Abstract
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is highly expressed on the surface of prostate cancer (PC) cells, making it an excellent radiotracer for both therapeutic and diagnostic purposes. In this prospective study, we investigated the efficacy and toxicity of 177Lutetium (Lu)-PSMA in metastatic castration-resistant PC (mCRPC) patients for the establishment and approval of this therapy in Iran. Fourteen mCRPC patients (mean age 70.57 ± 7.3 years) were treated with a single dose of 177Lu-PSMA. Complete blood count, liver function tests (aspartate aminotransferase and alanine aminotransferase), alkaline phosphatase levels, renal function tests (urea and creatinine), and prostate-specific antigen (PSA) levels were obtained for the patients at baseline and every 2 weeks. A majority of the patients (11 patients, 64.2%) experienced a decline in their PSA levels; in 5 (45.4%) of these patients, the PSA levels declined > 50%.The severity of pain decreased in 8 (57.1%) patients, and performance status was improved in 5 (45.4%) patients. The treatment was well tolerated, and no severe hematological or nonhematological side effects were observed. Our findings show that 177Lu-PSMA had a high efficacy and a low toxicity in an Iranian population and is a promising treatment option for PC patients.
Keywords: 177Lutetium, metastatic castration-resistant prostate cancer, prostate cancer, prostate-specific membrane antigen, radionuclide therapy, theranostic
INTRODUCTION
Prostate cancer (PC) is the second most common cancer in men in the world; in Iran, it is the third most common cancer in men and the sixth most common cancer in the country.[1] The American Cancer Society estimates that there will be 164,690 new cases of PC and 29,430 deaths in the United States in 2018.[2]
Metastatic castration-resistant PC (mCRPC) is a lethal disease with a 5-year survival rate of about 31%.[3] mCRPC is defined as disease progression despite castrated levels of testosterone and may present with a continuous rise in serum prostate-specific antigen (PSA) levels, the progression of preexisting disease, and/or the appearance of new metastases.[4] Therefore, it is important to develop new therapies. The approved therapies for mCRPC include abiraterone and enzalutamide, as well as, chemotherapy with docetaxel, cabazitaxel, and radium-223.[5,6,7,8,9,10]
Prostate-specific membrane antigen (PSMA), also known as glutamate carboxypeptidase II, is a type II transmembrane protein that is highly expressed on prostate adenocarcinoma cells,[11,12] particularly on dedifferentiated tumors.[13,14] This unique characteristic makes PSMA a suitable radiopharmaceutical for PC diagnosis and therapy.
177Lutetium (Lu)-PSMA radioligand therapy (RLT) was first introduced by the German Cancer Research Centre at the University Hospital Heidelberg in 2015.[15] Initial studies have shown promising results with 177Lu-PSMA RLT for patients in advanced stages of PC.[16,17,18,19,20,21,22,23,24,25,26,27] Most of the published efficacy and safety data on 177Lu-PSMA have been derived from retrospective analyses. To our knowledge, only one prospective trial has investigated this treatment's efficacy and toxicity.[28] Since this is a new treatment option, and a limited number of studies have been conducted outside Europe, further studies are needed, especially in different ethnic groups. The aim of this study was to prospectively evaluate the toxicity and response to this novel therapeutic treatment in Iranians for the establishment and approval of this therapy in Iran.
MATERIALS AND METHODS
Patient selection and preparation
Inclusion criteria
mCRPC patients with distant metastases and progressive disease (as determined by PSA levels) after standard treatment (second-line antiandrogen treatment, if the patient could afford it, and chemotherapy, if the patient was medically eligible)
PSMA-positive metastases identified in pretherapeutic low-dose 177Lu-PSMA imaging; and
An Eastern Cooperative Oncology Group (ECOG) performance status score of 2 or lower and a life expectancy >12 weeks.
Exclusion criteria
Patients with urinary obstructive disease
White blood cell count ≤2000/ml, platelet count ≤75 × 103/ml, and creatinine >2 mg/dl; and
Chemotherapy or external radiation therapy within the last 4 weeks.
The decision to apply this therapy was made by an interdisciplinary tumor board according to the Iranian guidelines.[29]
99mTc-DTPA renal scintigraphy was performed to rule out obstructive disease. Patients with urinary obstructions were referred to the urologist before therapy.
Blood samples were obtained at baseline and every 2 weeks up to 8 weeks of therapy for the following evaluations: complete blood count (CBC), liver function tests (including aspartate aminotransferase [AST] and alanine transaminase [ALT]), alkaline phosphatase level, renal function tests (urea and creatinine), and PSA level. An ECOG performance status score and a Visual Analog Scale score for pain were used to evaluate the patients' general well-being and daily activities.
Pretherapeutic imaging
Pretherapeutic low-dose 177Lu-PSMA imaging was performed on all patients to ensure adequate PSMA expression on the tumoral lesions. Whole-body images were obtained 24 h after intravenous injection of 185 MBq of 177Lu-PSMA-617 [Figure 1].
Figure 1.
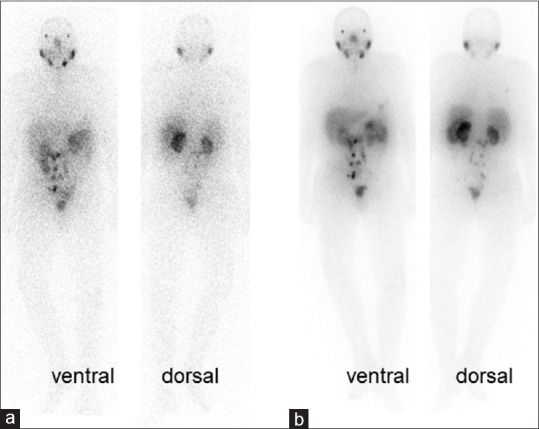
Patient number 8: 177Lutetium-prostate-specific membrane antigen whole-body scans in anterior and posterior projection 24 h after injection of diagnostic dose of 185 MBq (a) and therapeutic dose of 6 GBq (b) of 177Lutetium-prostate-specific membrane antigen, which are comparable in showing multiple lymph node involvement in the abdomen and pelvic cavity. He is a 78-year-old man who had undergone radical prostatectomy and radiation therapy. He could not afford second-line hormone therapy (abiraterone and enzalutamide) and was not a suitable candidate for chemotherapy. The baseline prostate-specific antigen = 3.1 ng/ml declined to 0.43 ng/ml after radioligand therapy
177 Letetium-prostate-specific membrane antigen preparation
PSMA-617 precursor (Advanced biochemical compounds, ABX, Germany) was radiolabelled with no-carrier added Lu-177 chlorides (Isotope technologies garching (ITG), Germany) in Pars Isotope radiopharmaceutical company, according to the manufacturer's instructions. Quality control was performed by an expert radiochemist before the administration of each dose and double-checked by the attending physician.
177 Letetium-prostate-specific membrane antigen administration
177Lu-PSMA was administrated by slow intravenous injection (5.5–6.5 GBq) within 30–-60 s followed by injection of 1000 ml isotonic saline solution. All patients were instructed to drink enough liquid before and after 177Lu-PSMA administration to stay hydrated. Cold compression of salivary glands with ice packs was started 30 min before to 4 h after injection of the radiopharmaceutical. According to the Iranian radiation exposure rules, the therapies were done in the outpatient setting of the department of nuclear medicine. The patients were observed until 4 h after injection of the Lu-PSMA and then discharged.
Posttherapeutic 177Letetium-prostate-specific membrane antigen imaging
At least one whole-body scintigraphic imaging was performed within 24 h of the 177Lu-PSMA administration to ensure uptake of the 177Lu-PSMA by the tumoral lesions.
Patient follow-up
All patients were seen every 2 weeks for at least 2 months. CBC, liver function tests (AST and ALT), alkaline phosphatase levels, renal function tests (urea and creatinine), and PSA levels were subsequently recorded.
A treatment response was defined by a decline in the patient's PSA levels and pain score according to the Visual Analog Scale. The ECOG status score was also documented at baseline and 8 weeks after therapy.
Toxicity
The Common Terminology Criteria for Adverse Events, version 4.03, was used to evaluate hematological toxicity and other adverse effects.
Ethical issues
This study was approved by the ethics committee of the University of Medical Sciences (ethic code: ir.sbmu.rec. 1396.135), and all local regulations for radiation protection were followed. Before initiating the therapy, a written informed consent for therapy and imaging studies was obtained from each patient. All patients received a consult from a nuclear medicine physician and were informed of the side effects and harms of this new therapy. The patients received this therapy under a compassionate use.
Statistical analysis
Statistical analyses were performed with the SPSS Statistics program version 21 (IBM Corp., Armonk, NY, USA). Data are shown as medians and ranges or as frequencies. The Kolmogorov–Smirnov test demonstrated that the variable distribution was skewed, so the Wilcoxon's signed rank test was used to compare the different groups. P ≤ 0.05 was considered statistically significant.
RESULTS
Between May 2017 and May 2018, among the 24 patients with mCRPC who were referred to the Nuclear Medicine department, 15 patients were eligible for enrollment in this study according to the inclusion and exclusion criteria. The patients' characteristics are shown in Table 1. All the 15 patients received at least one cycle of 177Lu-PSMA with an average dose of 5.7 GBq (range, 4.4–6.6 GBq). Seven patients agreed to continue treatment (receiving 2–6 further treatments). Two patients died during the study due to extensive metastatic disease: one patient died during the 7th week and one patient died during the 12th week after treatment (patient number 5). The first patient's data were excluded from the study because the 8-week follow-up was not completed. To our knowledge, the death of these patients was not related to the 177Lu-PSMA therapy. Table 2 shows the detailed characteristics of the 14 included patients.
Table 1.
Patient characteristics at baseline (n=14)
| Characteristics | Data |
|---|---|
| Age (year) | 70.57±7.3 (60-83) |
| Previous therapies, n (%) | |
| Second-line antiandrogen | 11 (78.6) |
| Chemotherapy | 11 (78.6) |
| Docetaxel + cabazitaxel | 5 (35.7) |
| Docetaxel | 6 (42.8) |
| External-beam radiation therapy | 8 (57.1) |
| Prostatectomy | 12 (78.5) |
| Gleason score (median) | 7.5 (4-10) |
| PSA (ng/ml) | 217.31±395.8 (0.4-1533) |
| Hemoglobin (g/dL) | 11.41±1.88 (7.3-13.3) |
| WBC (/dL) | 5475.7±1145.02 (2900-7000) |
| Platelets (×103/dL) | 224.86±55.752 (104.0-308.0) |
| Creatinine (mg/dL) | 1.0 (0.7-2.0) |
| Aspartate aminotransferase (U/L) | 21.0 (15.0-44.0) |
| Alanine transaminases (U/L) | 13.0 (7.0-25.0) |
| Pain (visual analog scale score) | |
| No pain | 5 |
| Mild pain (1-3) | 5 |
| Moderate-to-severe pain (4-10) | 4 |
| ECOG performance status | |
| 0 | 3 |
| 1 | 6 |
| 2 | 5 |
Qualitative data are expressed as numbers, followed by percentages in parentheses; continuous data are expressed as median, followed by range in parentheses. Pain (Visual Analog Scale score) is defined by score under 2, or 2 and more. PSA: Prostate-specific antigen; WBC: White blood cell; ECOG: Eastern Cooperative Oncology Group
Table 2.
Characteristic of 14 included patients with summary of therapeutic result
| n | Age | Cycles | GS | Previous therapy |
Known metastases |
PSA |
Pain score |
ECOG |
Hematologictoxicity |
Others | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUR | RT | CTx | Enz/Ab | Bone | LN | Lung | Liver | Brain | Pre | Post | Change (%) | Pre | Post | Pre | Post | PLT | WBC | Hb | |||||
| 1 | 66 | 6 | 8 | + | + | D/C | Enz | + | + | + | + | + | 0.4 | 0.05 | −87.5 | 0 | 0 | 2 | 1 | - | - | G1 | |
| 2 | 81 | 2 | 7 | - | + | D | Enz/Ab | + | - | - | - | + | 243 | 166 | −31.7 | 5 | 1 | 2 | 1 | G2 | G1 | G2 | |
| 3 | 69 | 1 | 4 | - | - | D/C | Enz | + | + | - | - | - | 47 | 24 | -48.9 | 0 | 0 | 0 | 0 | - | G1 | G1 | G1 nephrotoxicity |
| 4 | 69 | 1 | 6 | + | - | - | - | + | - | - | - | - | 14 | 12 | −14.3 | 2 | 1 | 0 | 0 | G1 | - | G1 | |
| 5 | 61 | 1 | 9 | + | - | D | Ab | + | - | - | - | - | 300 | 384 | 28.0 | 9 | 9 | 2 | 2 | G1 | G2 | G2 | Died at 12th week |
| 6 | 83 | 1 | 9 | + | + | D | Ab | + | + | - | - | - | 19 | 3.6 | −81.0 | 3 | 0 | 1 | 1 | - | - | - | |
| 7 | 60 | 1 | 10 | + | - | D | - | + | + | - | - | - | 59 | 34 | −42.4 | 3 | 1 | 1 | 1 | - | - | - | |
| 8 | 78 | 1 | 6 | + | + | - | - | - | + | + | - | - | 3.1 | 0.43 | −86.1 | 0 | 0 | 0 | 0 | - | - | - | |
| 9 | 71 | 3 | 7 | - | - | D/C | Ab | + | - | - | - | - | 218 | 100 | −54.1 | 0 | 0 | 1 | 1 | - | - | G1 | |
| 10 | 65 | 2 | 10 | + | + | D/C | Ab | + | - | - | - | - | 1533 | 2394 | 56.2 | 3 | 2 | 1 | 1 | - | G1 | G2 | |
| 11 | 67 | 3 | 7 | + | - | D | Enz | + | - | - | - | - | 138 | 31.9 | −76.9 | 3 | 0 | 1 | 1 | - | - | G1 | |
| 12 | 77 | 2 | 7 | + | + | - | Enz | + | - | - | - | - | 1.9 | 1.2 | −36.6 | 6 | 3 | 1 | 1 | - | G1 | G1 | |
| 13 | 76 | 1 | 9 | + | + | D | Ab | + | + | - | - | - | 325 | 283 | −12.9 | 0 | 0 | 1 | 1 | - | - | G1 | |
| 14 | 65 | 2 | 9 | + | + | D/C | Enz/Ab | + | - | - | - | - | 141 | 357 | 153.2 | 8 | 5 | 2 | 1 | G1 | - | G2 | |
GS: Gleason score; SUR: Surgery; RT: Radiation therapy; CTx: Chemotherapy; D: Docetaxel; C: Cabazitaxel; LN: Lymph node; PLT: Platelet; WBC: White blood cell; Hb: Hemoglobin; G: Grade, + : Positive, -: Negative
Of the patients included in the study, 11 (78.6%) had previously undergone prostatectomy and 11 (78.6%) had received chemotherapy (docetaxel and cabazitaxel in 7 [50%] and only docetaxel in 4 [28.5%] patients). The mean and median PSA levels prior to the therapy commencement were 217.3 and 59.8 ng/ml, respectively (range, 0.4–1533 ng/ml).
The extent of the patients' metastases is shown in Table 3. All but 1 (92.9%) of the patients had bone metastases based on previous conventional imaging (computed tomography scan, whole-body bone scan, magnetic resonance imaging, and PSMA scan). Lymph node metastases in three (21.4%) patients, lung metastases in two patients (14.3%), and brain and liver in one (7.1%) patient were detected.
Table 3.
Site of metastases
| Tissue or organ | Number of patients (%) |
|---|---|
| Skeletal system | 13 (92.9) |
| Lymph node | 6 (42.8) |
| Brain | 2 (14.3) |
| Liver | 1 (7.1) |
| Lung | 1 (7.1) |
Five patients (35.7%) were taking analgesics prior to the 177Lu-PSMA therapy. The Visual Analog Scale score for pain severity was 2 or more in eight patients. The blood, renal, and liver parameters before treatment are shown in Table 1.
Scintigraphy with 177Letetium-prostate-specific membrane antigen after therapy
As the radiopharmaceutical is primarily excreted through the kidneys, physiologic uptake was observed in the kidneys and the bladder as well as in the lacrimal and salivary glands and the liver, spleen, and the small intestine. Higher uptake of 177Lu-PSMA was detected in sites with metastases and residual tumors [Figures 2 and 3].
Figure 2.
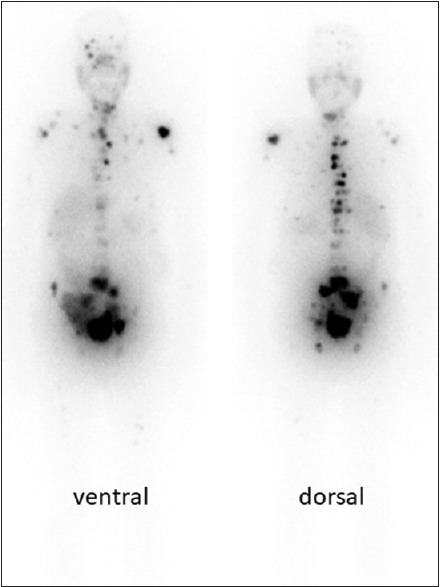
Patient number 11: 177Lutetium-prostate-specific membrane antigen scan 24 h after administration of the radiotracer (4440 MBq). He is a 67-year-old man with widespread bone metastases. He had undergone prostatectomy and been treated with docetaxel and enzalutamide. Gleason score = 7, prostate-specific antigen = 138, and creatinine = 0.8. Note the patient's nonfunctioning native kidneys and the transplanted kidney in the right hemipelvis
Figure 3.
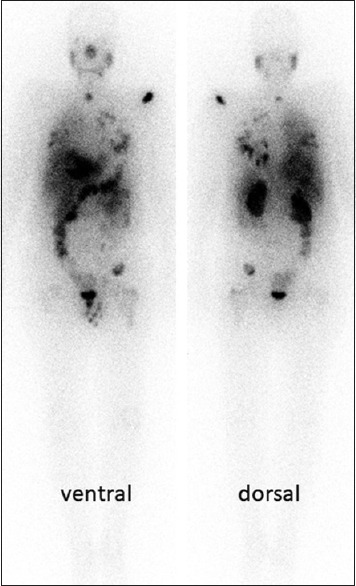
Patient number 1: 177Lutetium-prostate-specific membrane antigen scan 24 h after administration of the radiotracer (6.6 GBq). He is a 66-year-old man with multiple metastases in his lungs, bones, liver, and lymph nodes. His brain metastases had been treated by surgery and radiotherapy before radioligand therapy. He had also undergone treatment with docetaxel, cabazitaxel, abiraterone, and enzalutamide. Despite the diffuse metastases, the patient's baseline prostate-specific antigen dropped to 0.4 ng/ml, indicating a significant decline (−87.5%) after radioligand therapy
Response evaluation 2 months after therapy
Biochemical response
Over the 8-week follow-up period, PSA levels declined in 11 out of 14 (78.6%) patients, with PSA levels declining by 50% or more in 5 (45.4%) patients. Progressive disease, defined by more than a 25% rise in PSA, occurred in three (21.4%) patients. Figure 4 displays waterfall plots that indicate the percentage change in PSA at the 8-week follow-up. A subset of seven patients received repeated treatment, and five of those (patient numbers 1, 2, 9, 11, and 12) had a further PSA reduction (91.2%, 35.5%, 83.5%, 94.2%, and 81%, respectively).
Figure 4.
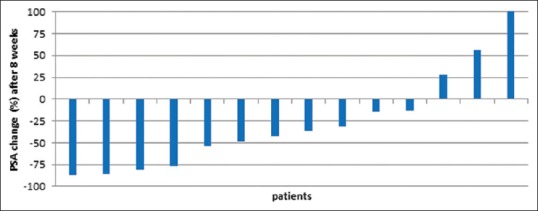
Waterfall curve showing percentage change in prostate-specific antigen values. Prostate-specific antigen increase >100% was cropped due to simplification
The two nonresponders (numbers 10 and 14) did not show any response during re-treatment and a continued PSA rise was recorded. However, one of these patients (patient number 14) had an improved pain score and a reduced analgesic consumption. In summary, 27 cycles were conducted [Table 2].
The mean serum alkaline phosphatase level declined from 569.5 U/L (range, 79–2667 U/L) to 498.4 U/L (range, 59–2023 U/L), but the difference was not statistically significant (P = 0.17).
Clinical symptoms
At baseline, nine (64.3%) patients with bone metastases reported skeletal bone pain. Among these, pain improved in eight (88.9%) patients after treatment. One patient showed no significant change in pain severity and required the continued use of analgesics (patient number 5). The remaining five patients had no pain complaints at the beginning of the study and did not complain of pain throughout the treatment.
The ECOG performance score improved in five (35.7%) patients. One patient's score worsened after therapy (patient number 5).
Toxicity and side effects
Complaints
There were no adverse effects immediately after the 177Lu-PSMA injections. No concerning changes in body temperature or blood pressure were recorded. There were no side effects in nine (64.2%) patients. During the 1st week after injection, one (7.1%) patient experienced myalgia; this was successfully treated with two 400-mg doses of ibuprofen administered daily for 1 week. Mild nausea was experienced by three (21.4%) patients and was the most common side effect within the first 2 days after injection. Ondansetron was used to effectively control the nausea. Mouth dryness was experienced by one (7.1%) patient; the condition showed significant improvement by the 8-week follow-up. Fatigue was reported in two (14.2%) patients. One patient complained of multiple side effects (nausea, fatigue, bone pain, myalgia, and parotiditis). Diarrhea did not occur in any patient. None of the patients asked to skip future doses due to intolerable side effects [Figure 5].
Figure 5.

Bar chart shows complaints of patients after administration of 177Lutetium-prostate-specific membrane antigen
Hematotoxicity
Table 4 illustrates the patients' toxicity values based on version 4.03 of the Common Toxicity Criteria for Adverse Events. There was no significant change in hemoglobin values (median ± standard deviation [SD]; prior to therapy: 11.7 ± 1.8 g/L; after therapy: 11.2 ± 1.8 g/L; P = 0.20). Three patients needed packed red blood cells prior to or after the therapy due to extensive bone metastases. Only two (14.2%) patients worsened one grade. None of the patients decreased more than one grade.
Table 4.
Toxicity based on Common Toxicity Criteria For Adverse Events, version 4.0
| Event | Baseline |
Post-RLT |
||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade4 | |
| Hemoglobin (%) | 8 (57.1) | 2 (14.2) | 0 (0.0) | 0 (0.0) | 7 (50.0) | 4 (28.5) | 0 (0.0) | 0 (0.0) |
| WBCs (%) | 1 (7.1) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 4 (28.5) | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| Platelets (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (21.1) | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| Creatinine (%) | 1 (7.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (14.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
WBCs: White blood cells
There was no statistically significant change in the white blood cell counts (median ± SD; prior to therapy: 5.4 ± 1.1 × 103/dL; after therapy: 4.6 ± 1.6 × 103/dL; P = 0.10) or the platelet counts (median ± SD; prior to therapy: 233.8 ± 55.7 × 103/dL; after therapy: 178.0 ± 78.4 × 103/dL; P = 0.10). Grade 1 or 2 leukocytopenia was observed in five (35.8%) patients. At the baseline, all patients had normal platelet counts, but after treatment, three (21.4%) patients with Grade 1 thrombocytopenia and one (7.1%) patient with Grade 2 thrombocytopenia were detected. None of the patients reported significant hemorrhagic episodes.
Nephrotoxicity and hepatotoxicity
There were no significant changes in the median values for serum creatinine or for the liver parameters as follows: serum creatinine (median ± SD; prior to therapy: 1.00 ± 0.34 mg/dL; after therapy: 0.91 ± 0.41 mg/dL; P = 0.38), AST (median ± SD; prior to therapy: 21.0 ± 8.6 U/L; after therapy: 19.5 ± 10.8 U/L; P = 0.97), and ALT (median ± SD; prior to therapy: 13.0 ± 4.8 U/L; after therapy: 12.5 ± 3.2 U/L; P = 0.26). Only one (7.1%) patient developed Grade 1 nephrotoxicity after therapy. Notably, one patient (number 11) had received a kidney transplant 9 years earlier. This patient was treated with three cycles of 177Lu-PSMA, with a cumulative activity of 13.3 GBq, and no change in the patient's creatinine value was noted during the follow-up period.
DISCUSSION
Cumulative data have confirmed the promising response rates and favorable safety profile of 177Lu-labeled PSMA ligands for the treatment of mCRPC patients, but this therapy has not yet received clinical approval.[11,30] Additional prospective studies are needed to make 177Lu-PSMA a first-line therapeutic.
In our study, PSA levels declined in a majority of patients (11 patients, 78.6%) after a single dose of 177Lu-PSMA therapy, and 5 (45.4%) of these patients experienced a PSA decline of more than 50%. In addition to the demonstrated efficacy and improved quality of life, 177Lu-PSMA was well tolerated, and no high-grade toxicity was recorded. Since a majority of the patients were anemic (Grades 1 or 2) at the beginning of the study, possibly due to progressive disease and extensive skeletal metastases, anemia (Grades 1 or 2) was commonly seen in the patients.
Since the primary excretion route of 177Lu-PSMA is through the kidneys, nephrotoxicity is a potential side effect. An optimal agent for renal protection is not yet available, since the routine use of nephroprotective agents for the blocking of PSMA binding sites in the kidneys has not been established in clinical studies.[31] In our study, repeated RLT cycles were well tolerated even in a patient with a transplanted kidney.
Previous retrospective studies on 177Lu-PSMA have reported similar results. Ahmadzadehfar et al. reported that 70% of ten patients experienced a decrease in PSA levels; of those, 71.4% experienced more than a 50% decline. Severe hematotoxicity (Grade 3 or 4) and Grade 2 leukopenia was only seen in one case, and these side effects were attributed to the patient's compromised bone marrow.[17] Another study by the same group investigated the efficacy and toxicity of repeated 177Lu-PSMA therapy on mCRPC patients and reported that PSA levels declined in 79.1% of the patients, with the PSA levels in 41.6% of these patients decreasing more than 50%. No severe hemato- or nephro-toxicity effects were detected except in two patients who experienced Grade 3 anemia.[16] A multicentric retrospective study by Rahbar et al. analyzed the data of 74 mCRPC patients treated with a single dose of 177Lu-PSMA. They found that PSA levels declined in 64% of the patients; in 31% of these patients, the PSA levels declined more than 50%. Again, no high-grade toxicity was reported.[23] A German multicentric study[20] investigated 145 patients with advanced PC; the data for 99 of these patients were available for analysis. The researchers found that after the first cycle of 177Lu-PSMA, 66% of the patients experienced a decline in PSA levels, while 40% of these patients experienced a decrease of more than 50%. After the second cycle, 72% of the patients experienced a decline in PSA levels and 57% experienced a PSA decline of more than 50%. This study also evaluated factors affecting the patients' biochemical response. The presence of visceral metastases and alkaline phosphatase levels of >220 resulted in lower biochemical response, whereas higher number of treatment cycles was associated with higher biochemical response.[20] In a recently published prospective trial, Hofman et al.[28] studied thirty patients with PSMA-avid mCRPC who had failed standard therapies and received up to four cycles of 177Lu-PSMA-617 every 6 weeks. This study found high response rates and low toxicity with improved quality of life and pain reduction in men with mCRPC who had failed conventional therapies.[28]
In accordance with the previous studies, our study confirmed the safety of 177Lu-PSMA treatment and demonstrated a favorable biochemical response and a low toxicity profile of RLT in patients with advanced PC. This efficacy and low toxicity may be due to the radiotracer's specific targeting of the tumor cells.
This prospective study was the first to investigate RLT with 177Lu-PSMA in mCRPC patients in our region. Most of the previous 177Lu-PSMA studies were conducted in Europe and Australia. Therefore, there is a need for more regional studies to evaluate the treatment response in different ethnic groups. The main limitation of our study was the lack of a control group. This would have allowed us to investigate whether the observed side effects were due to the therapy or whether they could be attributed to the disease progression.
Our patients entered this study after all the other available therapies had failed; therefore, they were at the end stage of the disease. Further studies should investigate the use of RLT in patients at earlier stages of the disease. Important questions should be addressed in future studies, including the optimal number of treatment cycles as well as the effect of patient characteristics (such as age, baseline PSA level, metastasis sites, type of bone metastases, Gleason score, and prior therapies) on the therapeutic response.
CONCLUSION
This prospective study demonstrated that treatment with 177Lu-PSMA was a safe and effective therapy in patients with mCRPC.
Financial support and sponsorship
Financial support was obtained in the form of research grant from the Cancer Research Center, Shahid Beheshti University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pakzad R, Rafiemanesh H, Ghoncheh M, Sarmad A, Salehiniya H, Hosseini S, et al. Prostate cancer in Iran: Trends in incidence and morphological and epidemiological characteristics. Asian Pac J Cancer Prev. 2016;17:839–43. doi: 10.7314/apjcp.2016.17.2.839. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Chi KN, Finelli A, Hotte SJ, Izawa J, Kapoor A, et al. The 2015 CUA-CUOG guidelines for the management of castration-resistant prostate cancer (CRPC) Can Urol Assoc J. 2015;9:90–6. doi: 10.5489/cuaj.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 8.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 9.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 10.Parker C, Sartor O. Radium-223 in prostate cancer. N Engl J Med. 2013;369:1659–60. doi: 10.1056/NEJMc1310231. [DOI] [PubMed] [Google Scholar]
- 11.Rahbar K, Afshar-Oromieh A, Jadvar H, Ahmadzadehfar H. PSMA theranostics: Current status and future directions. Mol Imaging. 2018;17:1536012118776068. doi: 10.1177/1536012118776068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoni M, Scarpelli M, Mazzucchelli R, Lopez-Beltran A, Cheng L, Cascinu S, et al. Targeting prostate-specific membrane antigen for personalized therapies in prostate cancer: Morphologic and molecular backgrounds and future promises. J Biol Regul Homeost Agents. 2014;28:555–63. [PubMed] [Google Scholar]
- 13.Wright GL, Jr, Haley C, Beckett ML, Schellhammer PF. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol. 1995;1:18–28. doi: 10.1016/1078-1439(95)00002-y. [DOI] [PubMed] [Google Scholar]
- 14.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 15.Kratochwil C, Giesel FL, Eder M, Afshar-Oromieh A, Benešová M, Mier W, et al. [177Lu] Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:987–8. doi: 10.1007/s00259-014-2978-1. [DOI] [PubMed] [Google Scholar]
- 16.Ahmadzadehfar H, Eppard E, Kürpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88. doi: 10.18632/oncotarget.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadzadehfar H, Rahbar K, Kürpig S, Bögemann M, Claesener M, Eppard E, et al. Early side effects and first results of radioligand therapy with (177) Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015;5:114. doi: 10.1186/s13550-015-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [177Lu] Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8:103108–16. doi: 10.18632/oncotarget.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadehfar H, Wegen S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu] Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–54. doi: 10.1007/s00259-017-3716-2. [DOI] [PubMed] [Google Scholar]
- 20.Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schäfers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. doi: 10.2967/jnumed.116.183194. [DOI] [PubMed] [Google Scholar]
- 21.Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligand therapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45:12–9. doi: 10.1007/s00259-017-3848-4. [DOI] [PubMed] [Google Scholar]
- 22.Rahbar K, Bögeman M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:243–6. doi: 10.1007/s00259-017-3877-z. [DOI] [PubMed] [Google Scholar]
- 23.Rahbar K, Schmidt M, Heinzel A, Eppard E, Bode A, Yordanova A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: A Multicenter retrospective analysis. J Nucl Med. 2016;57:1334–8. doi: 10.2967/jnumed.116.173757. [DOI] [PubMed] [Google Scholar]
- 24.Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: The Bad Berka experience since 2013. J Nucl Med. 2016;57:97S–104S. doi: 10.2967/jnumed.115.170167. [DOI] [PubMed] [Google Scholar]
- 25.Ahmadzadehfar H, Zimbelmann S, Yordanova A, Fimmers R, Kürpig S, Eppard E, et al. Radioligand therapy of metastatic prostate cancer using 177Lu-PSMA-617 after radiation exposure to 223Ra-dichloride. Oncotarget. 2017;8:55567–74. doi: 10.18632/oncotarget.15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yordanova A, Becker A, Eppard E, Kürpig S, Fisang C, Feldmann G, et al. The impact of repeated cycles of radioligand therapy using [177Lu] Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1473–9. doi: 10.1007/s00259-017-3681-9. [DOI] [PubMed] [Google Scholar]
- 27.Kabasakal L, AbuQbeitah M, Aygün A, Yeyin N, Ocak M, Demirci E, et al. Pre-therapeutic dosimetry of normal organs and tissues of (177) Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:1976–83. doi: 10.1007/s00259-015-3125-3. [DOI] [PubMed] [Google Scholar]
- 28.Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. doi: 10.1016/S1470-2045(18)30198-0. [DOI] [PubMed] [Google Scholar]
- 29.Ahmadzadehfar H, Aryana K, Pirayesh E, Farzanehfar S, Assadi M, Fallahi B, et al. The Iranian society of nuclear medicine practical guideline on radioligand therapy in metastatic castration-resistant prostate cancer using 177Lu-PSMA. Iran J Nucl Med. 2018;26:2–8. [Google Scholar]
- 30.Rahbar K, Ahmadzadehfar H, Boegemann M. 177Lu-PSMA-617 radioligand therapy in mCRPC: Ready for phase III trial? Eur J Nucl Med Mol Imaging. 2018;45:513–4. doi: 10.1007/s00259-017-3892-0. [DOI] [PubMed] [Google Scholar]
- 31.Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–8. doi: 10.2967/jnumed.114.147181. [DOI] [PubMed] [Google Scholar]


