones, such as glucocorticoids have devastating effects on the biochemical, anatomical, and physiological processes of the CNS (5). Chronic stress changes the release of neurotransmitters, negatively alters the number of synapses and neuroplasticity in the brain. One of the most important systems in the brain affected by stress is the dopaminergic system. Indeed, stress can accelerate deterioration of dopaminergic cells, reduces dopaminergic neurons and dopamine levels in the CNS (6,7).
Disruption of the dopaminergic system, especially in the nigra-striatal pathway, can be presented as mobility disorders. In the animal-based studies, the restraint stress procedure is frequently used as a reliable method to impose psychological and physical stress (8).
Obviously, many toxic effects show a time dependent algorithm. In addition, simultaneous exposure to two or more neurotoxicants can cause different complications which essentially are not similar to individual neurotoxicant toxicity. The co-exposure can lead to additive, synergistic, neutral, or decreasing toxic effect. Elimination of one neurotoxic agent can occasionally stop the toxicity of other concomitant agents (9).
As mentioned, in industrial cities some people concomitantly experience the chronic lead exposure and the stress, in different period of times. Although there are several evidences indicating the destroying effect of lead or stress alone on dopaminergic and glutamate system (6,7,10), no study has investigated lead and restraint stress clinical manifestation like motor disorders. In addition, the effect of lead and restraint stress interaction on the movement behavior has not been clarified.
In the present study, we evaluated the time-dependent effect of lead or restraint stress as well as their combination on the incidence of movement disorders.
MATERIALS AND METHODS
Animals
Adult male albino mice, weighing 28 ± 2 g were purchased from the animal house, Isfahan University of Medical Sciences, Isfahan, I.R. Iran. Animals were randomly divided into the experimental groups of 6 each. They were kept in the standard cages under normal conditions of temperature (25 ± 5 °C), humidity (60 ± 5%), and 12/12-h light/dark cycles. Mice were given food and water ad libitum. The experimental protocol was according to the international animal right guidelines and approved by the Animal Ethics Committee of Isfahan University of Medial Science (IR.MUI.REC.1396.3.654).
Chemicals and agents
1-methyl - 4 - phenyl - 1, 2, 3, 6-tetrahydro -pyridine (MPTP) hydrochloride from Sigma Aldrich (Germany, CAS Number 23007-85-4) and Lead acetate from Merck Company (Germany, CAS Number 6080-56-4).
Restraint stress procedure
A porous plastic tube (1.5 × 4 cm) was used to impose restraint stress on mice. It could be adjusted in length according to the mice size. In the restrainer, animal just could slightly go back and stretch their legs. For the restraint stress procedure, the animal was held in the tube for 6 h per day in a well-ventilated room. After the restricted period, the animal was transferred to its home cage, and had enough access to food and water (5).
Animal treatment protocol
Animals divided into 5 groups of 6 each. Animals in the control group received daily doses of 0.3 mL saline intraperitoneally (i.p.) for 6 weeks but they underwent no restraint stress. In the restraint stress groups 3 groups of animals were injected with saline and exposed to the restraint stress based on the aforementioned procedure for 2, 4, or 6 weeks. In the lead-treated groups, 3 groups of animals were daily injected with lead acetate 15 mg/kg i.p. for 2, 4, or 6 weeks (2). In the co-exposure groups, 3 groups of animals received lead acetate daily at 15 mg/kg i.p. and 1 h later they exposed to restraint stress for 2, 4, or 6 weeks. In the MPTP group, a group of animals injected with four successive doses of MPTP 10 mg/kg i.p. with 1 h intervals (11) and considered as positive control group for the movement disorder to confirm method’s reliability. Twenty-four h after the last session of stress or injection, animals subjected to the mobility evaluation tests. If the training was needed for mobility test, it was done on the last two days of exposure period.
Mobility evaluation
To evaluate animal voluntary movements, different tests were used. Rotarod and elevated beam test showed motor imbalance, bar test showed the muscle rigidity, and the akinesia test showed the retardation in movement.
Rotarod test
The rotarod test is a widely used test to measure coordinated motor skills. It requires animals to balance and walk on a rotating cylinder. The rotarod unit consisted of a rotating rod (30 mm diameter) (12), which divided into five parts by compartmentalization, which allows examining five mice at a time. When the mice fell down from rotating rod, the time automatically stopped. In this study, the rotating speed of rotarod was constant (15 rpm). After training, the time for each mouse to remain on the rotating rod (rotarod latency) was recorded for three trials at 10 min intervals. The maximum time for each trial was 90 sec. The rotarod latency is directly dependent on the movement and balance skill of the animal. Twice daily training for two consecutive days was done before the test day (13).
Bar test
Bar is a 40 cm in length and 0.4 cm in diameter stainless steel rod which was horizontally elevated 4.5 cm above the surface. The bar test was used for the evaluation of catalepsy. Both forelimbs of mice were positioned on the bar. Catalepsy measured as the time duration each mouse hold the elevated bar by his both forelimbs (14). The catalepsy was scored into 5 different levels (12): 1, falling between 1-5 sec; 2, falling between 6-10 sec; 3, falling between 11-20 sec; 4, falling between 21-30 sec; 5, falling after 30 sec.
Akinesia
Akinesia measured using a wooden elevated (30 cm) platform (40 × 40 cm). Each animal initially put on the platform. The time required for animal to move all four limbs was recorded and called latency. The trial was terminated if the latency exceeded 180 sec. This test was repeated 3 times for each animal (15).
Elevated beam test
The beam apparatus consists of a flat surface wooden beam with 1 m length and 12 mm width which is elevated 50 cm above the table. A black box was placed at the end of the beam as the finish point and the other end was the start point. A lamp was used as an aversive stimulus on the starting point. The time required to cross the beam was recorded. The mouse was allowed to rest in the black box for 15 sec. This test repeated 3 times for each mouse. After that the mice returned to their home cages and taken back into their housing room. Twice-daily training for two consecutive days performed before the test day (16).
In the positive control group, tests started 24 h after the last dose of MPTP.
Statistical analysis
The data of different mobility evaluating test presented as mean ± SEM. The values were analyzed using one way analysis of variance (ANOVA). For statistical analysis the Graphpad Prism V.5 software was used. For multiple comparisons we used the Tukey post hoc tests. P < 0.05 was considered statistically significant.
RESULTS
Effects of lead on the movement disorder
The lead acetate injection at 15 mg/kg for 2 weeks did not show significant differences in rotarod, bar, elevated beam, and akinesia tests compared to the control group. Nevertheless, administration of lead acetate for 4 weeks significantly decreased the rotarod latency (P < 0.001), while increased bar test score (P < 0.01), akinesia latency (P < 0.01), and elevated beam time (P < 0.01) compared to the normal saline control group. In addition, animals received lead acetate for 6 weeks exhibited remarkable diminution in the rotarod latency (P < 0.001) and significantly elevated bar score (P < 0.001), akinesia latency (P < 0.001) and elevated beam time (P < 0.001) compared to the control group.
The animals who received lead acetate for 4 weeks demonstrated considerable decrease in the rotarod latency (P < 0.01) and significant increase in the bar test score (P < 0.01) and elevated beam time (P < 0.05) compared to the 2-week lead acetate-treated mice. There was no significant difference in akinesia latency between 2 or 4 weeks lead acetate administration (Figs. 1-4). As shown in Figs. 1-4, exposure to 15 mg/kg lead acetate for 6 weeks significantly decreased the rotarod latency (P < 0.01) while remarkably increased the bar test score (P < 0.01), akinesia latency (P < 0.01) and elevated beam time (P < 0.01) in comparison with animals received lead acetate for 2 weeks.
Fig. 1.
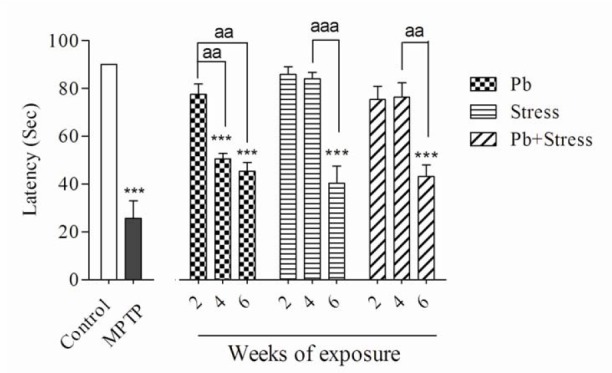
Effects of 2, 4, and 6 weeks of lead exposure, restraint stress, and their co-exposure on rotarod test. Columns represent mean ± SEM. Six weeks of lead exposure, restraint stress, and and their co-exposure significantly (***P < 0.001) decreased the rotarod latency compared to the control group. Co-exposure of lead and restraint stress did not show significant differences compared to lead or restraint stress group individually. There are statistical differences between periods of exposures (aaP < 0.01, aaaP < 0.001). MPTP was used as positive control. Pb, Lead; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Fig. 4.
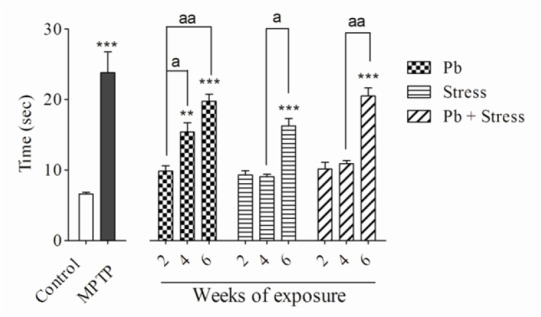
Effects of 2, 4, and 6 weeks of lead exposure, restraint stress, and their co-exposure on elevated beam test. Columns represent mean ± SEM. Six weeks of all exposures significantly (***P < 0.001) increased beam time in comparison with control group. Four weeks of lead exposure increased the elevated beam time compared with the control group (**P < 0.01). There are statistical differences between periods of exposures (aP < 0.05, aaP < 0.01). Combination of lead and restraint stress did not change the beam time significantly compared to each parameter alone. Pb, Lead; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Fig. 2.
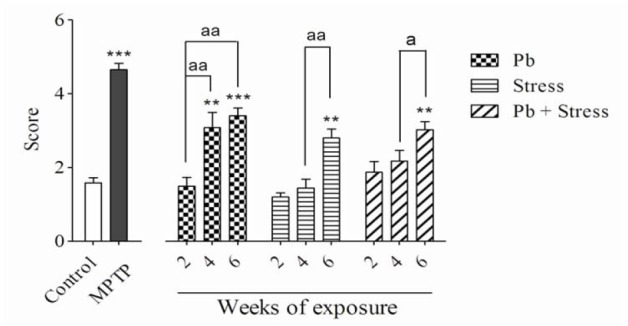
Effects of 2, 4, and 6 weeks of lead exposure, restraint stress and their combination on bar test. Columns represent mean ± SEM. Four or six weeks of lead exposure, six weeks of restraint stress, and six weeks of their co-exposure significantly (**P < 0.01, ***P < 0.001) elevated bar score compared to the control group. There are statistical differences between periods of exposures (aP < 0.05, aaP < 0.01). Pb, Lead; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Fig. 3.
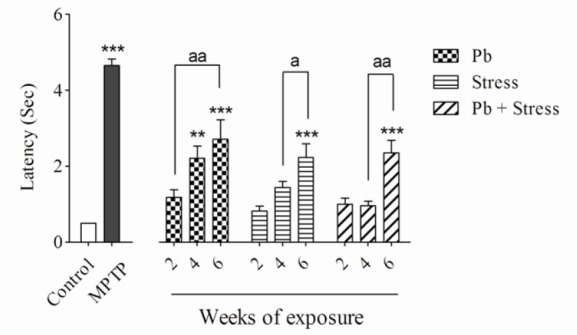
Effects of 2, 4, and 6 weeks of lead exposure, restraint stress, and their combination on akinesia test. Columns represent mean ± SEM. Six weeks of all exposures show significant change (***P < 0.001) compared to control group. Four weeks of lead exposure increased latency vs. control group (**P < 0.01). There are statistical differences between periods of exposures (aP < 0.05, aaP < 0.01). Pb, Lead; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Effects of restraint stress exposure on the movement disorder
Exposure to restraint stress for 2 or 4 weeks did not noticeably alter rotarod, bar, akinesia, and elevated beam test values in comparison with the control group. The animals experienced 6 weeks restraint stress showed significant decrease in the rotarod latency (P < 0.001) compared to the non-stress animals. They displayed a remarkable increase in the bar test score (P < 0.01), akinesia latency (P < 0.001), and elevated beam time (P < 0.001).
In addition, animals exposed to 6-week restraint stress showed remarkable decrease in the rotarod test values (P < 0.001) but exhibited statistical elevation in their bar score (P < 0.01), akinesia latency (P < 0.05), and elevated beam time (P < 0.05) compared to the animals exposed to the restraint stress for four weeks (Figs. 1-4).
Effects of co-exposure of lead acetate and restraint stress on the movement disorder
Although concomitant exposure to lead acetate and restraint stress for 2 or 4 weeks, in comparison with control group, did not change the value of movement evaluating tests, their exposure for 6 weeks significantly decreased the rotarod latency (P < 0.001) and increased the bar test score (P < 0.01), the akinesia latency (P < 0.001), and the elevated beam time (P < 0.001). Animals who experienced 6-week co-exposure of lead and restraint stress showed statistically significant decrease in rotarod latency (P < 0.01) but significant increase in bar test score (P < 0.05), the akinesia latency (P < 0.01), and the elevated beam time (P < 0.01) compared to the 4-week co-exposed animals (Figs. 1-4).
The comparison of lead acetate, restraint stress, or their co-exposure on the movement disorder tests
Co-exposure to both lead acetate and restraint stress for 2, 4 or 6 weeks caused no significant differences in all experimental tests compared to individual exposures in their respective periods of time (Figs. 1-4).
DISCUSSION
The effect of lead or stress exposure as two common toxic conditions on the movement manifestation has not yet been clarified. The present study evaluated their effects, either alone or in combination on the mice mobility. In addition the time dependency of possible movement deteriorations has been examined.
The results indicated that all of the evaluated movement disorder symptoms appeared after 4-week exposure to the lead acetate and further increase after 6 weeks. Therefore, the impaired mobility effect of lead showed a time dependency manner. Our findings are in agreement with other studies with regard to the lead neuronal toxicity and behavioral effects. At the molecular level, in microdialysate samples from rats who consumed 250 ppm lead for 3-6 weeks, it has been shown that the lead exposure significantly decreased the dopamine release and the dopamine D1 receptor sensitivity post-synaptically (17). Lead increases the lipid peroxidation and reduces the antioxidant cell capacity in the CNS (18). In addition, it has been reported that lead changes the corticosterone level and alters the hypothalamic-pituitary-adrenal (HPA) axis activity. HPA can play a mechanistic role in behavioral and neurochemical effects of lead exposure (19).
In the current study, the movement disorder observed after 6 weeks of restraint stress exposure. These findings are consistent with a large body of studies which demonstrated the deteriorating effect of stress on the dopaminergic pathways (6,7,20,21). It has been shown that the psychological stress and elevated corticosterone levels accelerate nigral neuronal loss and change behavioral responses in rats and the stress disrupted expression of anti-apoptotic, and neurotrophic factors (20). Down-regulation of neurotrophic factor expression can explain the lack of compensatory behavior found in stress-treated animals (6). Exposure to chronic restraint stress significantly reduced the number of dopaminergic neurons in the pars compacta of substantia nigra and noradrenergic neurons in the locus coeruleus (7). An increase in the glucocorticoids and neuroinflammation plays an important role in stress-induced neurodegenerative effects (21).
Significant disorder in the movement observed after 6 weeks to stress exposure, whereas this time for lead exposure was 4 weeks. This demonstrates that both lead and restraint stress need a lag time to begin their adverse effects on mice movements. Presumably the side effects of lead or restraint stress are the time-dependent process. Another possibility is that it takes a while for the CNS to perform its protective effects against lead or stress. The shorter lag time for the lead exposure could be because of more toxic intensity of lead, the wide variety of its toxic mechanisms, lipid peroxidation, and apoptosis (22,23,24). Based on the previous literatures, lead involves major toxic cascades like lipid peroxidation which causes neurodegeneration while stress shows moderate toxicity through increasing brain corticoid levels result in neuroinflammation (25,26)
We found that co-exposure to the lead and restraint stress caused movement disorders after 6 weeks. Interestingly, the addition of restraint stress to the lead exposure did not show a synergistic or additive effect compared to the lead or restraint stress alone. It can be assumed that the lead is a more potent toxic substance than stress. The lead triggers more crucial consequences and involves a wide range of mechanisms in the CNS (22,23,24,27). Therefore, restraint stress as a weaker component failed to augment the lead induced mobility disorder.
Concomitant stress could modulate the involving mechanism in lead toxicity after 4 weeks. For example, stress will cause HPA axis dysregulation and modulate its sensitivity to the lead exposure (25). This may explain why the movement impairment induced by lead was not observed until 6 weeks when the stress was added.
Based on previous studies the stress and lead can modify each other’s effects. Lead exposure causes alteration in HPA axis and consequently result in abnormal response to the stress (25,28). The combination of lead and stress in males does not elevate corticosterone level as much as lead or stress does individually (29). It has been shown that lead exposure modifies the effect of prenatal stress on stress responsivity in adulthood (30).
Our findings are in agreement with the findings of Virgolini et al. They demonstrated that restraint stress can decrease sensitivity to lead toxic responses in male mice (25). It was previously mentioned that exposure to a repeated stress can cause tolerance to an exogenic substance response (31,32).
It should be noted that the nature of lead/stress interaction is not easily predictable because numerous factors influence these interactions, including gender, brain region, time of measurement, and complexity of HPA axis interactions with neurotransmitters (e.g. serotonergic, norepinephrine, dopamine, and GABA) (33,34). Other molecular studies are needed to clarify the concomitant lead and stress underlying mechanism.
CONCLUSION
According to this study, although either of lead or restraint stress alone caused observable movement disorders, their combination did not show necessarily additive effects. Both lead and stress showed a lag time to begin their effects on movement and this time for stress is longer than lead. The mechanism of lead and restraint stress interaction is not easily predictable because their effects are not selective and are influenced by several factors.
ACKNOWLEDGEMENTS
We would like to acknowledge the School of Pharmacy of Isfahan University of Medical Sciences, Isfahan, I.R. Iran Research Department in for their co-operation and financial supports (Grant No. 396654). We also thank Mrs. Moradi and Mr. Sharifi for their help in the pharmacology laboratory.
REFERENCES
- 1.Tong S, von Schirnding YE, Prapamontol T. Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ. 2000;78(9):1068–1077. [PMC free article] [PubMed] [Google Scholar]
- 2.Moosavirad SA, Rabbani M, Sharifzadeh M, Hosseini-Sharifabad A. Protective effect of vitamin C, vitamin B12 and omega-3 on lead-induced memory impairment in rat. Res Pharm Sci. 2016;11(5):390–396. doi: 10.4103/1735-5362.192490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones DC, Miller GW. The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol. 2008;76(5):569–581. doi: 10.1016/j.bcp.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Lauretti E, Di Meco A, Merali S, Praticò D. Chronic behavioral stress exaggerates motor deficit and neuroinflammation in the MPTP mouse model of Parkinson’s disease. Transl Psychiatry. 2016;6:e733. doi: 10.1038/tp.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith LK, Jadavji NM, Colwell KL, Katrina Perehudoff S, Metz GA. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. Eur J Neurosci. 2008;27(8):2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugama S, Sekiyama K, Kodama T, Takamatsu Y, Takenouchi T, Hashimoto M, et al. Chronic restraint stress triggers dopaminergic and noradrenergic neurodegeneration: possible role of chronic stress in the onset of Parkinson's disease. Brain Behav Immun. 2016;51:39–46. doi: 10.1016/j.bbi.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campos AC, Fogaça MV, Aguiar DC, Guimarães FS. Animal models of anxiety disorders and stress. Braz J Psychiatry. 2013;5(Suppl 2):S101–11. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 9.Coors A, De Meester L. Synergistic, antagonistic and additive effects of multiple stressors: predation threat, parasitism and pesticide exposure in Daphnia magna. J Appl Ecol. 2008;45(6):1820–1828. [Google Scholar]
- 10.Xu J, Yan HC, Yang B, Tong LS, Zou YX, Tian Y. Effects of lead exposure on hippocampal metabotropic glutamate receptor subtype 3 and 7 in developmental rats. J Negat Results Biomed. 2009;8(1):5–9. doi: 10.1186/1477-5751-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibrat C, Saint-Pierre M, Bousquet M, Lévesque D, Rouillard C, Cicchetti F. Differences between subacute and chronic MPTP mice models: investigation of dopaminergic neuronal degeneration and α‐synuclein inclusions. J Neurochem. 2009;109(5):1469–1482. doi: 10.1111/j.1471-4159.2009.06072.x. [DOI] [PubMed] [Google Scholar]
- 12.Deacon RM. Measuring motor coordination in mice. J Vis Exp. 2013;75:e2609. doi: 10.3791/2609. DOI: 10.3791/2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan MM, Kempuraj D, Thangavel R, Zaheer A. Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem Int. 2013;62(4):379–388. doi: 10.1016/j.neuint.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheidaei H. Buspirone improves haloperidol-induced Parkinson disease in mice through 5-HT1A recaptors. Daru. 2010;18(1):41–45. [PMC free article] [PubMed] [Google Scholar]
- 15.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res. 2001;125(1-2):109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 16.Luong TN, Carlisle HJ, Southwell A, Patterson PH. Assessment of motor balance and coordination in mice using the balance beam. J Vis Exp. 2011;49 doi: 10.3791/2376. pii:2376 DOI: 10.3791/2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavakoli-Nezhad M, Barron AJ, Pitts DK. Postnatal inorganic lead exposure decreases the number of spontaneously active midbrain dopamine neurons in the rat. Neurotoxicology. 2001;22(2):259–269. doi: 10.1016/s0161-813x(01)00010-9. [DOI] [PubMed] [Google Scholar]
- 18.Patra RC, Swarup D. Effect of antioxidant ascorbic acid, l-methionine or α tocopherol alone or along with chelator on cardiac tissue of lead-treated rats. Vet Arh. 2004;74(3):235–244. [Google Scholar]
- 19.Canfield RL, Gendle MH, Cory-Slechta DA. Impaired neuropsychological functioning in lead-exposed children. Dev Neuropsychol. 2004;26(1):513–540. doi: 10.1207/s15326942dn2601_8. [DOI] [PubMed] [Google Scholar]
- 20.Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124(4):985–992. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39(3):579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- 22.Ercal N, Gurer-Orhan H, Aykin-Burns N. Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem. 2001;1(6):529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Dubey RS. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003;164(4):645–655. [Google Scholar]
- 24.Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003;126(Pt 1):5–19. doi: 10.1093/brain/awg014. [DOI] [PubMed] [Google Scholar]
- 25.Virgolini MB, Chen K, Weston DD, Bauter MR, Cory-Slechta DA. Interactions of chronic lead exposure and intermittent stress: consequences for brain catecholamine systems and associated behaviors and HPA axis function. Toxicol Sci. 2005;87(2):469–482. doi: 10.1093/toxsci/kfi269. [DOI] [PubMed] [Google Scholar]
- 26.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 27.Stewart WF, Schwartz BS. Effects of lead on the adult brain: a 15-year exploration. Am J Ind Med. 2007;50(10):729–739. doi: 10.1002/ajim.20434. [DOI] [PubMed] [Google Scholar]
- 28.Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Parsons PJ, et al. Low-level prenatal and postnatal blood lead exposure and adrenocortical responses to acute stress in children. Environ Health Perspect. 2007;116(2):249–255. doi: 10.1289/ehp.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112(6):717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virgolini MB, Rossi-George A, Weston D, Cory-Slechta DA. Influence of low level maternal Pb exposure and prenatal stress on offspring stress challenge responsivity. Neurotoxicology. 2008;29(6):928–939. doi: 10.1016/j.neuro.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva Torres IL, Cucco SN, Bassani M, Duarte MS, Silveira PP, Vasconcellos AP, et al. Long-lasting delayed hyperalgesia after chronic restraint stress in rats-effect of morphine administration. Neurosci Res. 2003;45(3):277–283. doi: 10.1016/s0168-0102(02)00232-8. [DOI] [PubMed] [Google Scholar]
- 32.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 33.Van de Kar LD, Blair ML. Forebrain pathways mediating stress-induced hormone secretion. Front Neuroendocrinol. 1999;20(1):1–48. doi: 10.1006/frne.1998.0172. [DOI] [PubMed] [Google Scholar]
- 34.Rougé-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress‐induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998;10(12):3903–3907. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]


