Abstract
Gallium-68 labeled prostate-specific membrane antigen (Ga-68 PSMA) ligand (HBED-CC) is a novel tracer used for prostate cancer imaging. The aim of the study was to investigate the performance of Ga-68 PSMA positron emission tomography/computed tomography (PET/CT) in patients with biochemical recurrence (BCR) after definitive treatment. Scans of 96 consecutive patients were analyzed. Sixty-two patients received external beam radiotherapy, 34 underwent radical prostatectomy (RP), and 20 patients were on androgen deprivation therapy. Patients with prostate-specific antigen (PSA) level ≥>0.2 ng/mL following RP and PSA rise by 2 ng/mL or more above the nadir PSA following RT (Phoenix criteria) was considered as BCR, respectively. All patients underwent contrast-enhanced PET/CT after injection of 67–111 MBq Ga-68 PSMA ligand. Detection rates were correlated with serum PSA level. Detection rate for nodal metastases was compared with CT. Results of the scan were validated by using either biopsy or follow-up imaging or clinical follow-up. Seventy-four (77%) patients showed abnormal finding in Ga-68 PSMA PET/CT. The median serum PSA level of the population was 5.5 ng/ml (range 0.2–123 ng/ml). The median PSA of the positive scans was higher than that of the negative scans (6 vs. 1.7 ng/ml) and was statistically significant (P = 0.001 by Mann–Whitney U-test). In post-RP group, the detection rates were 23%, 50%, and 82% for PSA <1, 1–2, and >2 ng/ml, respectively. For post-RT, the detection was 86%, 85%, and 95% for PSA 2–5, 5.1–10, and >10 ng/ml, respectively. PSMA PET/CT revealed nodal metastases in 52 (54%) patients while CT showed pathological nodes only in 27 (28%) patients. Overall PSMA PET/CT revealed more number of nodes than CT (111 vs. 48 nodal station). PSMA PET/CT showed relapse in prostate/prostatic bed in 26 (27%) patients, nodal metastases in 50 (52%), skeletal metastases in 20 (21%), and other sites in 4 (4%) patients. Ga-68 PSMA PET/CT has high detection rate for localizing the site of recurrence in patients with biochemical failure and is superior to CT scan in the detection of nodal disease.
Keywords: Biochemical failure, biochemical recurrence, Gallium-68 Prostate-specific membrane antigen, positron emission tomography, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the second-most frequent cancer and sixth leading cause of cancer death in men worldwide.[1] Reports from 13 different population-based cancer registries in India showed a significant increase in the incidence of PCa.[2] Definitive treatment for nonmetastatic disease includes radical prostatectomy (RP) (with or without pelvic node dissection) or radiotherapy (RT) (with or without hormone therapy). About 35% of patients receiving definitive treatment present with rise in serum prostate-specific antigen (PSA) level (biochemical recurrence [BCR]) within 10 years of treatment. Among them, one-third of patients progress to metastatic disease within 8 years.[3]
The evaluation of patients with BCR includes calculation of PSA doubling time, imaging, and TRUS biopsy. Conventional imaging used for evaluation of BCR, such as abdominal and pelvic computed tomography (CT), magnetic resonance imaging (MRI), and bone scan have poor sensitivity for detecting recurrence especially at low-PSA level. Until recently, only bone scintigraphy and F-18 or C-11 Choline positron emission tomography/CT (PET/CT) scan (Choline PET) were the molecular imaging recommended by cancer societies for evaluation of BCR.[4] The yield of a bone scan is very poor unless the patient is symptomatic for bone pain or the PSA is more than 40 ng/ml.[5] Choline PET was previously used extensively for detecting disease in BCR, but studies have demonstrated limited sensitivity and specificity particularly in patients with PSA levels below 3 ng/mL.[6,7]
Recently, Gallium-68 Prostate-specific membrane antigen PET/CT (Ga-68 PSMA PET/CT) has shown promising potential in patients with BCR. PSMA is a transmembrane glycoprotein that is found on prostate epithelial cells, the small intestine, renal tubular cells, celiac ganglia, and salivary glands, but the expression is about 100-fold–1000-fold higher in PCa than in benign tissue.[8,9] Ga-68 PSMA PET/CT uses PSMA targeting ligand (Glu-urea-Lys (Ahx) conjugated through a acyclic radiometal chelator (HBED-CC), to the radioisotope Ga-68. This retrospective study was performed with an aim to calculate the detection rate of Ga-68 PSMA PET/CT in BCR and correlate it with serum PSA level. The detection rate of Ga-68 PSMA PET/CT for nodal metastases was compared to CT scan.
MATERIALS AND METHODS
Patients
Data of all PCa patients (from January 2014 to December 2017) with BCR following RP or RT who underwent Ga-68 PSMA PET/CT were extracted from institution's database. Patients with PSA level ≥0.2 ng/mL following RP and PSA rise by 2 ng/mL or more above the nadir PSA following RT (Phoenix criteria) was considered as BCR, respectively. Patients who were on hormonal treatment at the time of Ga-68 PSMA PET/CT were also included. A total of 96 patients were eligible for analysis. This single institute retrospective study was approved by IRB, and a waiver of consent was obtained.
Synthesis of tracer and acquisition of positron emission tomography/computed tomography
Ga-68 was obtained from a 68Ge/68Ga radionuclide generator (iTG generator) using dilute Hydrochloric acid (0.05N) as eluent. Labeling with the peptide (PSMA-HBED-CC) was done by using fluidic labeling module (iQS). Radiochemical purity (RCP) was tested using instant thin layer chromatography. Radiopharmaceutical was injected only if RCP was >95%.
No specific preparation was made for PSMA PET except for 4 h fasting before the scan since all patients underwent simultaneous contrast-enhanced CT scan. PET/CT acquisition was done on Philips Medical Systems, GEMINI TOF 64. Tracer was administered intravenously 60 min before the study and at a dose of 67–111 MBq. CT was acquired in caudocranial direction with parameters of slice thickness-2 mm, pitch-0.83, voltage 120 kV, FOV 600 mm, rotation time-0.5 s, automated mA, and image matrix-512 × 512. Eighty milliliters of low-osmolar nonionic intravenous contrast was administered in all eligible patients at a rate of 1.8 ml/s, and scan delay was 50 s. All PET scans were acquired in 3D-mode with an acquisition time of 2 min per bed position. Images were reconstructed iteratively using RAMLA algorithm.
Positron emission tomography/computed tomography image interpretation
Images were viewed on display system having extended brilliance workspace software version 4.5.3.40140 (Philips healthcare, cleveland, Ohio, USA), Philips healthcare. PET/CT studies were reviewed by two Nuclear Medicine Physicians. In case of discrepancy, a blind review by a third nuclear medicine physician was taken into consideration to reach a consensus. Focal increased uptake more than background corresponding to the anatomical structure (node, prostate/prostatic bed, and skeletal system) were considered positive for disease recurrence. For a node to be considered positive in CT, short axis measurement of 1 cm for oval and 8 mm for round node was taken as the threshold. PET/CT findings were validated by correlative imaging (CT/bone scan/MRI) or biopsy. In patients where correlative imaging or biopsy was not available, follow-up imaging or clinical follow-up was used for validation.
Statistical analysis
The data were represented as mean, median, or frequency (%) as appropriate. Demographic data were represented as mean or median. Detection rates were represented as frequency. The median PSA level of the positive scans and the negative scans were compared by using Mann–Whitney U-test. P < 0.05 was considered statistically significant.
RESULTS
Patient characteristics are shown in Tables 1 and 2. Of the total 96 patients, 34 were post-RP, 62 were post-RT, and 20 patients were on androgen deprivation therapy (ADT) at the time of PET/CT [Table 1]. The median PSA level of the population was 5.5 ng/ml [Table 2].
Table 1.
Prior treatment
| Prior treatment | No of patients |
|---|---|
| Total number of patients | 96 |
| Postradical prostatectomy | 34 |
| Postradiotherapy | 62 |
| On ADT at the time of scan | 20 |
ADT: Androgen deprivation therapy
Table 2.
Patient’s characteristics
| Patient characteristics | Median | Range |
|---|---|---|
| Age (years) | 71 | 45-84 |
| PSA level (ng/ml) | 5.5 | 0.2-123 |
| Gleason’s score* | 7 | 4-10 |
*Gleason’s score was not available for 10 patients. PSA: Prostate-specific antigen
Detection efficiency of Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography
Ga-68 PSMA PET/CT localized the site of recurrence in 74 (77%) patients [Table 3]. It revealed relapse in prostatic bed [Figure 1] in 26 (27%) patients, nodal metastases [Figure 2] in 50 (52%), skeletal metastases [Figure 3] in 20 (21%), and other visceral metastases [Figure 4] in 4 (4%) patients [Figure 5]. Statistically significant difference was noted between the median PSA level of the abnormal scans (6 ng/ml) and the normal scans (1.7 ng/ml) (P = 0.001 by Mann–Whitney U-test).
Table 3.
Detection rate of gallium-68 Prostate-specific membrane antigen positron emission tomography/computed tomography
| PSMA PET positive | PSMA PET negative | |
|---|---|---|
| Number of patients (%) | 74 (77) | 22 (23) |
| Median PSA level (ng/ml) | 6 | 1.7 |
PSA: Prostate-specific antigen; PSMA PET: Prostate-specific membrane antigen positron emission tomography
Figure 1.
Recurrence in prostate: Patient presented with serum prostate-specific antigen of 3 ng/ml following radical external beam radiotherapy. Maximum intensity projection image (a) shows intense focal tracer uptake in the region of prostate (arrow). Axial view of CT (b) and Fused (c) images confirms confirms the focal uptake in the prostate. Subsequently, the patient responded to salvage Brachytherapy and the serum prostate-specific antigen fell to 0.86 ng/ml
Figure 2.
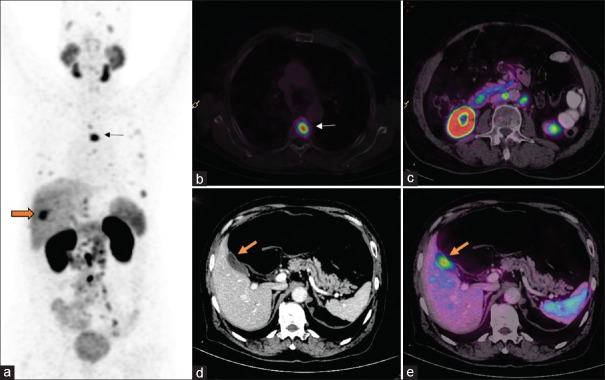
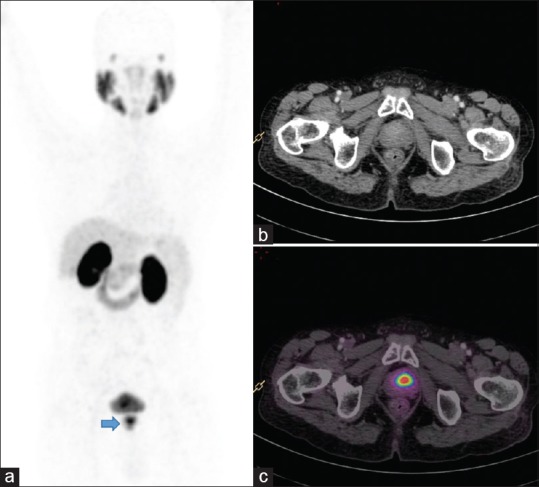
Nodal recurrence: Postexternal beam radiotherapy, the patient presented with serum prostate-specific antigen of 4 ng/ml. Whole body maximum intensity projection (a) image shows multiple focal tracer uptake in mediastinum, abdomen, and pelvis. Axial fusion images (c and e) show intense uptake in subcentimeter-sized retroperitoneal (block arrow) and mediastinal nodes (arrow), respectively. The size of all these nodes was not abnormal in CT (b and d). Patients were subsequently started on androgen deprivation therapy but progressed to castrate resistant disease
Figure 3.
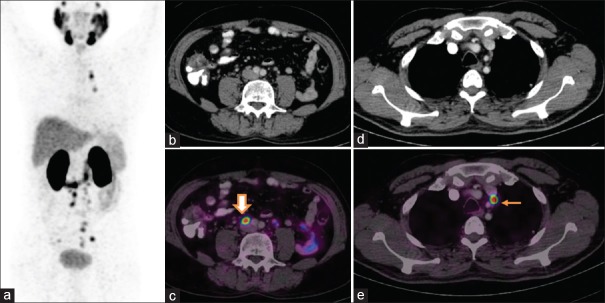
Skeletal metastases: Postexternal beam radiotherapy and adjuvant androgen deprivation therapy presented with prostate-specific antigen of 5.5 ng/ml. Maximum intensity projection images (a) show focal increased uptake in thorax, abdomen, and pelvis. The fused axial images reveal uptake in thoracic vertebrae (arrow) (b) and subcentimeter-sized retroperitoneal nodes (c). (Note: Increased tracer uptake noted in liver (block arrow) actually corresponds to gall bladder (d and e), and is not pathological but a physiological variant)
Figure 4.
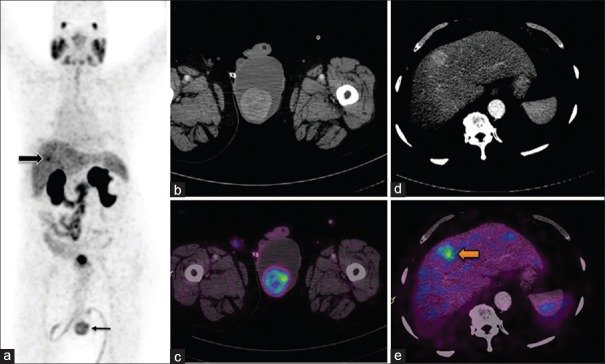
Visceral metastases: Following external beam radiotherapy, patient presented with scrotal swelling and rising prostate-specific antigen of 11.2 ng/ml. Maximum intensity projection (a) image show focal tracer uptake in liver (block arrow) and in scrotum (line arrow). Axial computed tomography (b) and fused images (c) show increased tracer uptake noted in the left testicular swelling. Images (d and e) show focal uptake in enhancing lesion in right lobe of liver. Subsequently, bilateral orchidectomy was done and the histopathology report confirmed metastatic adenocarcinoma and were positive for prostate-specific antigen in immunohistochemistry
Figure 5.
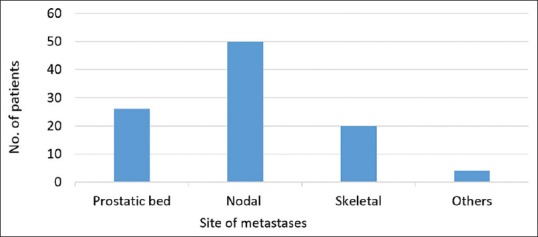
Histogram chart of number of patients with disease recurrence at various sites
Comparison with computed tomography scan
Out of the total 52 patients, who were positive for nodal recurrence in Ga-68 PSMA PET/CT, only 27 patients were detected by CT scan, demonstrating incremental value for PET/CT in 46% of patients [Figure 6]. Overall Ga-68 PSMA PET/CT revealed more number of nodes than CT (111 vs. 48 nodal station).
Figure 6.
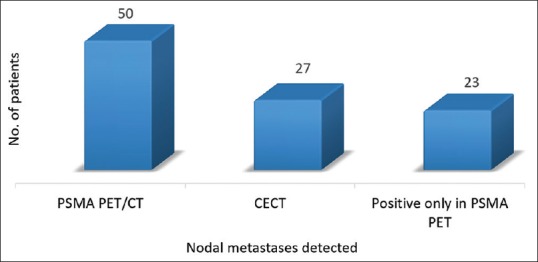
Comparison of detection rate of prostate-specific membrane antigen positron emission tomography/computed tomography and contrast-enhanced computed tomography for nodal metastases
Validation of results of Gallium-68 prostate-specific membrane antigen positron emission tomography/computed tomography scan
Out of the 74 patients that were positive for the PET scan, biopsy was done in five patients (7%), correlative imaging was available in 36 (49%), follow-up Ga-68 PSMA PET/CT scan in 11 (15%) [Figure 7], and clinical follow-up with serum PSA was available in 16 (22%). However, in 6 (8%) patients, the PET scan results could not be validated due to lack of further data.
Figure 7.
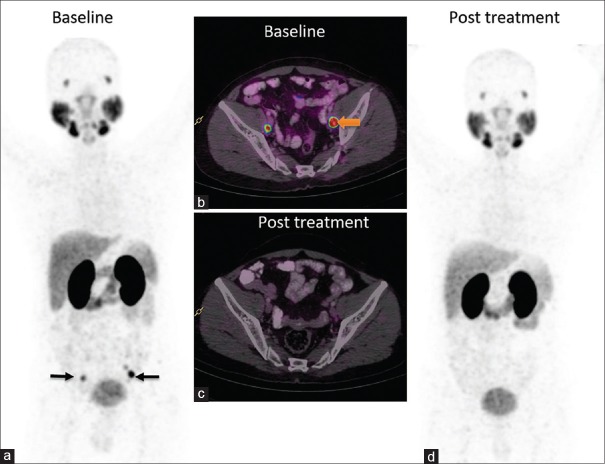
Postradical prostatectomy patient presented with prostate-specific antigen of 3 ng/ml. Whole body maximum intensity projection image (a) and fusion image (b) reveal intense uptake in subcentimeter-sized bilateral pelvic nodes (arrows). Following 6 months of androgen deprivation therapy, prostate-specific antigen decreased to 0.09 and follow-up prostate-specific membrane antigen positron emission tomography images (c and d) revealed complete resolution of prostate-specific membrane antigen expression in the pelvic nodes
Detection rate in postradical prostatectomy and radiotherapy group
In the post-RP cohort, the detection rates for Ga-68 PSMA PET/CT at various PSA levels were 23%, 50%, and 82% for PSA <1, 1–2, and >2 ng/ml, respectively, with an overall detection rate of 56% [Figure 8]. Out of 14 patients, with nodal metastases detected by Ga-68 PSMA PET/CT, only 6 (43%) patients were positive in CT scan.
Figure 8.
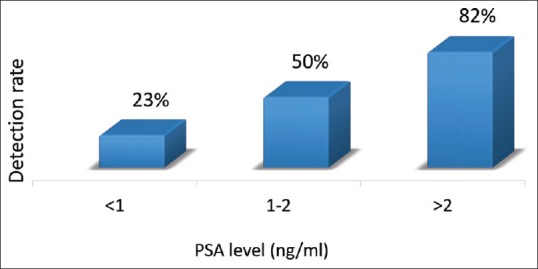
Frequency histogram of detection rate of prostate-specific membrane antigen positron emission tomography at various prostate-specific antigen level in postradical prostatectomy group
Overall detection rate of Ga-68 PSMA PET/CT for the post-RT group was 89%. Detection rates at various PSA levels were 86%, 85%, and 95% for PSA 2–5, 5.1–10, and >10 ng/ml, respectively [Figure 9]. Nodal recurrence was detected by Ga-68 PSMA PET/CT in 65% with an incremental value of 42% over CT.
Figure 9.
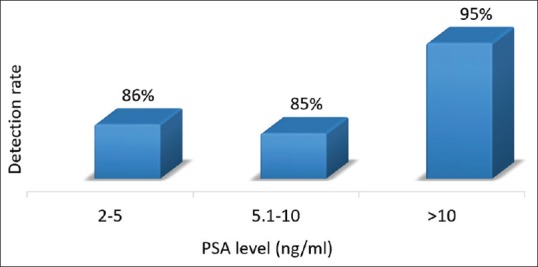
Frequency histogram of detection rate of prostate-specific membrane antigen positron emission tomography at various prostate-specific antigen level in postradical prostatectomy group
DISCUSSION
Salvage treatments of BCR are effective when the PSA level at the time of treatment is low.[10,11] Determining whether recurrence is local (within the prostate or prostatic bed), regional (pelvic), or distant (outside the pelvis) is critical when considering appropriate further treatment options. Conventional imaging modalities, CT, MRI, and bone scintigraphy have not been much useful for selecting patients for salvage treatments, owing to their low-detection rate at low-PSA level.[12] Threshold size used for defining metastatic node in CT/MRI varied in literature from 0.5 to 2 cm. In general, 1 cm in the short-axis nodal diameter for oval nodes and 0.8 cm for round nodes has been recommended as criteria for the diagnosis of lymph node metastases. A meta-analysis by Hövels et al. concluded that both CT and MRI performed poor in detecting nodal metastases in PCa patients with the pooled sensitivity of 42% (26%–56%, 95% confidence interval).[13] Biopsy is recommended whenever possible, but it is invasive, and not all suspicious lesions can be sampled. Hence, a whole body imaging which detects disease recurrence with high sensitivity in all sites is highly desirable. Ga-68 PSMA PET/CT is one such novel imaging modality which detects recurrence with high sensitivity.
In this study, Ga-68 PSMA PET/CT scan localized the disease in 77% of the patients (74 of 96). The detection rate reported in literature varies from 33% to 94%.[14] One of the main reasons for such wide range reported is the difference in the median PSA of the population under study. In the post-RP cohort, our overall detection rate was 56% (19 of 34) with median PSA level of the cohort being 2 ng/ml. The detection at various PSA level were 23%, 50%, and 82% for PSA <1, 1–2, and >2 ng/ml, respectively. Our results were relatively lower when compared to those reported by Eiber et al.[15] Possible explanations could be small study population and influence of other independent variables such as PSA doubling time, Gleason score, and surgical margin positivity which could not be analysed individually. However, similar results were also observed in studies which had smaller population.[16,17] About half of the patients with nodal metastases identified in Ga-68 PSMA PET/CT in post-RP group were negative in CT scan by size criteria [Figures 2 and 7].
The detection rate for the post-RT was 89% (55 of 62) and the median PSA level of the population was 6.7 ng/ml. Only few studies investigated the detection efficacy of Ga-68 PSMA PET/CT in patients with BCR as defined by phoenix criteria following RT. Einspieler et al. had reported similar detection rate of 90.7% in 118 patients with BCR as defined by Phoenix criteria.[18] The median PSA level (6.4) of their population was also similar to our cohort. The detection rate was higher for higher PSA level (i.e., 86%, 85%, and 95% for PSA 2–5, 5.1–10, and >10 ng/ml, respectively) as reported in literature. About 21% of the patients had relapse only in prostatic bed, and 10% had only regional node metastases. These patients were eligible for salvage treatment. For detecting nodal metastases, Ga-68 PSMA PET/CT had incremental value over CT in 42% (15 of 36) of the patients.
Due to the limited sensitivity of conventional imaging and slow progression of natural history of PCa, nomograms are generally used for predicting BCR and metastases.[19] With the development of new sensitive imaging modalities such as Ga-68 PSMA PET/CT, there is a scope for the development of new nomograms. We believe earlier detection of relapse using PSMA PET may offer the option of further targeted therapy with curative intent. Metastases identified in PSMA PET could be treated by novel treatment like PSMA-based radionuclide therapy, especially in patients not responding to conventional treatment.[20]
One of the main limitations of our study is histopathological correlation was not done in all patients to confirm the disease recurrence. Furthermore, the effect of ADT, Gleason's score, and PSA kinetic could not be analyzed due to small number of patient and lack of data.
CONCLUSION
Ga-68 PSMA PET/CT has high detection rate for identifying disease recurrence and can detect recurrence in prostate/prostatic bed, nodes, skeletal system, and other viscera. Serum PSA level at the time of scan has positive correlation to the scan finding. For detecting nodal metastases, Ga-68 PSMA PET/CT is superior to CT scan.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Jain S, Saxena S, Kumar A. Epidemiology of prostate cancer in India. Meta Gene. 2014;2:596–605. doi: 10.1016/j.mgene.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce JY, Lang JM, McNeel DG, Liu G. Current controversies in the management of biochemical failure in prostate cancer. Clin Adv Hematol Oncol. 2012;10:716–22. [PubMed] [Google Scholar]
- 4.Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–42. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Cher ML, Bianco FJ, Jr, Lam JS, Davis LP, Grignon DJ, Sakr WA, et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J Urol. 1998;160:1387–91. [PubMed] [Google Scholar]
- 6.Beer AJ, Eiber M, Souvatzoglou M, Schwaiger M, Krause BJ. Radionuclide and hybrid imaging of recurrent prostate cancer. Lancet Oncol. 2011;12:181–91. doi: 10.1016/S1470-2045(10)70103-0. [DOI] [PubMed] [Google Scholar]
- 7.Castellucci P, Ceci F, Graziani T, Schiavina R, Brunocilla E, Mazzarotto R, et al. Early biochemical relapse after radical prostatectomy: Which prostate cancer patients may benefit from a restaging 11C-choline PET/CT scan before salvage radiation therapy? J Nucl Med. 2014;55:1424–9. doi: 10.2967/jnumed.114.138313. [DOI] [PubMed] [Google Scholar]
- 8.Bařinka C, Rojas C, Slusher B, Pomper M. Glutamate carboxypeptidase II in diagnosis and treatment of neurologic disorders and prostate cancer. Curr Med Chem. 2012;19:856–70. doi: 10.2174/092986712799034888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52:637–40. doi: 10.1016/s0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 10.Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs. observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299:2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amling CL, Lerner SE, Martin SK, Slezak JM, Blute ML, Zincke H. Deoxyribonucleic acid ploidy and serum prostate specific antigen predict outcome following salvage prostatectomy for radiation refractory prostate cancer. J Urol. 1999;161:857–62. [PubMed] [Google Scholar]
- 12.Kane CJ, Amling CL, Johnstone PA, Pak N, Lance RS, Thrasher JB, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology. 2003;61:607–11. doi: 10.1016/s0090-4295(02)02411-1. [DOI] [PubMed] [Google Scholar]
- 13.Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin Radiol. 2008;63:387–95. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Fitzpatrick C, Lynch O, Marignol L. 68Ga-PSMA-PET/CT has a role in detecting prostate cancer lesions in patients with recurrent disease. Anticancer Res. 2017;37:2753–60. doi: 10.21873/anticanres.11627. [DOI] [PubMed] [Google Scholar]
- 15.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–74. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 16.Lengana T, van de Wiele C, Lawal I, Maes A, Ebenhan T, Boshomane T, et al. 68Ga-PSMA-HBED-CC PET/CT imaging in black versus white South African patients with prostate carcinoma presenting with a low volume, androgen-dependent biochemical recurrence: A prospective study. Nucl Med Commun. 2018;39:179–85. doi: 10.1097/MNM.0000000000000791. [DOI] [PubMed] [Google Scholar]
- 17.Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–90. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 18.Einspieler I, Rauscher I, Düwel C, Krönke M, Rischpler C, Habl G, et al. Detection efficacy of hybrid 68Ga-PSMA ligand PET/CT in prostate cancer patients with biochemical recurrence after primary radiation therapy defined by phoenix criteria. J Nucl Med. 2017;58:1081–7. doi: 10.2967/jnumed.116.184457. [DOI] [PubMed] [Google Scholar]
- 19.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score: A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117:5039–46. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferdinandus J, Violet J, Sandhu S, Hofman MS. Prostate-specific membrane antigen theranostics: Therapy with lutetium-177. Curr Opin Urol. 2018;28:197–204. doi: 10.1097/MOU.0000000000000486. [DOI] [PubMed] [Google Scholar]