Abstract
The aim of this study was to evaluate if prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) has a higher detection rate compared to standard contrast CT imaging for patients with a rising prostate-specific antigen (PSA) following definitive treatment (i.e., curative radical prostatectomy, radiotherapy, and brachytherapy) for prostate cancer in a private hospital setting. A retrospective single-site clinical audit was conducted on 150 PSMA PET/CT scans done for patients with a rising PSA after definitive treatment for prostate cancer. All studies were performed using I and T Ga-68 PSMA produced on a Scintomics radiopharmaceutical unit (Munich). All scans were performed on a GE 710 PET/CT scanner. All studies were compared to standard CT and other imaging. Of the 150 patients who had a 68Gallium (Ga)-PSMA PET/CT for a rise in their PSA levels, 102/150 (68%) of patients had PSMA-avid scans compared to the conventional imaging group which had an overall detection rate of 42% (63/150). The rates of detection were 100%, 90%, 92%, 67%, and 25% at PSA levels of >10 μg/L, 5–10 μg/L, >1.5 μg/L, 0.5–1.5 μg/L, and <0.5 μg/L, respectively. PSMA PET/CT also solely picked up 39/102 (38%) of prostate cancer relapses compared to the conventional imaging group. In our study of 150 patients with biochemical recurrence of prostate cancer, 68Ga-PSMA PET/CT demonstrated a superior detection rate (P < 0.05) compared to conventional imaging, including patients with low PSA levels (<0.5 μg/L).
Keywords: 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography, biochemical relapse, prostate cancer
INTRODUCTION
Prostate cancer is a major and growing health issue in Australia. It is the most commonly diagnosed cancer in males and is estimated to become the third most commonly diagnosed malignancy in Australia, after breast and colorectal cancers.[1] The estimated number of new cases of prostate cancer diagnosed in Australia in 2017 is 16,665 cases, while the projected percentage of prostate cancer in all new male cancer cases in 2017 is 23.1%.[1] Of patients with treated primary prostate cancer, it is estimated that 35% develop relapse within 10 years.[2] From the group of patients who develop biochemical recurrence, 30% will go on to develop radiographic evidence of metastatic disease within 8 years from the time of prostate-specific antigen (PSA) elevation.[3] This is more common in patients who present with high-grade disease (Gleason 8+)[4] or have a positive surgical margin following prostatectomy.[5] With new imaging modalities such as positron emission tomography (PET) and magnetic resonance imaging (MRI), there is emerging evidence that the disease may be more widespread at diagnosis than originally suspected.[6,7,8]
68Gallium (Ga)-PSMA PET/computed tomography (CT) is a PET molecular imaging technique, which targets prostate-specific membrane antigen (PSMA), a cell surface target that is highly expressed by nearly all prostate cancer cells when compared to nonmalignant conditions such as benign prostatic hyperplasia.[9] There have been a few studies which have shown Ga-PSMA hybrid imaging with PET/CT, which combines anatomical and functional aspects and has a higher metastatic detection rate in biochemical recurrence of prostate cancer.[10,11,12,13] This has resulted in patients not only being able to receive treatment earlier, but also having more targeted treatment to the site of recurrence.[14,15] The aim of this study was to evaluate if PSMA PET/computed tomography (CT) has a higher detection rate compared to standard contrast CT imaging for patients with a rising PSA following definitive treatment (i.e. curative radical prostatectomy, radiotherapy, and brachytherapy) for prostate cancer in a private hospital setting.
MATERIALS AND METHODS
A retrospective analysis was done on patients who had a 68Ga-PSMA PET/CT scan from May 2015 to April 2016 for a rising PSA after definitive treatment for prostate cancer. Patients were selected from the Oceanic Molecular Ga-68 PSMA PACS database. All patients had given written consent to data being utilized for research and audit purposes. Studies were performed using I and T, 68Ga-PSMA produced on a Scintomics radiopharmaceutical unit (Munich). All scans were performed on a GE 710 PET CT scanner and compared to whole-body diagnostic CT at the time of PET imaging.
Imaging
68Ga-labeled 1,4,7,10-tetraazacyclododecane-1-(glutamic acid)-4,7,10-triacetic acid-conjugated 68Ga-PSMA-I&T (here with referred to as 68Ga-PSMA-I&T) was produced by a qualified radiochemist in an in-hospital laboratory, operating under good laboratory practice conditions, using a Scintomics® (Munich, Germany) radiopharmaceutical synthesis unit. Quality control of the 68Ga-PSMA-I&T product was performed by thin-layer and high-performance liquid chromatography according to methods developed by others and described elsewhere.[16] All patients were fasted for a minimum of 2 h prior to administration of radiopharmaceutical. The 68Ga-PSMA-I&T was administered to patients via an intravenous bolus with an average activity of 180 MBq (±25%). The variation in injected tracer activity was due to the variable elution activities and efficiencies during the lifetime of the 68Ge/68Ga generator. No patient was administered <120 MBq of 68Ga-PSMA-I&T. PET acquisition was started after a mean uptake time of 45 min. All patients underwent a PET/CT scan on a GE Discovery 710 scanner (USA). All patients underwent a diagnostic CT of the abdomen and pelvis first after intravenous contrast. Total acquisition time was between 20 and 30 min with 3-min and 4-min bed positions for whole body and local images, respectively. CT acquisition parameters were as follows: helical, 100, 120, or 140 keV depending on patient morphology; coverage speed: 110 mm/s; pitch: speed: 1.375: 1/55; and rotation time 0.5 s.
The PET reconstruction methodology was as follows: Iterative using time of flight (GE VUE Point FX); three iterations, 18 subsets with the filter cutoff set at 6.4; a standard z-axis filter; 256 matrix; and measured attenuated correction and nonattenuated correction reconstructions.
Some patients had a delayed pelvic PET/CT scan when high urinary retention of 68Ga-PSMA-I&T was present in initial images. All images were read and reported by an experienced PET radiologist with over 10 years' experience in reporting PET and with specific training in 68Ga-PSMA PET/CT reporting. Subjective analysis and correlation with anatomical imaging was carried out to determine whether PSMA-avid disease was present.
Lesions that were visually considered as suggestive for relapses or bony metastases of prostate cancer were counted and analyzed with respect to their PSMA avidity, with visual analysis being used with the bladder as the reference for most intense PSMA uptake. There was no cutoff value of SUVmax used, as the values were found to be very variable. Equivocal results were reviewed by a second experienced nuclear physician with training in 68Ga-PSMA PET/CT, who was blinded from the report by the 1st reviewer. Correlative diagnostic CT was available for all studies, with a lymph node being considered positive for a nodal metastasis, when it was larger than 10 mm. Bone scintigraphy images were also available for comparison where relevant.
RESULTS
The mean age of patients in this study was 68.5 years, with a range of 45–84 years. The average PSA for the seventy patients was 9.22 ng/ml, ranging from 0.04 to 256 ng/ml. The detection rates for PSMA PET/CT and contrast diagnostic CT based on PSA levels are shown in Table 1. For statistical analysis of different parameters, a Chi-square test was used and a P < 0.05 was considered statistically significant.
Table 1.
Detection rates for PSMA PET/CT and contrast diagnostic CT based on PSA level
| PSA levels (µg/L) | 68Ga-PSMA PET/CT (%) | Contrast diagnostic CT (%) |
|---|---|---|
| <0.5 | 11/44 (25) | 2/44 (5) |
| 0.5-1.5 | 18/27 (67) | 11/27 (41) |
| >1.5 | 73/79 (92) | 50/79 (63) |
| 5-10 | 19/21 (90) | 12/21 (57) |
| >10 | 30/30 (100) | 18/30 (60) |
PET: Positron emission tomography; CT: Computed tomography; PSA: Prostate-specific antigen
Of the 150 patients who had a 68Ga-PSMA PET/CT for a rise in their PSA levels, 102/150 (68%) of patients had PSMA-avid scans compared to the diagnostic CT group which had an overall detection rate of 42% (63/150). In terms of PSA levels, the patients who has a 68Ga-PSMA PET/CT for PSA levels of <0.5 ug/L, only 11/44 (25%) of patients had a positive scan. The detection rate for PSA levels below 0.5 μg/L in conventional imaging group was 2/44 (5%) (P = 0.0017, 95% confidence interval [CI]: 7.7929–38.4637).
For patients with a PSA level between 0.5 and 1.5 μg/L, 68Ga-PSMA PET/CT detected 18/27 (67%) patients with disease and conventional imaging detection rate for PSA levels between <0.5–1.5 μg/L was 11/27 (41%) (P = 0.0576, 95%, CI: 3.04–50.87). For patients with PSA >1.5 μg/L, 68Ga-PSMA PET/CT detected 73/79 (92%) and conventional imaging detected 50/79 (63%) of recurrences (P < 0.0001, 95% CI: 15.53–41.60).
For patients who had a PSA between 5 and 10 ug/L, 68Ga-PSMA PET/CT detected 19/21 (90%), while conventional imaging detected 12/21 (57%) of recurrences (P = 0.0167, 95% CI: 3.26–57.68).
In the group of patients with a PSA >10 ug/L, 68Ga-PSMA PET/CT detected 30/30 (100%), while conventional imaging detected 18/30 (60%) of recurrences (P = 0.0001, 95% CI: 19.15–59.39).
PSMA PET/CT had a higher detection rate compared to conventional imaging, with 39/102 (38%) of prostate cancer relapses being solely picked up on PSMA PET/CT.
The smallest PSMA-avid lymph node detected was 5 mm, while the lowest PSA level in which the PSMA PET/CT showed recurrence of disease was 0.34 μg/L.
Detection rates for PSMA PET/CT compared to diagnostic CT for PSA levels of <0.5 μg/L, 0.5–1.5 ug/L, and >1.5 ug/L were found to favor PSMA PET/CT at all PSA levels [Figure 1].
Figure 1.
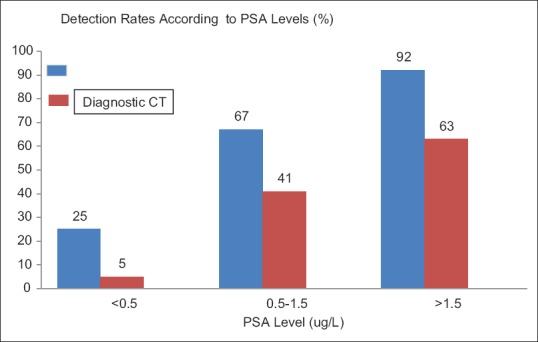
Comparative analysis between prostate-specific membrane antigen positron emission tomography/computed tomography and diagnostic computed tomography using prostate-specific antigen levels of <0.5 μg/L, 0.5–1.5 μg/L, and >1.5 μg/L
PSMA PET was found to be superior in its detection of disease recurrence postprostatectomy compared to conventional CT resulting in salvage radiotherapy to the prostatic bed [Figure 2]. This was also mirrored in another case study where PSMA PET allowed detection of disease activity with rising PSA levels postdefinitive treatment [Figure 3].
Figure 2.
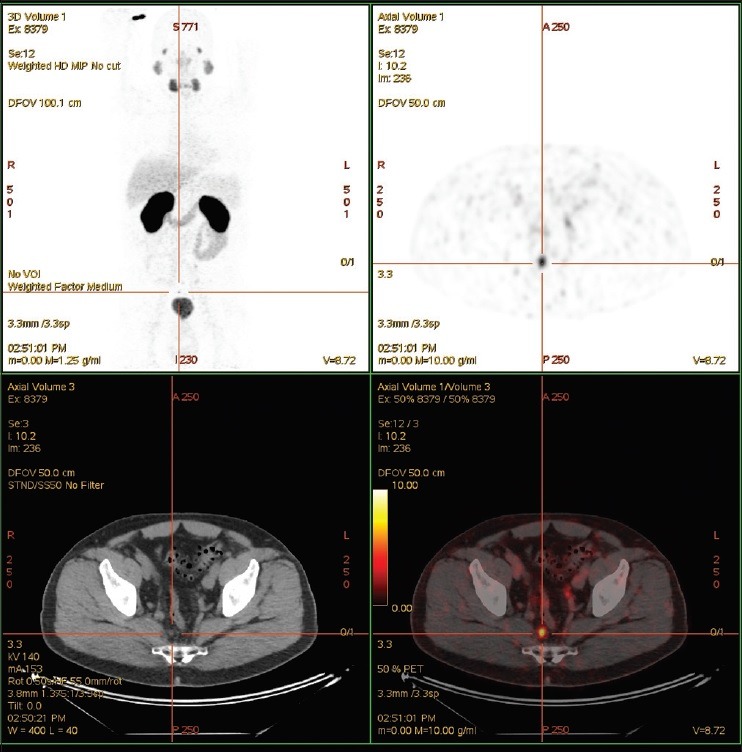
Patient had salvage radiotherapy to prostatic bed alone postradical prostatectomy. Prostate-specific antigen subsequently dropped to nadir of 0.08 in October 2012 and started to slowly rise. The last prostate-specific antigen in June 2015 was 1.87 μg/L. The patient had various staging scans over the last 2 years, all of which were normal. Prostate-specific membrane antigen positron emission tomography detected an avid pelvic lymph node. The patient went on to have targeted radiotherapy
Figure 3.
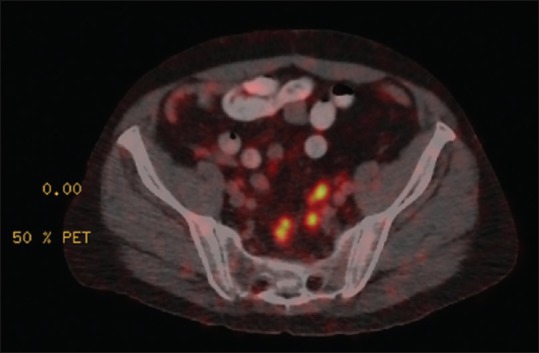
Initial treatment for this patient's prostate cancer was radical prostatectomy and radiotherapy. The prostate-specific antigen increased to 1.25 μg/L posttreatment. Prostate-specific membrane antigen positron emission tomography demonstrated avid lymph nodes in pelvis
PSMA PET was also able to detect skeletal metastases efficiency, which correlated with high PSA levels [Figure 4].
Figure 4.
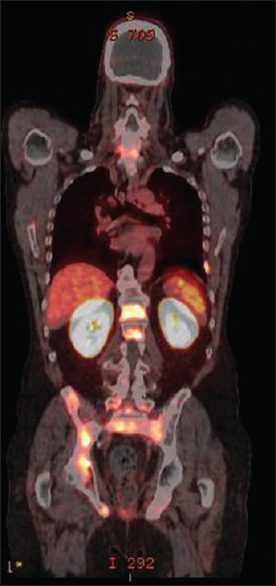
Skeletal metastases to the spine, rib, femur, and pelvis with a prostate-specific antigen level of 50 μg/L
DISCUSSION
As the life expectancy in Australia increases, the prevalence of prostate cancer continues to rise. At the end of 2012, there were 191,896 males living who had been diagnosed with prostate cancer in the previous 29 years (from 1982 to 2012).[1]
The definition of a biochemical recurrence by the American Association of Urology is detectable or rising PSA value after surgery that is ≥0.2 ng/ml with a second confirmatory level ≥0.2 ng/ml, while the European Association of Urology guidelines define biochemical recurrence after radical prostatectomy as an increase in the serum PSA value above 0.2 ng/ml and over a threshold of 2 ng/ml above the nadir value after radiation therapy.
There have been several studies which have shown that 68Ga-PSMA PET/CT imaging is superior to conventional imaging in the detection of recurrence of prostate cancer.[10,11,12,13]
This is mainly because other forms of imaging such as CT or MRI is primarily focused on morphological information of surrounding structures and in particular the size of regional lymph nodes in the determination of presumptive malignant involvement. Even when compared to other PET tracers such as 18F-fluoromethylcholine, there was a higher detection rate with 68Ga-PSMA PET/CT, as choline metabolism is not increased in a considerable number of cases in prostate cancer[17] and because PSMA appears particularly to be overexpressed in prostate cancer metastases.[18]
The advantage of 68Ga-PSMA PET/CT compared to diagnostic CT is its ability to detect prostate cancer recurrence with low PSA level.[19] As a molecular imaging technique, it can detect PSMA avidity at sites of locoregional and more distant lymph node involvement, whether these nodes are enlarged or not.[20] Prostate cancer most often metastasizes to pelvic lymph nodes, but as has been now appreciated with Ga-PSMA, PET/CT may often be present in more distant nodal stations.[21,22] 68Ga-PSMA PET/CT has also been shown to be superior to standard techniques such as bone scintigraphy at sites of osseous involvement in the restaging of relapsed prostate cancer.[23]
Often small-volume nodal metastasis is the reason for a small rise of PSA from a nadir postdefinitive treatment. This new imaging technique thus raises the possibility of potential early intervention with curative intent, i.e., patients with rising PSA levels will be able to get treatment at low PSA levels and at an earlier stage in their relapsed disease.[14] This requires further inquiry, but early intervention may lead to improvements in long-term outcome and possibly survival. This has been shown in previous radiotherapy studies whereby the 6-year progression-free survival in patients undergoing salvage radiotherapy ranged between 18% and 48% depending on their PSA levels prior to salvage treatment.[24]
There is a potential for false positives being reported in 68Ga-PSMA PET/CT,[25,26] and this is because PSMA receptors are also expressed in newly formed blood vessels and in some solid tumors such as renal cell carcinoma and glioblastoma also having overexpression of PSMA. It is often recommended that PSMA-avid lesions which are suspicious and not fitting into the clinical context be further evaluated.
This study found that 68Ga-PSMA PET/CT is a useful tool in the detection of recurrence prostate cancer, especially when PSA levels were >1.5 μg/L, where the detection rate was 92% in patients with a rising PSA postdefinitive therapy. However, with the high prevalence of patients with prostate cancer in the community, this scan's limitation is detecting relapsed prostate cancer in patients with low PSA levels (<0.5 μg/L) and thus may not be as cost-effective as an imaging test as compared with someone with a higher PSA level.
There has been a recent published study comparing 68Ga-PSMA-HBED-CC and 68Ga-PSMA-I&T as diagnostic agents for prostate cancer[27] which found that 96% of the lesions were found to have concordant uptake of each of these agents on PET/CT. Studies which used PSMA-HBED-CC had increased lesion binding in 2 out of the 47 lesions identified, and both lesions were lymph nodes which were <4 mm that were not visualized with PSMA-I&T. The inability to identify small nodal lesions could be a potential limitation of 68Ga-PSMA-I&T compared to 68Ga-PSMA-HBED-CC in restaging patients with a rising PSA. There has also been published data on a second-generation PSMA-targeted PET radiotracer for detection of metastatic prostate cancer, namely 18 F-PSMA-DCFPyL, which can provide a high image quality and visualize small prostate lesions with excellent sensitivity.[28,29] The advantage of using 68Ga-PSMA-I&T however is that it can be bound to both 68Ga and 177Lu, and can be used in a theranostic setting.
Only 25% (11/44) of patients had avid scans at PSA levels <0.5 ug/L, with the lowest PSA being measured at 0.34 μg/L. Although this highlights the limited utility of using GA-PSMA PET/CT in patients with very low PSA levels, the sensitivity of this test in this series was greater than diagnostic CT, and 68Ga-PSMA PET/CT is still the investigation of choice when patients are being considered for salvage treatment when there is concern of a relapse of their prostate cancer.[19]
Not all our patients from this study had accessibility to a whole-body bone scan, which in itself is of limited value in staging of patients with prostate cancer.[30] 68Ga-PSMA PET/CT has also been shown to be superior and more sensitive than standard bone scintigraphy in the detection of skeletal metastasis in patients with prostate cancer.[23]
Of particular note in our study, the relapsed prostate cancer was detected in almost 40% (39/102) of patients solely by 68Ga-PSMA PET/CT when compared to diagnostic CT. This finding is significant as again it demonstrates the superiority of 68Ga-PSMA PET/CT when prostate cancer recurrence is suspected. The smallest detectable lymph node using Ga-PSMA PET/CT was 5 mm in size (PSA level for the patient being 0.5 μg/L). This highlights the increased sensitivity compared with CT, which bases pathology predominantly on size criteria. The increased sensitivity and specificity of GAPSMA is also now been shown to improve diagnostic accuracy in primary staging by our group[31] and others.[32] This may alter the treatment paradigm and may improve results of primary therapeutic strategies though this has yet to be proven. Whether more accurate staging upfront will lead to lower rates of relapse/recurrence in the future needs to be proven. There has already been a published study on the impact of 68Ga-PSMA PET/CT in not only detecting suspected biochemical relapse but also influencing the planned clinical management in these patients.[15]
CONCLUSION
In our study of 150 patients with biochemical recurrence of prostate cancer, 68Ga-PSMA PET/CT demonstrated a superior detection rate (P < 0.05) compared to diagnostic CT, including patients with low PSA levels (<0.5 μg/L). The limitations of this study include it being a retrospective analysis and not a randomized controlled study. This study also had a small cohort of patients which often results in a relatively low statistical powered study.
The study however compares favorably with other studies when looking at detection rates for biochemical recurrence of prostate cancer and was undertaken to try and reciprocate the detection rates of 68Ga-PSMA PET/CT in a private health setting, in which to our knowledge, is one of the largest single private institution studies in Australia for patients with suspected biochemical relapse.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Australian Institute of Health and Welfare. Australian Cancer Incidence and Mortality (ACIM) Books. Australian Institute of Health and Welfare: Canberra, Australia; 2016 [Google Scholar]
- 2.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: Long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell DL, Russo JK, Mott SL, Tracy CR, Smith MC, Buatti JM, et al. Can high-grade prostate cancer (Gleason 8-10) Be cured with definitive local therapy without hormone suppression? Disease control and survival outcomes after up-front radical prostatectomy in patients with high-grade clinically localized disease. Clin Oncol. 2016;1:1057. [Google Scholar]
- 5.Silberstein JL, Eastham JA. Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol. 2014;30:423–8. doi: 10.4103/0970-1591.134240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamboglou C, Drendel V, Jilg CA, Rischke HC, Beck TI, Schultze-Seemann W, et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7:228–37. doi: 10.7150/thno.16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eiber M, Weirich G, Holzapfel K, Souvatzoglou M, Haller B, Rauscher I, et al. Simultaneous 68Ga-PSMA HBED-CC PET/MRI improves the localization of primary prostate cancer. Eur Urol. 2016;70:829–36. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 8.Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate cancer: Value of multiparametric MR imaging at 3 T for detection – Histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapidus RG, Tiffany CW, Isaacs JT, Slusher BS. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate. 2000;45:350–4. doi: 10.1002/1097-0045(20001201)45:4<350::aid-pros10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albisinni S, Artigas C, Aoun F, Biaou I, Grosman J, Gil T, et al. Clinical impact of 68 Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: Preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. doi: 10.1111/bju.13739. [DOI] [PubMed] [Google Scholar]
- 13.Sahlmann CO, Meller B, Bouter C, Ritter CO, Ströbel P, Lotz J, et al. Biphasic 68Ga-PSMA-HBED-CC-PET/CT in patients with recurrent and high-risk prostate carcinoma. Eur J Nucl Med Mol Imaging. 2016;43:898–905. doi: 10.1007/s00259-015-3251-y. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen PJ, Stricker P, Hruby G, Kneebone A, Ting F, Thompson B, et al. (68) Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 2016;117:732–9. doi: 10.1111/bju.13397. [DOI] [PubMed] [Google Scholar]
- 15.Roach PJ, Francis R, Emmett L, Hsiao E, Kneebone A, Hruby G, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: Results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–8. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 16.Weineisen M, Simecek J, Schottelius M, Schwaiger M, Wester HJ. Synthesis and preclinical evaluation of DOTAGA-conjugated PSMA ligands for functional imaging and endoradiotherapy of prostate cancer. EJNMMI Res. 2014;4:63. doi: 10.1186/s13550-014-0063-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasperzyk JL, Finn SP, Flavin R, Fiorentino M, Lis R, Hendrickson WK, et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:2354–63. doi: 10.1158/1055-9965.EPI-13-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabasakal L, Demirci E, Nematyazar J, Akyel R, Razavi B, Ocak M, et al. The role of PSMA PET/CT imaging in restaging of prostate cancer patients with low prostate-specific antigen levels. Nucl Med Commun. 2017;38:149–55. doi: 10.1097/MNM.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 20.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, et al. Diagnostic efficacy of (68)Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–43. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Jilg CA, Drendel V, Rischke HC, Beck T, Vach W, Schaal K, et al. Diagnostic accuracy of Ga-68-HBED-CC-PSMA-ligand-PET/CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics. 2017;7:1770–80. doi: 10.7150/thno.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, et al. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: Comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–9. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 23.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–21. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 24.Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobe C, Maintz D, Fischer T, Drzezga A, Chang DH. Prostate-specific membrane antigen PET/CT in splenic sarcoidosis. Clin Nucl Med. 2015;40:897–8. doi: 10.1097/RLU.0000000000000827. [DOI] [PubMed] [Google Scholar]
- 26.Rischpler C, Maurer T, Schwaiger M, Eiber M. Intense PSMA-expression using (68)Ga-PSMA PET/CT in a paravertebral schwannoma mimicking prostate cancer metastasis. Eur J Nucl Med Mol Imaging. 2016;43:193–4. doi: 10.1007/s00259-015-3235-y. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy M, Langton T, Kumar D, Campbell A. Comparison of PSMA-HBED and PSMA-I&T as diagnostic agents in prostate carcinoma. Eur J Nucl Med Mol Imaging. 2017;44:1455–62. doi: 10.1007/s00259-017-3699-z. [DOI] [PubMed] [Google Scholar]
- 28.Dietlein M, Kobe C, Kuhnert G, Stockter S, Fischer T, Schomäcker K, et al. Comparison of [(18)F] DCFPyL and [(68)Ga] Ga-PSMA-HBED-CC for PSMA-PET imaging in patients with relapsed prostate cancer. Mol Imaging Biol. 2015;17:575–84. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-based [(18)F] DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–9. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carmona Echeverria LM, Drudge-Coates L, Wilkins CJ, Muir GH. Bone scan is of doubtful value as a first staging test in the primary presentation of prostate cancer. ISRN Oncol. 2012;2012:585017. doi: 10.5402/2012/585017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyrick DP, Asokendaran M, Skelly LA, Lenzo NP, Henderson A. The role of 68Ga-PSMA-I&T PET/CT in the pretreatment staging of primary prostate cancer. Nucl Med Commun. 2017;38:956–63. doi: 10.1097/MNM.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 32.Fendler WP, Schmidt DF, Wenter V, Thierfelder KM, Zach C, Stief C, et al. 68Ga-PSMA PET/CT detects the location and extent of primary prostate cancer. J Nucl Med. 2016;57:1720–5. doi: 10.2967/jnumed.116.172627. [DOI] [PubMed] [Google Scholar]


