Abstract
Background:
Corticosteroids are the most common agents used in the treatment of alopecia areata (AA), however, their long-term use is associated with severe side effects. Therefore, other immunosuppressive agents have been tried and azathioprine appears to be an effective and promising alternative.
Objective:
The main objective of this study was to compare the efficacy of 300 mg once weekly azathioprine pulse (WAP) and 5 mg betamethasone on 2 consecutive days every week in the management of AA.
Materials and Methods:
In this open-label, randomized comparative study, 50 patients of AA with >10% scalp area involvement were treated with either 300 mg WAP or 5 mg betamethasone on 2 consecutive days every week for 4 months or till complete scalp hair regrowth and followed up for next 5 months. Primary efficacy parameters were average percentage scalp hair regrowth and change in average Severity of Alopecia Tool (SALT) score at 4 months.
Results:
Twenty patients in WAP group and 21 patients in betamethasone group completed the study. The median percent scalp hair regrowth and the median change in SALT score was 44.52 and 9.5 in WAP group compared to 71.43 and 14 in betamethasone group at 4-month, respectively, which were statistically similar in two groups, however, side effects were significantly higher in betamethasone group. On further follow-up at 9 months, 10 (50%) patients in WAP group and 13 (62%) patients in betamethasone group achieved complete hair regrowth. Lack of control group was a limitation of our study.
Conclusion:
WAP and betamethasone therapy, both appear to be effective in the treatment of AA. However, betamethasone caused several side effects; therefore, WAP can be used as a better alternative to corticosteroids in AA.
KEY WORDS: Alopecia areata, betamethasone, pulse therapy, severity of alopecia tool, weekly azathioprine
Introduction
Alopecia areata (AA) is a recurrent noncicatricial, autoimmune, chronic inflammatory patchy hair loss on the scalp and/or body.[1] Oral corticosteroids have shown high efficacy in moderate-to-severe disease, however, their long-term use results in serious side effects. Azathioprine has been shown to be efficacious in the treatment of this disease.[2]
Pulse regimens of immunosuppressive agents have been used in various immune-mediated dermatological conditions such as lichen planus, vitiligo, and parthenium dermatitis, with good results, favorable side effect profile, better compliance, and reduced cost of treatment.[3,4,5,6,7,8] Oral and intravenous corticosteroid pulse therapy has been used in the treatment of AA.[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23] Weekly azathioprine pulse (WAP) therapy has been shown to have better compliance and reduced cost of treatment, yet comparable efficacy, when compared to daily azathioprine in parthenium dermatitis.[7,8] In this open-label, randomized controlled, blinded, comparative and prospective study, we evaluated the effectiveness and side effect profile of WAP and betamethasone 5 mg on 2 days every week in the treatment of moderate-to-severe AA.
Materials and Methods
The study was approved by the Institutional Ethics Committee and written informed consent was obtained from each patient before enrollment.
Fifty consecutive patients of AA attending the outpatient department of our center were recruited. Patients of ≥18 years of age, with AA affecting ≥10% scalp area for at least 3 months, were included in the study [Table 1]. Patients having significant spontaneous regrowth of terminal hair, history of use of topical or intralesional therapy in the past 1 month and/or phototherapy and/or systemic therapy in the last 2 months, uncontrolled renal, hepatic, hematological or heart disease, or absolute contraindication to corticosteroids or azathioprine were excluded. Patients with alopecia universalis and pregnant or lactating women were also excluded. The diagnosis of AA in each patient was confirmed clinically by two dermatologists.
Table 1.
Demographic data and disease profile of the two groups
| Patient profile | Median (IQR) | P | |
|---|---|---|---|
| WAP (n=20) | Betamethasone (n=21) | ||
| Age* (years), mean±SD | 25.85±1.84 | 25.90±1.17 | 0.98 |
| Gender | |||
| Male** | 16 (80) | 13 (61.9) | 0.31 |
| Female** | 4 (20) | 8 (38.1) | |
| Total duration of illness (years) | 3.5 (1.58-8) | 5 (1-7) | 0.69 |
| Duration of current episode (years) | 1.58 (0.63-3) | 1 (0.4-2) | 0.39 |
| Average SALT score at baseline | 24 (14-58) | 19 (14-55) | 0.94 |
| Involvement of body sites other than scalp | 6 (30) | 7 (33.33) | 0.82 |
| Number of patients with first episode of AA | 13 (65) | 11 (52.4) | 0.41 |
| Number of patients with recurrent AA | 7 (35) | 10 (47.6) | |
*Data expressed in mean±SD, **Data expressed as frequency (%). IQR: Interquartile range, SD: Standard deviation, SALT: Severity of Alopecia Tool, AA: Alopecia areata, WAP: Weekly azathioprine pulse
Recruited patients were randomized into two groups using random number table generated online, to receive either 300 mg azathioprine once weekly (WAP) or 5 mg betamethasone on 2 consecutive days every week for at least 4 months or till complete regrowth, whichever was earlier. Patients showing no regrowth or further decline at 4 months were shifted to a combination therapy consisting azathioprine 100 mg daily and betamethasone 5 mg on 2 consecutive days in a week. The treatment was withdrawn in patients who achieved complete scalp hair regrowth and they were further followed up for 5 months.
Clinical details of each patient were recorded and severity of the disease was determined using severity of alopecia tool (SALT) score.[11] Baseline photographs of the standard 4 views of scalp under standard lighting conditions with a standard camera and camera settings were taken at first and subsequent visits. Baseline routine laboratory investigations such as complete blood counts, liver and renal function tests, routine and microscopic urine and stool examination, chest X-ray, and electrocardiogram were performed. Female patients were counseled about the need for contraception during the treatment period and 3 months following treatment. Complete blood count and liver and renal function tests were repeated on follow-up at every 4 weeks for initial 3 months and then every 8 weeks.
Primary efficacy parameters were comparison of average percentage scalp hair regrowth, average change in SALT score from baseline and side effects in two groups at the end of 4 months of treatment. Secondary efficacy parameters included comparison of percentage of patients who achieved SALT-50, median global assessment score, patient global assessment based on visual analog scoring from 0 to 100 after 4 months of treatment, percentage of patients showing cosmetically acceptable hair regrowth, Investigator Global Assessment by evaluation of photographs on a VAS of 0–100, proportion of patients not responding or worsening, and percentage of patients showing relapse. Relapse was defined as any loss of regrown hair or new AA patches, during the follow-up period.
Statistical analysis was done using STATA v11.2. (StataCorp, Texas, USA). Wilcoxon rank-sum test/t-test were applied for analyzing continuous variables. Chi-square test/Fisher exact test were used for analyzing categorical variables and a P<0.05 was considered statistically significant.
Results
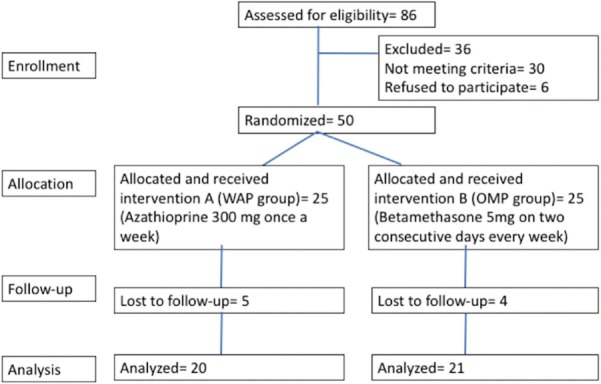
A total of 50 patients, 36 males and 14 females, between 18 and 46 years of age (mean age 26.6 ± 7.38 years) were included in the study. There were 25 patients in each group of which 20 patients in azathioprine pulse and 21 patients in betamethasone group completed the study [Figure 1]. Five patients in azathioprine group and 4 in betamethasone group were lost to follow-up. Three patients were lost to follow-up due to change in residence and inability to follow-up. One patient shifted to alternative form of therapy after taking 2 weeks of treatment and five patients could not be contacted despite repeated attempts. The demographic data of the two groups were comparable [Table 1]. The per-protocol analysis of the outcome was done.
Figure 1.
Flowchart showing the progression of the study
Primary efficacy parameter
The median SALT score at baseline (WAP=24 [14–58]; betamethasone group=19 [14–55]) and at 4 months (WAP=13.5 [5.5–26]; betamethasone group=8 [0–20]) as well as the percentage regrowth (WAP=44.52 [0-75.43]; betamethasone group=71.43 [11.11-100]) and change in SALT score from baseline to 4 month (WAP=9.5 [0-13.5]; betamethasone group=14 [4-19]) were comparable between the two groups [Table 2]. Sixteen (76.2%) patients in betamethasone group (n=21) developed facial puffiness/moon facies, 7 had weight gain, 5 each had reflux gastritis and steroid acne, 2 had striae distensae, and 1 patient had dermatophytic infection, whereas transient nausea lasting for a day with initial 1–2 doses was observed in 7 (35%) patients in WAP group (n=20). The side effects did not necessitate discontinuation of treatment in any patient. No significant changes in biochemical parameters were noted in either group.
Table 2.
Comparison of median percent scalp hair regrowth based on Severity of Alopecia Tool score and change in average Severity of Alopecia Tool score from baseline to 4 month of treatment
| Efficacy parameters | Median (IQR) | |
|---|---|---|
| WAP (n=20) | Betamethasone (n=21) | |
| SALT score at baseline | 24 (14-58) | 19 (14-55) |
| SALT score at 4 month | 13.5 (5.5-26) | 8 (0-20) |
| Change in SALT score | 9.5 (0-13.5) (P=0.009) | 14 (4-19) (P=0.001) |
| Percentage regrowth at 4 month | 44.52 (0-75.43) | 71.43 (11.11-100) |
IQR: Interquartile range, WAP: Weekly azathioprine pulse, SALT: Severity of Alopecia Tool
Secondary efficacy parameter
Eight (40%) patients in WAP group and 13 (61.9%) in betamethasone group achieved SALT-50 which was comparable in two groups (P=0.16). The median global assessment score at 4 months of treatment was 2 (The interquartile range [IQR]: 0–4) in WAP and 3 (IQR: 1–5) in betamethasone group which was also comparable (P=0.15) [Figures 2, 3 and Table 2].
Figure 2.
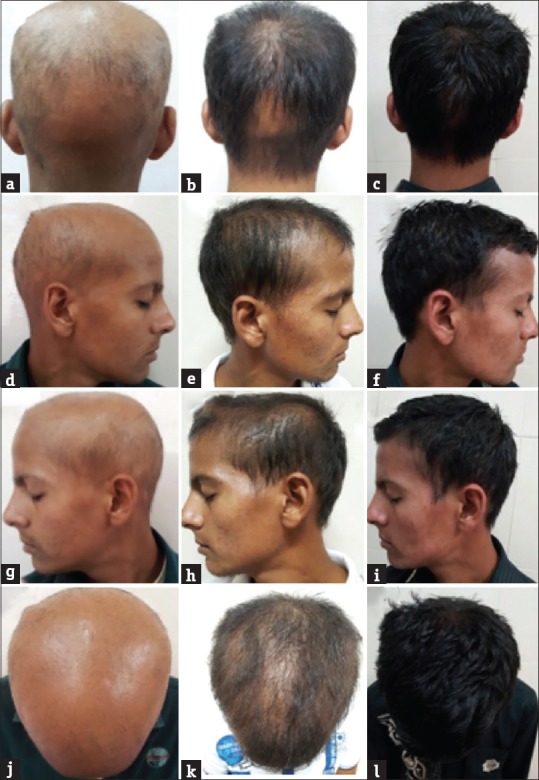
Hair regrowth seen with weekly azathioprine therapy, at 0 week (a, d, g, and j), 16 weeks (b, e, h, and k), and 36 weeks (c, f, i and l)
Figure 3.
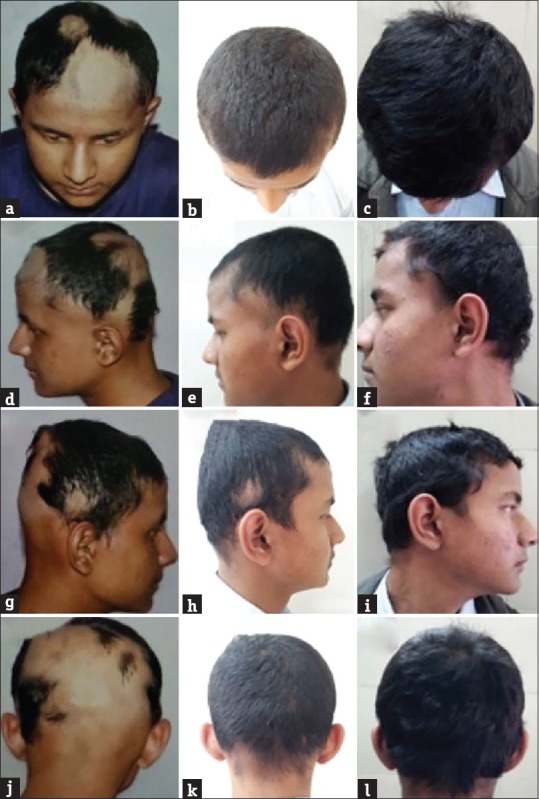
Hair regrowth seen with twice weekly betamethasone therapy, at 0 week (a, d, g, and j), 16 weeks (b, e, h, and k), and 36 weeks (c, f, i and l)
The median patient global assessment score at the end of 4 months of treatment was 45 (IQR: 0–82.5) in WAP group and 90 (IQR: 10–100) in betamethasone group which was statistically significant (P=0.03). The median investigator global assessment score at the end of 4 months of treatment was 32.5 (IQR: 0–72.5) in WAP group and 95 (IQR: 15–100) in betamethasone group (P=0.02). Seven (33.33%) patients in betamethasone group and none in WAP group achieved complete hair regrowth by 4 months of treatment. Cosmetically acceptable regrowth was seen in 8 (40%) patients in WAP group and 13 (61.9%) in betamethasone group (P=0.16). On further follow-up at 9 months, 10 (50%) patients in WAP group and 13 (62%) patients in betamethasone group achieved complete hair regrowth. One patient in each group relapsed at 2 month after complete regrowth of hair during follow-up period.
Seven (35%) patients in WAP group and 5 (23.8%) in betamethasone group did not experience any regrowth or had further decline in scalp hair coverage at the end of 4 months of treatment.
Discussion
Corticosteroids have been the most commonly used agent in the treatment of AA. These have been used in various doses and regimens with a success rate varying from 47% to 81.3%.[9,10,11,12,13,14,22,23,24,25,26,27] Pulse regimens of corticosteroids with methylprednisolone and dexamethasone used as monthly as well as weekly doses have resulted in improvements ranging from 37.8% to 81.3%.[12,13,14,15,16,17,18,19,20,21,22,23,27] Side effects are considered to be fewer with pulse regimens than daily corticosteroid doses [Table 3].[11,12,13,14,15,16,17,18,19,20,21]
Table 3.
Summary of studies using pulsed corticosteroid in alopecia areata
| Authors | Number of patients | Dose | Response | Side effects |
|---|---|---|---|---|
| Senila et al.[12] | 32 | 500 mg infusion of methylprednisolone on 3 days every month for 3 months | 26 (81.3%) | Facial flushing (17), headache and dizziness (9), asthenia (5), and palpitations (6) |
| Nakajima et al.[13] | 139 | Single cycle of 3 days methylprednisolone 500 mg infusion | 59.4% in disease duration <6 months, 15.8% in disease duration >6 months | No serious side effects |
| Friedli et al.[14] | 45 | Single cycle of 3 days methylprednisolone 500 mg infusion | 13 (65%) | No serious side effects |
| Seiter et al.[15] | 30 | 500 mg infusion of methylprednisolone on 3 days every month for 3 months | 12 (40%) | No serious side effects |
| Staumont-Sallé et al.[16] | 30 | 500 mg infusion of methylprednisolone on 3 days every month for 3 months | 10 (33.3%) | Tiredness (11), headache and flush (9), nausea and vomiting (7), and transient hyperglycemia (6) |
| Sharma and Gupta[17] | 30 | 5 mg dexamethasone two consecutive days every week | 16 (63.3%) | Minor side effects (8) |
| Lalosevic et al.[18] | 65 | Oral dexamethasone solution (equivalent to 5 mg/kg of prednisolone), in a single dose every 4 weeks for 6, 9, or 12 months with topical clobetasol propionate 0.05% ointment under occlusion | 37 (56.9%) | No serious side effects |
| Yeo et al.[19] | 82 | Methylprednisolone, 1 g/day, twice a day for 3 consecutive days for adults and 10 mg/kg/day weekly for 3 weeks for children for 6 months | 53 (64.6%) | GI discomfort, headache, dizziness, facial flushing, and palpitation in 16 (19.5%) patients |
| Yoshimasu et al.[20] | 55 | 500 mg/day of methylprednisolone infusion for 3 days for 6 months | Group 1 (<25%, 25%-49% hair loss): 100% growth Group 2 (50%-74%, 75%-99% hair loss):83% and 82% growth |
Myalgia and numbness (10), limbs edema (4), stomach discomfort (2), infection (2), arthralgia (1), burning sensation (1) |
| Khaitan et al.[11] | 16 | Betamethasone mini-pulse therapy for 6 months | 12 (75%) | Cushingoid facies and weight gain (2), acneiform eruption (2), mild gastric discomfort (3) |
| Jang et al.[21] | 37 | Betamethasone mini-pulse therapy for 3 months | 14 (37.8%) | Weight gain (11), facial edema (9), acneiform eruption (6), GI symptoms (4), headache/dizziness (4), skin atrophy (2) |
GI: Gastrointestinal
Betamethasone pulse has been used in a dose of 5 mg twice in a week in 16 patients of extensive AA, of which 12 (75%) patients experienced good to complete regrowth after 6 months of treatment, while the hair growth was unsatisfactory in 2 (12.5%) patients and 2 patients did not show any regrowth.[11] Jang et al. performed a comparative study of oral cyclosporine (n=51) and betamethasone mini-pulse therapy (n=37) and reported ≥50% of terminal hair regrowth in 54.9% and 34.8% patients, respectively.[21]
Although the corticosteroids used in various regimens have shown good response but have caused side effects even when used in pulsed regimens, and in some patients, the therapy had to be discontinued due to these side effects.[12,13,14,15,16,17,18,19,20,21,22,23] Azathioprine has been shown to be efficacious in the treatment of AA with lesser side effects.[2]
In our study, we used betamethasone 5 mg twice in a week and compared it with weekly 300 mg doses of azathioprine. Betamethasone is a long-acting corticosteroid (plasma half-life=300 min) and a dose of 0.75 mg is considered as an equivalent dose to 5 mg of prednisolone. Weekly 300 mg of azathioprine was used to improve the compliance and to reduce the cost of treatment.
On comparison with previous studies, our betamethasone group patients had >75% hair regrowth in 62% patients, which was comparable to Senila et al., Nakajima et al., Friedli et al., Sharma and Gupta, Khaitan et al., Lalosevic et al., and Yeo et al.,[10,12,13,17,18,19] but our results were better than the results reported by Seiter et al., Jang et al., and Staumont-Salle et al., which can be explained by a shorter duration of therapy in these studies.[14,15,21] Although corticosteroid-induced side effects were more frequently observed in our study than studies by Nakajima et al., Friedli et al., Seiter et al., Lalosevic et al., and Staumont-Salle et al., probably because of use of high dose shorter courses of corticosteroids in these studies, which also resulted in higher frequency of palpitations, headache, nausea, and dizziness in their studies.[12,13,14,15,21,27] Weight gain, Cushingoid appearance, and transient gastrointestinal discomfort were the most common side effects noted in our series as also with Khaitan et al. and Jang et al.[11,21] Relapse rate was much lower in our series (4.76%), compared to Nakajima et al. (16.7%), Friedli et al. (30.76%), Yeo et al. (20.0%), Lalosevic et al. (16.9%), and Seiter et al. (41.66%).[12,13,14,18,19]
Azathioprine, a purine antagonist, has been used in the treatment of AA.[28] In a study by Farshi et al., on 20 patients with moderate-to-severe AA, azathioprine in the dose of 2 mg/kg/day was administered.[2] There was 52.3% mean regrowth with a significant reduction in mean hair loss (72.7% to 33.5%) after 6 months of treatment and treatment had to be discontinued in one patient due to deranged liver function test. In our study, the mean hair regrowth was 44.52% which was comparable to the study by Farshi et al., however, none of the patients in our study experienced serious side effects warranting discontinuation of therapy, which suggested safety of weekly azathioprine regimen. The relapse rate and long-term follow-up were not systematically documented in the study by Farshi et al. In our study, one patient had relapse of disease after 2 months of discontinuing treatment. The thiopurine methyltransferase (TPMT) estimation was not done due to lack of laboratory facilities. Moreover, it has been shown that prior estimation of TPMT does not predict azathioprine-induced side effect/toxicity and routine hematological and biochemical investigations are adequate for follow-up monitoring.[29,30] In addition, a lower frequency of mutant TPMT alleles have been shown in Southwest Asian population (India, Pakistan), which further reduced the necessity of TPMT levels estimation.[31] Lack of a placebo group was a limitation of our study.
Conclusion
Both weekly betamethasone and WAP therapy appear to be effective in the treatment of AA. Although more patients in betamethasone group had regrowth after 4 months of treatment, which was associated with a higher global assessment score in this group, this difference was not statistically significant (P=0.15). However, several patients in betamethasone group had corticosteroids induced side effects; therefore, azathioprine can be used as a better tolerated and effective substitute for corticosteroids, particularly in patients who cannot be treated with corticosteroids due to any contraindication or to avoid corticosteroid-induced side effects. Further studies, however, are needed to confirm our findings.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Prof. R. M. Pandey, Department of Biostatistics, All India Institute of Medical Sciences, New Delhi, India, for his help in statistical analysis.
References
- 1.van der Steen P, Traupe H, Happle R, Boezeman J, Sträter R, Hamm H, et al. The genetic risk for alopecia areata in first degree relatives of severely affected patients. An estimate. Acta Derm Venereol. 1992;72:373–5. [PubMed] [Google Scholar]
- 2.Farshi S, Mansouri P, Safar F, Khiabanloo SR. Could azathioprine be considered as a therapeutic alternative in the treatment of alopecia areata? A pilot study. Int J Dermatol. 2010;49:1188–93. doi: 10.1111/j.1365-4632.2010.04576.x. [DOI] [PubMed] [Google Scholar]
- 3.Verma KK, Mittal R, Manchanda Y. Lichen planus treated with betamethasone oral mini-pulse therapy. Indian J Dermatol Venereol Leprol. 2000;66:34–5. [PubMed] [Google Scholar]
- 4.Pasricha JS, Khaitan BK. Oral mini-pulse therapy with betamethasone in vitiligo patients having extensive or fast-spreading disease. Int J Dermatol. 1993;32:753–7. doi: 10.1111/j.1365-4362.1993.tb02754.x. [DOI] [PubMed] [Google Scholar]
- 5.Verma KK, Manchanda Y, Pasricha JS. Azathioprine as a corticosteroid-sparing agent for the treatment of dermatitis caused by the weed Parthenium. Acta Derm Venereol. 2000;80:31–2. doi: 10.1080/000155500750012487. [DOI] [PubMed] [Google Scholar]
- 6.Verma KK, Pasricha JS. Azathioprine as a corticosteroid-sparing agent in air-borne contact dermatitis. Indian J Dermatol Venereol Leprol. 1996;62:30–2. [PubMed] [Google Scholar]
- 7.Verma KK, Bansal A, Sethuraman G. Parthenium dermatitis treated with azathioprine weekly pulse doses. Indian J Dermatol Venereol Leprol. 2006;72:24–7. doi: 10.4103/0378-6323.19713. [DOI] [PubMed] [Google Scholar]
- 8.Verma KK, Sethuraman G, Kalavani M. Weekly azathioprine pulse versus daily azathioprine in the treatment of Parthenium dermatitis: A non-inferiority randomized controlled study. Indian J Dermatol Venereol Leprol. 2015;81:251–6. doi: 10.4103/0378-6323.154788. [DOI] [PubMed] [Google Scholar]
- 9.Sharma VK. Pulsed administration of corticosteroids in the treatment of alopecia areata. Int J Dermatol. 1996;35:133–6. doi: 10.1111/j.1365-4362.1996.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 10.Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol. 2005;52:287–90. doi: 10.1016/j.jaad.2004.10.873. [DOI] [PubMed] [Google Scholar]
- 11.Khaitan BK, Mittal R, Verma KK. Extensive alopecia areata treated with betamethasone oral mini-pulse therapy: An open uncontrolled study. Indian J Dermatol Venereol Leprol. 2004;70:350–3. [PubMed] [Google Scholar]
- 12.Senila SC, Danescu SA, Ungureanu L, Candrea E, Cosgarea RM. Intravenous methylprednisolone pulse therapy in severe alopecia areata. Indian J Dermatol Venereol Leprol. 2015;81:95. doi: 10.4103/0378-6323.148608. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima T, Inui S, Itami S. Pulse corticosteroid therapy for alopecia areata: Study of 139 patients. Dermatology. 2007;215:320–4. doi: 10.1159/000107626. [DOI] [PubMed] [Google Scholar]
- 14.Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH, et al. Pulse methylprednisolone therapy for severe alopecia areata: An open prospective study of 45 patients. J Am Acad Dermatol. 1998;39:597–602. doi: 10.1016/s0190-9622(98)70009-x. [DOI] [PubMed] [Google Scholar]
- 15.Seiter S, Ugurel S, Tilgen W, Reinhold U. High-dose pulse corticosteroid therapy in the treatment of severe alopecia areata. Dermatology. 2001;202:230–4. doi: 10.1159/000051642. [DOI] [PubMed] [Google Scholar]
- 16.Staumont-Sallé D, Vonarx M, Lengrand F, Segard M, Delaporte E. Pulse corticosteroid therapy for alopecia areata: Long-term outcome after 10 years. Dermatology. 2012;225:81–7. doi: 10.1159/000341523. [DOI] [PubMed] [Google Scholar]
- 17.Sharma VK, Gupta S. Twice weekly 5 mg dexamethasone oral pulse in the treatment of extensive alopecia areata. J Dermatol. 1999;26:562–5. doi: 10.1111/j.1346-8138.1999.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 18.Lalosevic J, Gajic-Veljic M, Bonaci-Nikolic B, Nikolic M. Combined oral pulse and topical corticosteroid therapy for severe alopecia areata in children: A long-term follow-up study. Dermatol Ther. 2015;28:309–17. doi: 10.1111/dth.12255. [DOI] [PubMed] [Google Scholar]
- 19.Yeo IK, Ko EJ, No YA, Lim ES, Park KY, Li K, et al. Comparison of high-dose corticosteroid pulse therapy and combination therapy using oral cyclosporine with low-dose corticosteroid in severe alopecia areata. Ann Dermatol. 2015;27:676–81. doi: 10.5021/ad.2015.27.6.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimasu T, Kanazawa N, Yamamoto Y, Furukawa F. Multiple courses of pulse corticosteroid therapy for alopecia areata. J Dermatol. 2016;43:1075–7. doi: 10.1111/1346-8138.13388. [DOI] [PubMed] [Google Scholar]
- 21.Jang YH, Kim SL, Lee KC, Kim MJ, Park KH, Lee WJ, et al. A comparative study of oral cyclosporine and betamethasone minipulse therapy in the treatment of alopecia areata. Ann Dermatol. 2016;28:569–74. doi: 10.5021/ad.2016.28.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuyama M, Sato Y, Kinoshita-Ise M, Yamazaki Y, Ohyama M. Chronological clinicopathological characterization of rapidly progressive alopecia areata resistant to multiple i.v. corticosteroid pulse therapies: An implication for improving the efficacy. J Dermatol. 2018;45:1071–9. doi: 10.1111/1346-8138.14535. [DOI] [PubMed] [Google Scholar]
- 23.Shreberk-Hassidim R, Ramot Y, Gilula Z, Zlotogorski A. A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol. 2016;74:372–40. doi: 10.1016/j.jaad.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 24.Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467–73. [PubMed] [Google Scholar]
- 25.Joly P. The use of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia totalis or universalis. J Am Acad Dermatol. 2006;55:632–6. doi: 10.1016/j.jaad.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Kim BJ, Min SU, Park KY, Choi JW, Park SW, Youn SW, et al. Combination therapy of cyclosporine and methylprednisolone on severe alopecia areata. J Dermatolog Treat. 2008;19:216–20. doi: 10.1080/09546630701846095. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Aoshima M, Ito N, Uchiyama I, Sakamoto K, Kawamura T, et al. Combination therapy with oral PUVA and corticosteroid for recalcitrant alopecia areata. Arch Dermatol Res. 2009;301:373–80. doi: 10.1007/s00403-009-0936-8. [DOI] [PubMed] [Google Scholar]
- 28.Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711–34. doi: 10.1111/j.1365-2133.2011.10575.x. [DOI] [PubMed] [Google Scholar]
- 29.Sayani FA, Prosser C, Bailey RJ, Jacobs P, Fedorak RN. Thiopurine methyltransferase enzyme activity determination before treatment of inflammatory bowel disease with azathioprine: Effect on cost and adverse events. Can J Gastroenterol. 2005;19:147–51. doi: 10.1155/2005/470863. [DOI] [PubMed] [Google Scholar]
- 30.Benkov K, Lu Y, Patel A, Rahhal R, Russell G, Teitelbaum J, et al. Role of thiopurine metabolite testing and thiopurine methyltransferase determination in pediatric IBD. J Pediatr Gastroenterol Nutr. 2013;56:333–40. doi: 10.1097/MPG.0b013e3182844705. [DOI] [PubMed] [Google Scholar]
- 31.Collie-Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37–42. doi: 10.1097/00008571-199902000-00006. [DOI] [PubMed] [Google Scholar]