Abstract
We evaluated the influence of aerobic training on cardiac remodeling in untreated spontaneously hypertensive rats (SHR). Four experimental groups were used: sedentary (W‐SED, n=27) and trained (WEX, n=31) normotensive Wistar rats, and sedentary (SHR‐SED, n=27) and exercised (SHR‐EX, n=32) hypertensive rats. At 13 months old, trained groups underwent treadmill exercise five days a week for four months. Statistical analysis: ANOVA or Kruskal‐Wallis. Exercised groups had higher physical capacity. Hypertensive groups presented left ventricular (LV) concentric hypertrophy with impaired function. Left atrium diameter, LV posterior wall thickness and relative thickness, and isovolumetric relaxation time were lower in SHR‐EX than SHR‐SED. Interstitial collagen fraction and Type I‐Type III collagen ratio were higher in SHR‐SED than W‐SED. In SHR‐EX these parameters had intermediate values between W‐EX and SHRSED with no differences between either group. Myocardial matrix metalloproteinase‐2 activity, evaluated by zymography, was higher in SHR‐SED than W‐SED and SHR‐EX. TIMP‐2 was higher in hypertensive than normotensive groups. In conclusion, low intensity aerobic exercise reduces left atrium dimension and LV posterior wall thickness, and improves functional capacity, diastolic function, and metalloproteinase‐2 activity in adult SHR.
Keywords: arterial hypertension, metalloproteinase, myocardial fibrosis, TIMP
1. INTRODUCTION
Physical exercise plays an important role in attenuating pathological cardiac remodelling.1 However, the effects of exercise under persistent arterial hypertension are less understood. Spontaneously, hypertensive rats (SHR) subjected to long‐term voluntary wheel running 2, 3, 4 have presented worse cardiac remodelling than sedentary SHR. In voluntary, wheel running SHR usually perform short series of relatively high‐intensity stints reaching many kilometres a day.5 It is therefore possible that a high‐intensity exercise programme produces deleterious cardiac effects when performed under increased afterload. In fact, low‐intensity swimming improved adverse remodelling and myocyte contractility in young SHR.6 We also observed that light treadmill exercise over four months reduced myocardial fibrosis and attenuated ventricular dysfunction in ageing SHR.7 In this study, we evaluated the influence of physical training performed before heart failure development on cardiac remodelling in adult SHR. As the effects of physical exercise on extracellular matrix changes during cardiac remodelling have been poorly addressed, our focus was on myocardial collagen tissue.
2. METHODS
The study protocol was approved by Botucatu Medical School Ethics Committee. Twelve‐month‐old male rats were divided into four groups: sedentary Wistar (W‐SED, n = 27); exercised Wistar (W‐EX, n = 31); sedentary SHR (SHR‐SED, n = 27); and exercised SHR (SHR‐EX, n = 32). Training protocol was started at 13 months of age and maintained for 16 weeks. We performed echocardiogram and assessed maximum functional capacity and blood pressure in non‐anesthetized rats before and at the end of the exercise.
Maximum exercise capacity was assessed on a graded treadmill as previously described.8 Exercise protocol consisted of 45 min/day treadmill running 5 days/week.9 The average treadmill velocity was 17 m/min.
2.1. Echocardiography
Echocardiogram was performed under anaesthesia as previously described.10, 11
2.2. Histology
Myocyte diameters were measured as the shortest distance between borders drawn across the nucleus.12 Sirius Red F3BA‐stained slides were used to quantify interstitial collagen fraction.13
2.3. Myocardial hydroxyproline
Hydroxyproline (HOP) concentration was assessed to estimate tissue collagen content (QuickZyme Hydroxyproline Assay, Leiden, Netherlands).
2.4. Western blotting
Protein levels were analysed by Western blotting 14 using antibodies Anti‐Type I collagen (sc‐8784‐r; Santa Cruz Biotechnology), Type III collagen (ab6310; Abcam), lysyl oxidase (ab60178; Abcam), TIMP‐1 (R&D Sistems, 150906), TIMP‐2 (Novus Biologicals, NB100‐92000), and GAPDH.
2.5. Zymography
Zymography of metalloproteinase (MMP)‐2 was performed as previously described.7
2.6. Statistical analysis
Results are expressed as mean and standard deviation or median and percentiles. Variables were compared by analysis of variance (ANOVA) for a 2 × 2 factorial design followed by Tukey's test or Dunn's test. Statistical significance: P < 0.05.
3. RESULTS
Blood pressure was higher in hypertensive groups than their respective controls and unchanged by exercise. Exercise increased functional capacity in exercised groups compared with sedentary rats (Figure S1).
Echocardiographic data are shown in Tables S1 and S2. Hypertensive groups presented higher LV diastolic diameter‐to‐body weight ratio, left atrium diameter (LA)‐to‐body weight ratio, LV mass index, LV posterior wall thickness, relative wall thickness, Tei index, and isovolumetric relaxation time (IVRT), and lower posterior wall shortening velocity than their controls. SHR‐EX had lower LV posterior wall thickness, LA, relative wall thickness, and IVRT than SHR‐SED. Exercise did not change systolic function.
Atrial weight was lower in SHR‐EX than SHR‐SED. Myocyte diameter was statistically larger in hypertensive groups than their controls (W‐SED 16.4 ± 1.11; W‐EX 15.5 ± 1.18; SHR‐SED 17.8 ± 0.96; and SHR‐EX 17.5 ± 0.45 µm). Interstitial collagen fraction (W‐SED 4.26 ± 1.19; W‐EX 5.20 ± 2.11; SHR‐SED 6.49 ± 1.00; and SHR‐EX 6.34 ± 1.92%) was higher in SHR‐SED than W‐SED. Hydroxyproline concentration did not differ between groups. Protein expression is shown in Table 1. Results from zymography of MMP‐2 are shown in Figure 1.
Table 1.
Protein expression
| W‐SED | W‐EX | SHR‐SED | SHR‐EX | |
|---|---|---|---|---|
| Collagen (Col) I | 1.00 ± 0.22 | 1.05 ± 0.10 | 1.45 ± 0.56 | 1.57 ± 0.64# |
| Col III | 1.00 ± 0.31 | 0.83 ± 0.15 | 0.82 ± 0.24 | 0.99 ± 0.24 |
| Col I/Col III | 1.00 ± 0.27 | 1.29 ± 0.33 | 1.79 ± 0.40* | 1.59 ± 0.53 |
| Lysyl oxidase | 1.00 ± 0.68 | 0.49 ± 0.15 | 1.10 ± 0.52 | 0.86 ± 0.39 |
| TIMP‐1 | 1.00 ± 0.28 | 1.03 ± 0.43 | 0.75 ± 0.45 | 0.70 ± 0.28 |
| TIMP‐2 | 1.00 ± 0.57 | 0.72 ± 0.25 | 3.68 ± 2.25* | 1.42 ± 0.67# |
Abbreviations: SHR‐EX: exercised SHR; SHR‐SED: sedentary spontaneously hypertensive rats (SHR); TIMP: tissue inhibitor of metalloproteinases; W‐EX: exercised Wistar rats; W‐SED: sedentary Wistar rats.
Data as mean ± SD.
ANOVA and Tukey's.
P < 0.05 vs W‐SED,
P < 0.05 vs W‐EX.
Figure 1.
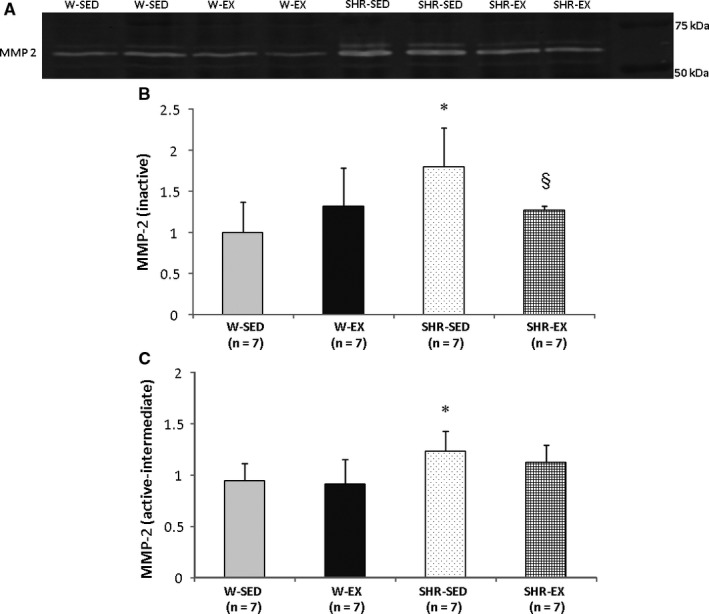
Representative zymography of myocardial matrix metalloproteinase (MMP)‐2 activity (A). Quantification of inactive metalloproteinase‐2 (MMP‐2) activity (B). Quantification of active and intermediate MMP‐2 activity (C). SHR‐EX: exercised SHR; SHR‐SED: sedentary spontaneously hypertensive rats (SHR); W‐EX: exercised Wistar rats; W‐SED: sedentary Wistar rats; Data are mean and standard deviation; ANOVA, and Tukey's; *P < 0.05 vs W‐SED; §P < 0.05 vs SHR‐SED
4. DISCUSSION
We applied a low‐intensity aerobic exercise protocol, previously shown to improve cardiac remodelling in ageing SHR.7 Final exercise test showed that the protocol was efficient in improving physical capacity. The fact that exercise did not change blood pressure is in accordance with previous studies in aged SHR 2, 7 and suggests that uncontrolled long‐term hypertension is not modulated by physical exercise.
Echocardiogram performed prior to exercise (data not shown) ensured a uniform assignment of rats between groups. As expected, a decrease in structural parameters observed in SHR‐EX was combined with improved diastolic function, characterized by a lower isovolumetric relaxation time in SHR‐EX than SHR‐SED.
Myocardial collagen fibres form a network regulating force transmission during myocyte shortening and resistance to pathological deformation.15 Type I collagen is formed of thick fibres with a high tensile strength, and Type III collagen is composed of fibres of small diameter with a lower tensile strength. The amount of collagen and the integrity of the extracellular matrix are modulated by MMPs and TIMPs. MMPS degrade components of the extracellular matrix and co‐ordinate tissue repair to normal and pathological growth. Excessive activation of MMP, especially MMP‐2 and MMP‐9, plays a key role in cardiac remodelling. TIMPs modulate MMPs by blocking their catalytic site.15
Interstitial collagen fraction and Type I‐Type III collagen ratio were higher in SHR‐SED than W‐SED. In SHR‐EX, these parameters had intermediate values between W‐EX and SHR‐SED with no significant differences with these groups, suggesting that exercise reduced the amount of collagen and attenuated change in the Type I‐Type III collagen ratio. Activity of inactive MMP‐2 was lower in SHR‐EX than SHR‐SED; TIMP‐2 expression was increased in both SHR‐SED and SHR‐EX compared with controls. Alterations in variables related to the extracellular matrix in SHR‐SED may be associated with changed collagen tissue, which may affect myocardial stiffness and contribute to LV dysfunction. The fact that SHR‐EX did not present these changes may have contributed to cardiac structural and functional improvement.
Studies on the effects of exercise in cardiac remodelling of hypertensive rats have produced disparate results. When subjected to voluntary wheel running, SHR had increased mortality and myocardial fibrosis with impaired LV dilatation, hypertrophy, and diastolic dysfunction.2, 3, 4 On the other hand, young 6 and old 7 SHR subjected to low‐intensity exercise showed improved cardiac remodelling and contractility with reduced fibrosis. Our study shows for the first time that light exercise is safe, reduces LA size, LV posterior wall thickness, and concentric cardiac remodelling, and improves functional capacity, diastolic function, and metalloproteinase‐2 levels in adult SHR. Therefore, data from this study and literature allow us to conclude that the intensity of physical training has a direct influence on adaptations occurring in cardiac geometry and function of untreated hypertensive rats.
CONFLICT OF INTEREST
Authors report no conflict of interest.
AUTHORS CONTRIBUTION
LUP and KO contributed to study design, manuscript writing, and fundraising; RLD, MJG, ARRL, MDMC, FCD, DRAR, BFP, TMMC, and MPO contributed to data collection. All authors have approved the final manuscript.
Supporting information
ACKNOWLEDGEMENTS
Financial support was provided by CNPq (308557/2018‐2, 310876/2018‐4); FAPESP (2014/00747‐1, 2015/17539‐5); CAPES; and UNESP.
Pagan LU, Damatto RL, Gomes MJ, et al. Low‐intensity aerobic exercise improves cardiac remodelling of adult spontaneously hypertensive rats. J Cell Mol Med. 2019;23:6504–6507. 10.1111/jcmm.14530
DATA AVAILABILITY
All data generated or analysed during this study are included in this published article.
REFERENCES
- 1. Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C. Exercise and heart failure: an update. ESC Heart Fail. 2018;5:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. da Costa Rebelo RM, Schreckenberg R, Schluter KD. Adverse cardiac remodelling in spontaneously hypertensive rats: acceleration by high aerobic exercise intensity. J Physiol. 2012;590:5389‐5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schreckenberg R, Horn A‐M, da Costa Rebelo RM, et al. Effects of 6‐months' exercise on cardiac function, structure and metabolism in female hypertensive rats‐the decisive role of lysyl oxidase and collagen III. Front Physiol. 2017;8:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schultz RL, Swallow JG, Waters RP, et al. Effects of excessive long‐term exercise on cardiac function and myocyte remodeling in hypertensive heart failure rats. Hypertension. 2007;50:410‐416. [DOI] [PubMed] [Google Scholar]
- 5. Schultz RL, Kullman EL, Waters RP, et al. Metabolic adaptations of skeletal muscle to voluntary wheel running exercise in hypertensive heart failure rats. Physiol Res. 2013;62:361‐369. [DOI] [PubMed] [Google Scholar]
- 6. Locatelli J, Paiva N, Carvalho S, et al. Swim training attenuates the adverse remodeling of LV structural and mechanical properties in the early compensated phase of hypertension. Life Sci. 2017;187:42‐49. [DOI] [PubMed] [Google Scholar]
- 7. Pagan LU, Damatto RL, Cezar M, et al. Long‐term low intensity physical exercise attenuates heart failure development in aging spontaneously hypertensive rats. Cell Physiol Biochem. 2015;36:61‐74. [DOI] [PubMed] [Google Scholar]
- 8. Gomes MJ, Martinez PF, Campos D, et al. Beneficial effects of physical exercise on functional capacity and skeletal muscle oxidative stress in rats with aortic stenosis‐induced heart failure. Oxid Med Cell Longev. 2016;2016:8695716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gimenes C, Gimenes R, Rosa CM, et al. Low intensity physical exercise attenuates cardiac remodeling and myocardial oxidative stress and dysfunction in diabetic rats. J Diabetes Res. 2015;2015:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reyes D, Gomes MJ, Rosa CM, et al. Exercise during transition from compensated left ventricular hypertrophy to heart failure in aortic stenosis rats. J Cell Mol Med. 2019;23:1235‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cezar M, Damatto RL, Martinez PF, et al. Aldosterone blockade reduces mortality without changing cardiac remodeling in spontaneously hypertensive rats. Cell Physiol Biochem. 2013;32:1275‐1287. [DOI] [PubMed] [Google Scholar]
- 12. Damatto RL, Lima A, Martinez PF, Cezar M, Okoshi K, Okoshi MP. Myocardial myostatin in spontaneously hypertensive rats with heart failure. Int J Cardiol. 2016;215:384‐387. [DOI] [PubMed] [Google Scholar]
- 13. Rosa CM, Gimenes R, Campos D, et al. Apocynin influence on oxidative stress and cardiac remodeling of spontaneously hypertensive rats with diabetes mellitus. Cardiovasc Diabetol. 2016;15:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lima A, Martinez PF, Damatto RL, et al. Heart failure‐induced diaphragm myopathy. Cell Physiol Biochem. 2014;34:333‐345. [DOI] [PubMed] [Google Scholar]
- 15. de Boer RA, De Keulenaer G, Bauersachs J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail. 2019;21:272‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.


