Abstract
Background:
Recent years have seen an alarming rise in the prevalence of recalcitrant and relapsing dermatophyte infections in India associated with lack of clinical response to standard antifungal regimens.
Aims and Objectives:
A study was undertaken to identify the antifungal susceptibility patterns of dermatophyte species isolated from lesions of dermatophytoses in patients examined at our center.
Materials and Methods:
A total of 85 patients with clinically diagnosed dermatophytoses were subjected to skin scrapings for potassium hydroxide mount (microscopic examination) and culture using Sabouraud's agar medium containing chloramphenicol and cycloheximide (incubated at 30°C). Antifungal susceptibilities [minimum inhibitory concentration-90 (MIC-90)] of the identified dermatophytes were tested for seven systemic and topical antifungal agents (terbinafine, griseofulvin, itraconazole, fluconazole, sertaconazole, ketoconazole, and clotrimazole) using Clinical and Laboratory Standards Institute broth microdilution method (M38-A).
Results:
Trichophyton rubrum (50%) and Trichophyton mentagrophytes complex (47.2%) were the two major species isolated. Isolates of both showed downy and granular forms (61.11%, 38.89% and 32.35%, 67.65%, respectively). The overall in-vitro susceptibility profiles (MIC-90 ranges in μg/mL) of the seven drugs for T. rubrum and T. mentagrophytes complex respectively were as follows: terbinafine (0.008–0256, 0.016–0.256), griseofulvin (0.03-1, 0.06–1), itraconazole (0.125-2, 0.25–2), fluconazole (0.125–1, 0.25–32), sertaconazole (0.03-1, 0.03-1), ketoconazole (0.06–1, 0.125–1), and clotrimazole (0.03–2, 0.06–1).
Conclusions:
This study indicates a rising proportion of T. mentagrophytes complex with increased proportion of granular form (T. mentagrophytes var. mentagrophytes). This study represents the current antifungal susceptibility profile of dermatophytic infections in a tertiary care medical center in western India with rising MICs to terbinafine and itraconazole.
KEY WORDS: Antifungal susceptibility, dermatophytes, minimum inhibitory concentration
Introduction
Within the past 5 years, there has been a sudden, unexplained surge in dermatophytoses in India.[1] This epidemic has been characterized by recurrent, recalcitrant infections characterized by unusual clinical patterns including erythrodermic dermatophytoses, pseudoimbricata patterns, and scalp involvement in adults.[2] These patterns of dermatophyte infections have been observed even in patients who have been compliant with standard antifungal therapies. However, there are very few studies documenting the present clinicoepidemiological patterns of dermatophytoses in India. More importantly, there are even fewer studies outlining antifungal susceptibility of common dermatophyte species in India. The paucity of epidemiological data, coupled with so far undetermined breakpoint minimum inhibitory concentration (MIC) values of antifungals against dermatophytes adds further to the difficulty in managing the current dermatophyte epidemic.
Hence, the present work was conducted to study the antifungal susceptibility patterns of dermatophytes isolated from lesions of dermatophyte infections at our tertiary care medical facility in western India.
Aims and Objectives
To identify the causative dermatophyte species responsible for dermatophytoses in patients reporting to the tertiary care center.
To determine the in-vitro MIC-90 of these dermatophyte species to seven antifungal agents: terbinafine, griseofulvin, itraconazole, sertaconazole, fluconazole, ketoconazole, and clotrimazole.
Materials and Methods
A total of 85 clinically diagnosed cases of dermatophytoses were included in this cross-sectional study. The study was conducted over a period of 1 year in a tertiary health care center of western India after institutional ethics committee approval. Their demographic details and clinical examination of cutaneous lesions were recorded.
Inclusion criteria
All clinically diagnosed cases of dermatophytoses.
Exclusion criteria
Any patient treated with oral/topical antifungals within 1 week prior to inclusion in the study
Any patient with suspected deep dermatophytoses
Any patient with secondary infection of dermatophytic lesions.
Mycology laboratory procedures
Scraping of the skin from the active border of the lesions was collected and transported under sterile conditions to the microbiology laboratory. In cases of tinea capitis, hair mount was taken and in tinea unguium, nail clippings were taken. Microscopic examination for the presence of fungi was performed after treatment of an aliquot of the skin scales with 10% potassium hydroxide (KOH). All specimens were also inoculated on Sabouraud's agar medium containing chloramphenicol and cycloheximide. These inoculated media plates were incubated at 30°C with daily observation for fungal colonies. Slide microcultures and lactophenol cotton blue mount were prepared for observing microscopic morphology of the fungal colonies. Species identification of dermatophytes was done on basis of gross and microscopic morphological characteristics including their pattern of conidiation and physiological tests (urease test and in-vitro hair perforation test).
Antifungal susceptibility testing
In-vitro antifungal susceptibility assay was performed by following the Clinical and Laboratory Standards Institute (CLSI) guidelines, document M38-A2 for filamentous fungi.[3] Dermatophytes were subcultured from the primary Sabouraud's agar plate to oat meal agar medium to induce conidiation. Plates were incubated at 35°C for 7 days or longer until colonies developed abundant spores.
The conidial spores were then carefully collected by gently flushing 5 ml phosphate buffer saline (pH = 7.4) on top of the colonies and aspirating the suspension into a sterile collection tube. The fungal spores were enumerated using a hemocytometer and cell numbers adjusted to 1 × 104 cells/ml. Susceptibility of isolates to seven antifungals was tested in the study; itraconazole, griseofulvin, and fluconazole were the systemic antifungals, while sertaconazole and clotrimazole were the topical antifungals. Ketoconazole and terbinafine are being used both as oral and topical preparations in India. The antifungal drug powders (terbinafine, griseofulvin, itraconazole, ketoconazole, fluconazole, clotrimazole, and sertaconazole) were purchased from Sigma-Aldrich. Stock solution of all drugs was prepared in dimethyl sulfoxide, at a concentration of 2 mg/ml (except fluconazole, which was dissolved in water).
Next, the drug stock solutions were diluted in Roswell Park Memorial Institute 1640 liquid medium (RPMI) buffered with MOPS (3-N-Morpholinopropanesulfonic acid), in twice the final concentration followed by addition of equal volume of the preadjusted inoculum of dermatophyte isolates, in 96-well microtiter plates.
The following final concentration ranges were tested: terbinafine (0.004–1.0 μg/ml), griseofulvin (0.03–4.0 μg/mL), itraconazole (0.06–32 μg/ml), ketoconazole (0.06–32 μg/ml), sertaconazole (0.007–4.0 μg/ml), fluconazole (0.125–64 μg/ml), and clotrimazole (0.06–16 μg/ml). All plates were incubated at 35°C for 4 days or longer, until sufficient growth (i.e., confluent hyphal growth covering the bottom of the well) was observed in control wells containing media without antifungal drug.[3] T. mentagrophytes ATCC MYA-4439 was chosen as a quality control strain and an in-house T. rubrum strain displaying consistently reproducible susceptibilities to MICs of all antifungal drugs tested were also used for comparative control purposes. MIC-90 (i.e., the concentration of the drug that inhibits 90% of growth of the fungus.) of the drug against the fungus in each well was detected visually by using a magnifying reading glass. The reading was taken at 4 days or until the day of sufficient growth of the fungus in the control well for comparison. Slide microscopy and culture identification by growth characteristics, pigment formation, and lactophenol cotton blue mount were used as criteria for nondermatophytes isolation).
Statistical analysis was performed by Mann–Whitney U test and Kruskal–Wallis tests using SPSS-16 software in order to find the significant differences between variables. P value of <0.05 was considered significant.
Results
Of the 85 patients diagnosed with dermatophytoses and included in the study, there were 41 females (48.2%) and 44 males (51.8%). Age range of the patients was 2–72 years (mean age: 30.42 ± 14.59). Extensive dermatophytic infection (lesions involving >20% body surface area or ≥ two noncontiguous sites) was present in 41 cases (48.23%) [Figure 1], while 44 cases (51.76%) had localized lesions. Among the 85 cases, co-occurrence of tinea corporis and tinea cruris (32 cases) was most common, followed by only tinea corporis (30 cases) and tinea cruris (10 cases). Face was involved in seven cases, whereas nail and scalp were the least common sites (5 and 1, respectively) [Table 1].
Figure 1.
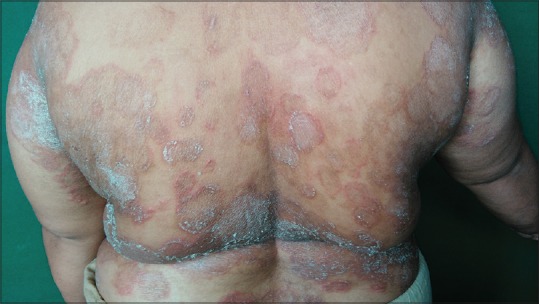
Extensive lesions of Tinea corporis in a middle-aged female patient
Table 1.
Various clinical presentations along with KOH and culture results
| Clinical presentation | (n) | KOH +ve | KOH -ve | Culture +ve | Culture -ve | TR | TM | Others | TRD | TRG | TMD | TMG |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. corporis | 30 | 27 | 3 | 27 | 3 | 10 | 17 | - | 4 | 6 | 3 | 14 |
| T. cruris | 10 | 8 | 2 | 7 | 3 | 5 | 2 | - | 1 | 4 | 1 | 1 |
| Onychomycosis | 3 | 2 | 1 | 2 | 1 | 1 | - | 1 | 1 | - | - | - |
| T. capitis | 1 | 1 | - | 1 | - | - | - | 1 | - | - | - | - |
| T. corporis + T. cruris | 32 | 29 | 3 | 26 | 6 | 15 | 11 | - | 12 | 3 | 4 | 7 |
| T. corporis + T. faciei | 4 | 4 | - | 4 | - | 2 | 2 | - | 1 | 1 | 2 | - |
| T. cruris + T. faciei | 1 | 1 | - | 1 | - | - | 1 | - | - | - | 1 | - |
| T. corporis + T. cruris + T. faciei | 2 | 2 | - | 2 | - | 1 | 1 | - | 1 | - | - | 1 |
| T. corporis + T. cruris + onychomycosis | 1 | 1 | - | 1 | - | 1 | - | - | 1 | - | - | - |
| T. cruris + onychomycosis | 1 | 1 | - | 1 | - | 1 | - | - | 1 | - | - | - |
TR: Trichophyton rubrum, TM: T. mentagrophytes complex, TRD: Trichophyton rubrum, downy form, TRG: T. rubrum, granular form, TRD: T. mentagrophytes, downy form, TMG: T. mentagrophytes, granular form
Microscopy and culture characteristics of dermatophytic fungi isolated from patient specimens
Out of 85 samples, 76 samples (89.4%) showed hyphae and spores on KOH examination, and 72 samples were culture positive (84.7%). Scrapings were taken from multiple sites so as to improve the culture outcomes and also to determine if different species cohabitated in one patient at a time.
There was an abundance of hyaline, long, smooth, septate, branching hyphae on KOH mounts of 72.36% of the specimens [Figure 2].
Figure 2.
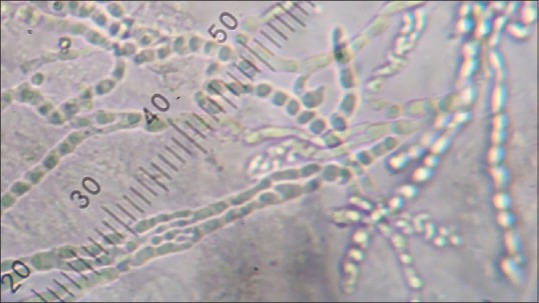
KOH mount: Showing abundant hyaline branching septate hyphae and chains of arthroconidia (×200)
T. rubrum (n = 36, 50%) and T. mentagrophytes complex (n = 34, 47.22%) were the predominant species identified with an almost equal frequency of occurrence; whereas T. violaceum was isolated in one patient of tinea capitis (1.39%). Acremonium was isolated from one suspected case of onychomycosis [Figures 3, 4 and Table 2].
Figure 3.
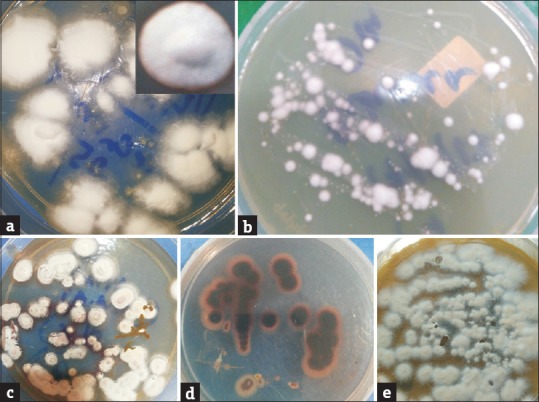
(a) Downy velvety white raised colonies of Trichophyton rubrum on SDA at 30°C at 2 weeks. Inset: close up view (b) White downy colonies of Trichophyton mentagrophytes at 30°C at 2 weeks (c) Thin powdery colonies of Trichophyton rubrum (Granular form) at 1 week with vinaceous-red pigment seen at the margins (Obverse view) (d) Vinaceous red pigment formed by the primary culture of the granular forms of Trichophyton rubrum seen distinctly (Reverse view) (e) Rapidly growing chalky-granular creamy white colonies of Trichophyton mentagrophytes on SDA at 30°C at 1 week
Figure 4.
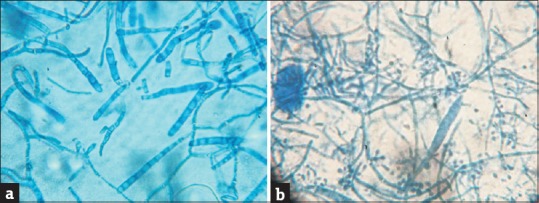
(a) Lactophenol cotton blue (LPCB) mount of Trichophyton mentagrophytes showing abundance of septate, elongated, and cylindrical macroconidia (×100). (b) LPCB mount of Trichophyton rubrum showing plenty of pear-shaped, microconidia arranged in engreppe and enthyrse pattern with a long thin cylindrical macroconidium (×100)
Table 2.
Species isolated on culture with types of colonies
| Species isolated in our study (Culture positive: n=72/85) | Colony type | Subtotal | Total (n=72) | |
|---|---|---|---|---|
| T. rubrum | Downy (TRD) | 22 (61.11%) | 36 (50%) | |
| Granular (TRG) | 14 (38.89%) | |||
| T. mentagrophytes | Downy (TMD) | 11 (32.35%) | 34 (47.2%) | |
| Granular (TMG) | 23 (67.65%) | |||
| T. violaceum | 1 (1.4%) | |||
| Acremonium | 1 (1.4%) | |||
TRD: Trichophyton rubrum, downy form, TRG: T. rubrum, granular form, TRD: T. mentagrophytes, downy form, TMG: T. mentagrophytes, granular form
Isolates of T. rubrum and T. mentagrophytes showed two distinctive types of colony forms [Table 2 and Figures 3, 4]
Downy form: Slow-growing colonies with velvety surface
Granular form: Rapidly growing (within a week) powdery/chalky colonies.
Antifungal susceptibility profiles of the isolated dermatophytic fungi
MIC-90 values were obtained for all the isolates of dermatophytes (single case of Acremonium isolate was excluded). In vitro susceptibility (MIC-90 ranges and median MIC-90) of seven oral and topical antifungal agents, against the isolated dermatophyte species is illustrated in Tables 3 and 4.
Table 3.
Susceptibility data (MIC-90 ranges) for T. rubrum (downy and granular), T. rubrum (downy), T. rubrum (granular), T. mentagrophytes (downy and granular forms), T. mentagrophytes (downy) and T. mentagrophytes (granular)
| MIC 90 range (μg/mL) | TR | TR colony types | TM | TM colony types | Overall | ||
|---|---|---|---|---|---|---|---|
| Downy | Granular | Downy | Granular | ||||
| TRB | 0.008-0.256 | 0.008-0.256 | 0.016-0.256 | 0.016-0.256 | 0.016-0.256 | 0.016-0.256 | 0.008-0.256 |
| GRF | 0.03-1 | 0.03-1 | 0.12-0.5 | 0.06-1 | 0.06-1 | 0.06-1 | 0.03-1 |
| ITZ | 0.125-2 | 0.125-2 | 0.125-1 | 0.25-2 | 0.5-2 | 0.25-2 | 0.125-2 |
| FLC | 0.125-1 | 0.125-1 | 0.5-1 | 0.25-32 | 0.25-1 | 0.25-32 | 0.125-32 |
| STC | 0.03-1 | 0.03-0.5 | 0.03-1 | 0.03-1 | 0.03-1 | 0.06-1 | 0.06-1 |
| KTC | 0.06-1 | 0.06-1 | 0.06-1 | 0.125-1 | 0.125-1 | 0.125-1 | 0.06-1 |
| CTZ | 0.03-2.0 | 0.03-2.0 | 0.125-0.5 | 0.06-1 | 0.06-1 | 0.06-1.0 | 0.03-2.0 |
TRB: Terbinafine, GRF: Griseofulvin, ITZ: Itraconazole, FLC: Fluconazole, STC: Sertaconazole, KTC: Ketoconazole, CTZ: Clotrimazole, TR: T. rubrum, TM: T. mentagrophytes
Table 4.
Susceptibility data (Median MIC-90) for T. rubrum (downy and granular), T. rubrum (downy), T. rubrum (granular), T. mentagrophytes (downy and granular forms), T. mentagrophytes (downy) and T. mentagrophytes (granular)
| Median MIC 90 (μg/mL) | TR | TR colony types | TM | TM colony types | ||
|---|---|---|---|---|---|---|
| Downy | Granular | Downy | Granular | |||
| TRB | 0.064 | 0.032 | 0.064 | 0.096 | 0.128 | 0.064 |
| GRF | 0.24 | 0.24 | 0.18 | 0.24 | 0.24 | 0.24 |
| ITZ | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 |
| FLC | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 |
| STC | 0.06 | 0.06 | 0.06 | 0.06 | 0.125 | 0.06 |
| KTC | 0.25 | 0.25 | 0.25 | 0.5 | 0.5 | 0.5 |
| CTZ | 0.25 | 0.125 | 0.25 | 0.125 | 0.125 | 0.125 |
TRB: Terbinafine, GRF: Griseofulvin, ITZ: Itraconazole, FLC: Fluconazole, STC: Sertaconazole, KTC: Ketoconazole, CTZ: Clotrimazole, TR: T. rubrum, TR: T. mentagrophytes
MIC-90 levels of the single isolate of T. violaceum were as follows: terbinafine-0.128 μg/ml, griseofulvin-0.24 μg/ml, itraconazole-0.5 μg/mL, fluconazole-1.0 μg/ml, sertaconazole-0.03 μg/ml, ketoconazole-0.25 μg/ml, and clotrimazole-0.5 μg/ml.
Statistical analysis
Comparison of median MIC-90 for the isolated dermatophyte species:
-
i)
T. rubrum vs. T. mentagrophytes complex
Median MIC-90 values for itraconazole were significantly higher in T. mentagrophytes complex group as compared to T. rubrum group (P<0.01, Mann–Whitney U test).
The median MIC-90 was again higher in the T. mentagrophytes complex group for terbinafine (0.096 vs. 0.064) and ketoconazole (0.5 vs. 0.25), whereas T. rubrum group showed higher median MIC-90 for fluconazole (1.0 vs. 0.5) and clotrimazole (0.25 vs. 0.125); however in each of these, the difference was not statistically significant. The MICs for griseofulvin and sertaconazole were similar for both the groups.
-
ii)
Comparison of downy vs. granular forms of T. rubrum The median MIC-90 values were higher in the T. rubrum, granular form for terbinafine and clotrimazole as compared to T. rubrum, downy form, while the latter showed higher MICs for fluconazole and griseofulvin. The differences were however not statistically significant.
-
iii)
Comparison of downy vs. granular forms of T. mentagrophytes:
T. mentagrophytes, downy form showed higher median MIC-90 than granular form for terbinafine and sertaconazole. The differences among both the groups were however not statistically significant.
-
iv)
Comparison of all four subspecies (granular and downy forms of T. rubrum and T. mentagrophytes for individual drug susceptibilities (Kruskal–Wallis test): showed highest MIC-90 for T. mentagrophytes downy group for itraconazole (P < 0.05), terbinabine, sertaconazole, and ketoconazole. T. rubrum downy group showed highest MIC-90 for griseofulvin and fluconazole. T. rubrum, granular form showed highest MIC-90 for clotrimazole. These differences were not statistically significant for any of the antifungals, except for itraconazole in T mentagrophytes downy group.
Discussion
The recent changing clinicoepidemiological scenario of dermatophyte infections in India has presented a challenge for identifying the factors responsible for this abrupt change. The rising trend of recalcitrant dermatophytoses could possibly be due to some or all of the followings: a) an epidemiological shift in the growth patterns of dermatophytes providing them with an advantage for better persistence in the human host, b) an evolution in the genetic make-up of the fungi that perhaps enhances its virulence traits and supports pathogenicity, c) a rapid emergence in the drug resistant species perhaps due to rampant use of potent antifungal drugs in inadequate doses, and d) human host factors. Obesity (due to changing food habits), climate change (longer exposure to the hot and humid climate), change in clothing habits (tight, synthetic material), and most importantly topical steroid misuse help in persistence of the fungi in its colonized niches.[4]
Our study has confirmed the recent increase in extensive forms of the disease with 48.23% of the patients having extensive dermatophytosis. The clinical pattern paralleled results from the mycological examination of the specimens with almost three fourths of fungal KOH mounts showing an overabundance of long branched intertwined dermatophytic hyphae clearly indicating a higher fungal load at the site of infection. This picture was reminiscent of a biofilm-like phenotype which is a concentrated community of organisms that build up on each other and develop vast amounts of hyphae in the niche. This microscopic picture is in stark contrast to that observed perhaps only a decade ago, wherein dermatophytic hyphae were found only after laboriously scanning several fields of one or more KOH mounts.
The changing scenario in the epidemiological growth pattern of dermatophytic fungi was additionally reflected in its growth phenotype on culture; almost half of the infections in the study were caused by T. mentagrophytes complex. Historically in India, and worldwide, T. rubrum used to be the primary pathogen isolated from cases of dermatophytoses.[5,6,7] In contrast, some of the recent studies have revealed an augmenting prevalence of T. mentagrophytes complex.[8,9,10] Thus, the recent explosive expansion of superficial dermatophytosis in India seems to have its base over a perceptible epidemiological shift in species, with an increasing proportion of T. mentagrophytes complex.
An interesting trend seen on culture growth was the preponderance of rapidly growing granular form, especially in case of T. mentagrophytes complex. Normal time required for growth of dermatophyte species on culture is at least 2 weeks. The colonies of these granular forms however grew extremely rapidly within a week. T. rubrum and T. mentagrophytes are known to show polymorphic growth of colonies. Downy form is known to be associated with chronic human infections, while granular form is associated with animal infections and acute inflammatory human infections.[11] Downy and granular forms of T. mentagrophytes are designated as T. mentagrophytes var. interdigitale and T. mentagrophytes var. mentagrophytes, respectively.[12] Downy form of the Trichophyton spp. used to be commonly found in the past. Rapid growth of granular forms of dermatophytes correlates with the recent clinical observations of rapidly spreading clinical lesions as well as rapid reappearance of lesions after clearance on therapy.
It is evident from Table 5 that recent Indian studies by Indira and Bhatia and Sharma show the MIC-90 ranges to be slightly higher in T. mentagrophytes as compared to T. rubrum for terbinafine.[13,14] Studies by Indira and Sowmya et al. showed higher MIC-90 ranges for T. mentagrophytes to griseofulvin and fluconazole, respectively.[13,15] The study by Sowmya et al. however shows T. rubrum to have higher MIC-90 ranges for itraconazole.[15] Our study showed MIC-90 (median) of itraconazole to be significantly higher in T. mentagrophytes complex as compared to T. rubrum (P<0.01, Mann–Whitney U test). Though this difference in susceptibility of the two species is variable across antifungals and also the regions involved, it may represent a trend of higher MIC-90 levels of T. mentagrophytes vs. T. rubrum in India.
Table 5.
Comparative susceptibility data MIC-90 ranges (μg/mL) of T. rubrum and T. mentagrophytes from studies in India and other countries
| Studies | T. rubrum | T. mentagrophytes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TRB | GRS | ITR | FLU | KTZ | TRB | GRS | ITR | FLU | KTZ | |
| Indira,[13] south India, 2014 | 0.001-0.08 | 0.16-5.12 | 0.03-3.84 | 0.16-20.48 | 0.01-3.84 | 0.002-0.16 | 0.32-5.12 | 0.03-1.92 | 0.08-20.48 | 0.01-0.96 |
| Sowmya et al.,[15] south India, 2015 | 0.001-0.008 | 0.25-0.5 | - | 0.125-2 | 0.03-1 | 0.001-0.008 | 0.25-0.5 | - | 1.0-8 | 0.25-1.0 |
| Bhatia and Sharma,[14] north India, 2015 | 0.03-4 | - | 0.015-1 | - | 0.015-0.5 | 0.06-4 | - | 0.015-1 | - | 0.015-2 |
| Dabas et al.,[19] north India, 2017 | 0.03-8 | 1.0-8 | 0.03-0.06 | - | - | 0.03-16 | 0.03-16 | 0.03-0.125 | - | - |
| Pathania et al.,[20] north India, 2017 | 0.015-16 | 0.5-128 | 0.03-0.5 | 0.03-16 | - | 0.015-8 | 0.5-128 | 0.015-1 | 0.12-32 | - |
| Present study, western India, 2016 | 0.008-0.256 | 0.03-1 | 0.125-2 | 0.125-1 | 0.06-1 | 0.016-0.256 | 0.06-1 | 0.25-2 | 0.25-32 | 0.125-1 |
| Santos and Hamdan,[18] Brazil, 2005 | <0.031 | 0.25-2 | <0.031-0.5 | 2-32 | 0.031-2 | - | - | - | - | - |
| Rodrigues et al.,[16] Brazil, 2006 | 0.03-0.5 | 0.25-2 | 0.03-4 | 2.0-32 | 0.03-4 | 0.03-0.5 | 0.25-1 | 0.03-0.25 | 4.0-16 | 0.03-1 |
| Barros et al.,[17] Brazil, 2006 | <0.007-0.031 | 0.062-1 | 0.015-0.25 | 1.0-64 | - | <0.007-0.031 | 0.062-1 | 0.015-0.25 | 1.0-64 | - |
TRB: Terbinafine, GRS: Griseofulvin, ITR: Itraconazole, FLU: Fluconazole, KTZ: Ketoconazole
While comparing this observation with two similar studies from a different tropical country (Brazil), no such difference in MIC-90 of T. rubrum and T. mentagrophytes is seen for terbinafine, while T. rubrum shows higher MIC-90 range for itraconazole in the study by Rodrigue et al.[16,17]
Since there are no specified breakpoints for antifungal susceptibility in dermatophytes, we compared the MICs in our study to similar studies in India as well as in other tropical countries. The present study showed higher MIC-90 ranges for terbinafine for both T. rubrum and T. mentagrophytes complex in comparison with those in studies by Indira and Sowmya et al.; also MIC-90 ranges in present study were several folds higher than those in the study by Barros et al. and Santos and Hamdan.[13,15,17,18] The studies by Bhatia and Sharma and Dabas et al. showed MIC-90 ranges to terbinafine to be even higher than in our study.[14,19]
On the contrary, MIC-90 ranges for fluconazole were higher in the study by Pathania et al. than in the present study, while those for griseofulvin were higher in the study by Indira in comparison with our study.[13,20] MIC-90 ranges for itraconazole were higher than those in studies by Bhatia and Sharma and Pathania et al.[14,20]
From these observations, we can infer that antifungal susceptibilities vary widely with geographical attributes even with identical testing methods. There is however an evident trend of rising MIC-90 to terbinafine in India though a clear statement about resistance to terbinafine cannot be made due to lack of CLSI guidelines defining the drug breakpoints. This emphasizes the need for several such studies from different regions in India along with in vivo correlation to define the MIC breakpoints. Recent Indian reports of mutation in squalene epoxidase gene in Trichophyton isolates support our observation of high MIC-90 to terbinafine.[21,22]
Limitations of the study
A larger sample size involving different geographical locations would have given a more diverse view of the present dermatophyte epidemic in India. Genomic studies on the isolated species would have given us an insight into possible mutations. However, this was not possible in view of cost and infrastructure constraints. The study did not include in vivo correlation of responsiveness to drugs. The ideal model of antifungal drugs susceptibility should be inclusive of in vitro sensitivity, followed by measuring the level of the drug reached in stratum corneum and then observing clinical response of the drugs in vivo.
Conclusion
This study points to rising proportion of strains of Trichophyton mentagrophytes complex with higher MICs to antifungals like itraconazole and terbinafine. This study also confirms the epidemiological shift of dermatophytes in India toward an upsurge of T. mentagrophytes complex. It demonstrates the increasing proportion of granular form over downy form especially in T. mentagrophytes complex.
Financial support and sponsorship
Research Grant from IADVL (Indian Association of Dermatologists, Venereologists and Leprologists).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
IADVL (Indian Association of Dermatologists, Venereologists and Leprologists) and IADVL academy for their support as well as providing the research grant for conducting the study
Dr. Dipak Patil, Assistant Professor in Department of Community Medicine, K. J. Somaiya Medical College and Research Centre for helping in statistical analysis.
References
- 1.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogra S, Narang T. Emerging atypical and unusual presentations of dermatophytosis in India. Clin Dermatol Rev. 2017;1(Suppl S1):12–8. [Google Scholar]
- 3.Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008. Clinical Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed. CLSI document M38-A2. [Google Scholar]
- 4.Segal E, Frenkel M. Dermatophyte infections in environmental contexts. Res Microbiol. 2015;166:564–9. doi: 10.1016/j.resmic.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Bhagra S, Ganju SA, Kanga A, Sharma NL, Guleria RC. Mycological pattern of dermatophytosis in and around Shimla Hills. Indian J Dermatol. 2014;59:268–70. doi: 10.4103/0019-5154.131392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakshmanan A, Ganeshkumar P, Mohan SR, Hemamalini M, Madhavan R. Epidemiological and clinical pattern of dermatomycoses in rural India. Indian J Med Microbiol. 2015;33:S134–6. doi: 10.4103/0255-0857.150922. [DOI] [PubMed] [Google Scholar]
- 7.Jain N, Sharma M, Saxena VN. Clinico-mycological profile of dermatophytosis in Jaipur, Rajasthan. Indian J Dermatol Venereol Leprol. 2008;74:274–5. doi: 10.4103/0378-6323.41388. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia VK, Sharma PC. Epidemiological studies on Dermatophytosis in human patients in Himachal Pradesh, India. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramaraj V, Vijayaraman RS, Rangarajan S, Kindo AJ. Incidence and prevalence of dermatophytosis in and around Chennai, Tamilnadu, India. Int J Res Med Sci. 2016;4:695–700. [Google Scholar]
- 10.Agarwal US, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in northwest India. Indian J Dermatol Venereol Leprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 11.Mohapatra LN, Gugnani HC, Shivrajan K. Natural infection in laboratory animals due to Trichophyton mentagrophytes in India. Mycopathol Mycol Appl. 1964;24:275–80. doi: 10.1007/BF02049291. [DOI] [PubMed] [Google Scholar]
- 12.Shukla NP, Agarwal GP. Antigenic relationship between downy and granular forms of Trichophyton mentagrophytes and Trichophyton rubrum. Microbiol Immunol. 1983;27:311–4. doi: 10.1111/j.1348-0421.1983.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 13.Indira G. In vitro antifungal susceptibility testing of 5 antifungal agents against dermatophytic species by CLSI micro dilution method. Clin Microbial. 2014;3:145. [Google Scholar]
- 14.Bhatia VK, Sharma PC. Determination of minimum inhibitory concentrations of itraconazole, terbinafine and ketoconazole against dermatophyte species by broth microdilution method. Indian J Med Microbiol. 2015;33:533–7. doi: 10.4103/0255-0857.167341. [DOI] [PubMed] [Google Scholar]
- 15.Sowmya N, Appalaraju B, Srinivas CR, Surendran P. Antifungal susceptibility testing for dermatophytes isolated from clinical samples by broth dilution method in a tertiary care hospital. JMR. 2015;1:64–7. [Google Scholar]
- 16.Araújo CR, Miranda KC, Fernandes Ode F, Soares AJ, Silva Mdo R. In vitro susceptibility testing of dermatophytes isolated in Goiania, Brazil, against five antifungal agents by broth microdilution method. Rev Inst Med Trop Sao Paulo. 2009;51:9–12. doi: 10.1590/s0036-46652009000100002. [DOI] [PubMed] [Google Scholar]
- 17.da Silva Barros ME, de Assis Santos D, Hamdan JS. Evaluation of susceptibility of Trichophyton mentagrophytes and Trichophyton rubrum clinical isolates to antifungal drugs using a modified CLSI microdilution method (M38-A) J Med Microbiol. 2007;56:514–8. doi: 10.1099/jmm.0.46542-0. [DOI] [PubMed] [Google Scholar]
- 18.Santos DA, Hamdan JS. Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J Clin Microbiol. 2005;43:1917–20. doi: 10.1128/JCM.43.4.1917-1920.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dabas Y, Xess I, Singh G, Pandey M, Meena S. Molecular identification and antifungal susceptibility patterns of clinical dermatophytes following CLSI and EUCAST guidelines? J Fungi. 2017;3:E17. doi: 10.3390/jof3020017. doi: 10.3390/jof3020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol. 2018;84:678–84. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 21.Singh A, Masih A, Khurana A, Singh PK, Gupta M, Hagen F, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61:477–84. doi: 10.1111/myc.12772. [DOI] [PubMed] [Google Scholar]
- 22.Rudramurthy SM, Shankarnarayan SA, Dogra S, Shaw D, Mushtaq K, Paul RA, et al. Mutation in the squalene epoxidase gene of trichophyton interdigitale and trichophyton rubrum associated with allylamine resistance. Antimicrob Agents Chemother. 2018;62:59. doi: 10.1128/AAC.02522-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


