Abstract
Background:
Aggregatibacter actinomycetemcomitans is involved in the etiology of localized aggressive periodontitis (LAP), a condition that frequently requires supplemental antibiotic therapy. Information on antimicrobial susceptibility pattern and guidelines for oral antibiotic therapy are not available on Indian patients.
Aim:
The main aim of the present study was to screen clinical isolates on a panel of antibiotics commonly used for oral/systemic therapy.
Materials and Methods:
The study included 40 strains of A. actinomycetemcomitans isolated from patients with LAP. The subgingival plaque was plated onto Trypticase Soy Serum Bacitracin Vancomycin Agar medium and incubated for 72 h, and suspected colonies were confirmed by phenotypic tests. Each isolate was tested against a panel of 12 antibiotics using MIC gradient strip test. ATCC strains of A. actinomycetemcomitans serotype A and C were used as standards. Performance and interpretation of the test were done according to the manufacturers’ instructions. Distribution of MICs among isolates (n = 40) were used to calculate concentrations inhibiting 50% (MIC50) and 90% (MIC90) of strains.
Results:
Moxifloxacin, cefotaxime and ceftriaxone showed excellent activity with 100% growth inhibition followed by amoxicillin, amoxiclav and doxycycline (>90% activity). The bacterial strains were moderately susceptible to cefuroxime, cefazolin and tetracycline but displayed poor susceptibility to clindamycin and azithromycin. All isolates were resistant to metronidazole.
Conclusion:
The isolates of A. actinomycetemcomitans displayed a high level of resistance to azithromycin and clindamycin. Development of resistance against tetracycline also appears to be significant. Variable resistance among the different members of the cephalosporin group is a factor to be investigated further since susceptibility profile against these antibiotics and interpretative criteria for oral bacteria are not available.
Keywords: Aggregatibacter actinomycetemcomitans, cephalosporins, enzyme test, MIC50, MIC90
INTRODUCTION
Aggregatibacter actinomycetemcomitans is a tiny, nonmotile, facultatively anaerobic, fastidious Gram-negative coccobacillus that requires an atmosphere with 5%–10% CO2 for its growth.[1] It occurs as a commensal in the human oral cavity and can be recovered from up to 20% of healthy individuals by the culture of the subgingival plaque.[2] Investigations over several years have convincingly shown the involvement of this organism in the etiology of localized aggressive periodontitis (LAP), a disease that mainly affects younger subjects leading to extensive periodontal tissue destruction and tooth loss.[1,3,4,5,6] It is also known to produce several nonoral infections and as a part of the HACEK group is frequently isolated from patients with bacterial endocarditis.[7]
In patients with aggressive periodontitis, adjunctive systemic therapy of antibiotics is known to offer better relief when used in combination with root planing and surgical intervention. Despite numerous research studies about the role of antibiotics in the treatment of periodontal diseases, clinicians are still not clear about what to prescribe for their patients.[8] One main reason for such a dilemma could be nonavailability of antimicrobial susceptibility pattern of oral bacterial pathogens. In addition, the susceptibility/resistance profile of an organism is largely dependent on the panel of antimicrobial agents most commonly used in that geographical area.[9]
So far, there are no reports on the antimicrobial susceptibility pattern of A. actinomycetemcomitans from India isolated from the oral cavity of our subjects. Keeping this in mind, we undertook the present study, wherein oral isolates of A. actnomycetemcomitans were tested against a panel of 12 antibiotics which are either recommended for treating oral infections and/or most commonly used in hospital settings in our area. The testing was performed with commercially available gradient diffusion test strips. This study is part of a research project titled “Determination of Antimicrobial susceptibility pattern and induced metronidazole resistance and prevalence of drug resistance genes in oral Gram-negative anaerobes” funded by the Indian Council of Medical Research, New Delhi.
MATERIALS AND METHODS
The present study was carried out in the Central Research Laboratory of our Institution after obtaining approval from the Institutional Ethics Committee. A total of 40 strains of A. actinomycetemcomitans isolated from clinically and radiologically confirmed patients with LAP were included in the study. Written informed consent was obtained from each patient before the collection of the clinical material for testing. Subgingival plaque specimens from each participant were collected with sterile curette and placed in a vial of reduced transport fluid and sent to the laboratory. The vials were then vortexed briefly to break the plaque and plated onto Dentaid[10] and Trypticase soy Serum Bacitracin Vancomycin Agar prepared as per the instructions of the original authors.[11] The plates were incubated in an atmosphere of 5% Co2 at 37°C for 72 h. The identity of the strains was confirmed by characteristic colony characters and various phenotypic tests such as catalase, oxidase, indole and fermentation of glucose, xylose, maltose and mannitol.[12] The isolates were then stored at −80°C in glycerol broth till tested.
Antimicrobial susceptibility testing was performed on each isolate using E-test gradient diffusion method (Ezy strip, Hi-Media). The antimicrobial agents used in the study included amoxicillin, amoxicillin/clavulanic acid (2:1) (amoxy-clav), tetracycline, doxycycline, clindamycin, azithromycin, moxifloxacin, cefazolin, ceftriaxone, cefuroxime, cefotaxime and metronidazole. Inocula of test strains were prepared in thioglycollate broth to a concentration of 0.5 MacFarland standard and inoculated onto brucella blood agar plates supplemented with hemin and menadione. One Ezy test strip of the respective antibiotic was placed in the center of the plate and the plates were then incubated anaerobically in a gas-pak jar at 37°C overnight. MICs were determined according to the manufacturer's instructions [Figure 1]. Two serotypes of A. actinomycetemcomitans serotype A (ATCC 29523) and serotype C (ATCC 43719) was used as standard strains in the assays.
Figure 1.
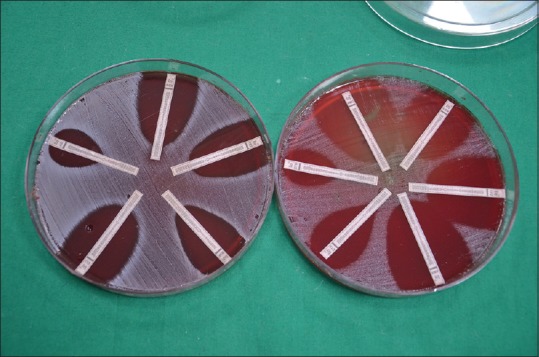
E-test showing zone of inhibition on blood agar
Data analysis
The interpretative criteria for the susceptibility of anaerobes were applied to determine the breakpoints for ampicillin, amoxyclav, ceftriaxone, cefotaxime, tetracycline, moxifloxacin, clindamycin and metronidazole.[13] Since guidelines for doxycycline, azithromycin, cefazolin and cefuroxime were not available for anaerobic bacteria, interpretative criteria for facultative anaerobic organisms were applied to these antibiotics[13] [Table 1].
Table 1.
Susceptibility/resistance interpretative criteria for antibiotics included in the study
| Antibiotic | Sensitive (S) (ug/ml) | Intermediate (I) (ug/ml) | Resistant (R) (ug/ml) |
|---|---|---|---|
| Amoxicillin | <0.5 | 1 | >2 |
| Amoxicillin-clavulinic acid | <4/2 | 8/4 | >16/8 |
| Azithromycin | <4 | 8 | >16 |
| Clindamycin | <2 | 4 | >8 |
| Cefazolin | <2 | 4 | >8 |
| Cefotaxime | <16 | 32 | >64 |
| Ceftriaxone | <16 | 32 | >64 |
| Cefuroxime | <4 | 8 | >16 |
| Doxycycline | <4 | 8 | >16 |
| Metronidazole | <8 | 16 | >32 |
| Moxifloxacin | <2 | 4 | >8 |
| Tetracycline | <4 | 8 | >16 |
Distribution of MICs among isolates (n = 40) were used to calculate concentrations inhibiting 50% (MIC50) and 90% (MIC90) of strains.
RESULTS
Both the standard strains of A. actinomycetemcomitans used in the study were susceptible to all the antibiotics tested except metronidazole. All clinical isolates were susceptible to moxifloxacin, cefotaxime and ceftriaxone. Only one strain had shown intermediate sensitivity to moxifloxacin, which persisted even after repeat testing.
Amoxicillin, amoxyclav and doxycycline showed very good effect inhibiting the growth of more than 90% strains [Table 2]. Tetracycline was slightly less effective with 5 strains showing intermediate susceptibility and 2 strains were resistant. Cefuroxime and cefazolin could exert inhibitory action on 77.5% and 75% of strains, respectively.
Table 2.
The susceptibility pattern of Aggregatibacter actinomycetemcomitans isolates studied
| Antibiotic | Sensitive (%) | Intermediate (%) | Resistant (%) |
|---|---|---|---|
| Amoxicillin | 36 (90) | 2 (5) | 2 (5) |
| Amoxicillin-clavulanic acid | 37 (92.5) | 1 (2.5) | 2 (5) |
| Azithromycin | 25 (62.5) | 3 (7.5) | 12 (30) |
| Clindamycin | 15 (37.5) | 9 (22.5) | 16 (40) |
| Cefazolin | 30 (75) | 5 (12.5) | 5 (12.5) |
| Cefotaxime | 40 (100) | 0 | 0 |
| Ceftriaxone | 40 (100) | 0 | 0 |
| Cefuroxime | 31 (77.5) | 6 (15) | 3 (7.5) |
| Doxycycline | 38 (95) | 2 (5) | 0 |
| Metronidazole | 0 | 0 | 40 (100) |
| Moxifloxacin | 39 (97.5) | 1 (2.5) | 0 |
| Tetracycline | 33 (82.5) | 5 (12.5) | 2 (5) |
The test strains showed only moderate susceptibility to azithromycin (30%) and clindamycin (40%). On the other hand, all the isolates showed complete resistance to metronidazole.
When the susceptibility pattern of isolates studied was compared with MIC50 and MIC90 results, it could be seen that moxifloxacin, amoxicillin, amoxyclav and ceftriaxone had MIC90 of 1 ug/ml showing excellent efficacy. On the other hand, MIC90 values of azithromycin, tetracycline, clindamycin and cefuroxime were quite high, falling between 8 ug/ml and 64 ug/ml [Table 3]. Other antibiotics except metronidazole showed moderate MIC90 values.
Table 3.
Minimum inhibitory concentration50, minimum inhibitory concentration90 values and mean of range for each antibiotic tested
| Antibiotic | MIC50 values (ug/ml) | MIC90 values (ug/ml) | Range (ug/ml) |
|---|---|---|---|
| Amoxicillin | 0.5 | 1 | 0.064-16 |
| Amoxicillin-clavulanic acid | 0.75 | 1 | 0.064-8 |
| Azithromycin | 4 | 16 | 0.125-64 |
| Cefazolin | 1 | 4 | 0.5-24 |
| Cefotaxime | 0.5 | 2 | 0.038-8 |
| Ceftriaxone | 0.125 | 1 | 0.094-1 |
| Cefuroxime | 2 | 8 | 0.2-48 |
| Clindamycin | 4 | 64 | 1->256 |
| Doxycycline | 1 | 2 | 0.064-8 |
| Metronidazole | >256 | >256 | 64-256 |
| Moxifloxacin | 0.125 | 0.5 | 0.047-4 |
| Tetracycline | 2 | 8 | 0.125-16 |
MIC: Minimum inhibitory concentration
DISCUSSION
Numerous investigations in the past two decades have established the definitive role played by A. actinomycetemcomitans in the etiology of aggressive periodontitis.[3] Studies have also shown that mechanical therapy alone cannot eliminate all the major periodontal pathogens such as A. actinomycetemcomitans from diseased sites mainly due to the inability of the periodontal instruments to access the deeper part of the gingival sulcus.[14] Hence in patients with aggressive periodontitis, supplemental antibiotic therapy is recommended by many clinicians.[8] However unfortunately, neither proper guidelines nor antimicrobial susceptibility pattern of A. actinomycetemcomitans are available from our country for adequate antimicrobial therapy.
The most common drugs used as part of the periodontal therapy include amoxicillin, amoxicillin-clavulanic acid, tetracycline, azithromycin, clindamycin, moxifloxacin and metronidazole.[8] However, keeping in mind, the most common antibiotics prescribed for systemic illnesses, we have made additions to this panel that include doxycycline, cefazolin, cefuroxime, cefotaxime and ceftriaxone. Even though A. actinomycetemcomitans is a facultative anaerobe, the antimicrobial susceptibility testing procedure adopted is that of anaerobic bacteria. There are three different methods for this purpose that include-agar dilution, broth microdilution and MIC gradient method by E-test strips.[9] In the present study, we have used gradient MIC test strips for antimicrobial testing of A. actinomycetemcomitans since the results are comparable to that of agar dilution method which is considered as the “gold standard.”
Several studies have examined the effect of different periodontal therapies on clinical and microbiological parameters in LAP.[8,15] To the best of our knowledge, there are no publications about antimicrobial susceptibility pattern of A. actinomycetemcomitans from India using the MIC gradient method. In our study, the isolates studied showed high level of susceptibility to amoxicillin (90%) and amoxyclav (92.5%). Other investigators have shown varying results with moderate-to-high susceptibility to amoxicillin and usually excellent efficacy of amoxyclav.[16,17,18,19]
We found high level of resistance to metronidazole among our isolates (100%) with MIC50 values of >256 and moderate resistance to clindamicin and azithromycin. This is in accordance to the results of several other studies.[16,17,18,20] In the present study, doxycycline had a very good inhibitory effect (95%) on A. actinomycetemcomitans compared to tetracycline (82.5%). Investigators from several countries have demonstrated rising level of resistance in A. actinomycetemcomitans whereas doxycycline is said to be having excellent activity, even against biofilms of this organism.[16,21,22,23]
Fluoroquinolones are known to be having very good action against oral bacteria including A. actinomycetemcomitans. Among various drugs in this group, moxifloxacin has been approved by the FDI.[8] Almost all the investigators have shown that moxifloxacin has excellent activity against oral microbes, a finding similar to our results.[17,18,19,20] In our study, all the strains were highly susceptible to this drug with MIC50 and MIC90 values of <1 ug/ml except one strain which showed intermediate susceptibility (4 ug/ml). The readings were similar even on repeated testing. This aspect should be looked into.
For the first time, we have tested the activity of different cephalosporins on A. actinomycetemcomitans strains. We found the results to be highly variable. While cefotaxime and ceftriaxone showed very good efficacy (100%), with MIC50 values of <1 ug/ml, cefazolin (75%) and cefuroxime (77.5%) displayed moderate activity. Even though cefoxitin is the preferred drug from this group to treat anaerobes,[24] we chose to include the cephalosporins most commonly prescribed in our area for systemic/nonoral conditions. The findings clearly show that cefotaxime and ceftriaxone which belong to the 3rd generation of cephalosporins and have a broader range of activity have superior effect in comparison to cefazolin (1st generation) and cefuroxime (2nd generation) in bacterial growth inhibition.
CONCLUSION
The data presented here demonstrate the level of resistance of A. actinomycetemcomitans to different commonly prescribed drugs. It appears that moxifloxacin, amoxy-clav, amoxicillin and doxycycline have definite benefits over the other antibiotics. Moderate level of resistance shown against clindamycin and azithromycin indicate the limited efficacy of these drugs in treatment of aggressive periodontitis. The MIC gradient method of testing, even though expensive, has the advantage of ease of performance and interpretation and can be applied to even a single isolate at a time. We feel more such studies should be taken up from other parts of our country to get information on effect of geographical distribution on resistance pattern of A. actinomycetmcomitans.
Financial support and sponsorship
Research grant of ICMR, registration no. 2014-0540.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We sincerely thank ICMR, New Delhi. Since this work is part of project funded by ICMR, New Delhi titled “determination of antimicrobial susceptibility pattern and induced metronidazole resistance and prevalence of drug resistance genes in oral Gramnegative anaerobes”.
We express special thanks to Central Research Laboratory, Maratha Mandal's NGH Institute of Dental Sciences and Research Centre, Belgaum, for providing infrastructure and additional equipment needed for the study.
REFERENCES
- 1.Henderson B, Ward JM, Ready D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontol 2000. 2010;54:78–105. doi: 10.1111/j.1600-0757.2009.00331.x. [DOI] [PubMed] [Google Scholar]
- 2.Asikainen S, Chen C. Oral ecology and person-to-person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol 2000. 1999;20:65–81. doi: 10.1111/j.1600-0757.1999.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 3.Könönen E, Müller HP. Microbiology of aggressive periodontitis. Periodontol 2000. 2014;65:46–78. doi: 10.1111/prd.12016. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida Y, Suzuki N, Nakano Y, Shibuya K, Ogawa Y, Koga T. Distribution of Actinobacillus actinomycetemcomitans serotypes and Porphyromonas gingivalis in Japanese adults. Oral Microbiol Immunol. 2003;18:135–9. doi: 10.1034/j.1399-302x.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 5.Yang HW, Asikainen S, Doǧan B, Suda R, Lai CH. Relationship of Actinobacillus actinomycetemcomitans serotype b to aggressive periodontitis: Frequency in pure cultured isolates. J Periodontol. 2004;75:592–9. doi: 10.1902/jop.2004.75.4.592. [DOI] [PubMed] [Google Scholar]
- 6.Joshi VM, Bhat KG, Kugaji MS, Ingalgi PS. Occurrence of Aggregatibacter actinomycetemcomitans in Indian chronic periodontitis patients and periodontally healthy adults. J Indian Soc Periodontol. 2016;20:141–4. doi: 10.4103/0972-124X.175171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Winkelhoff AJ, Slots J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontol 2000. 1999;20:122–35. doi: 10.1111/j.1600-0757.1999.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 8.Shaddox LM, Walker C. Microbial testing in periodontics: Value, limitations and future directions. Periodontol 2000. 2009;50:25–38. doi: 10.1111/j.1600-0757.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- 9.Brook I, Wexler HM, Goldstein EJ. Antianaerobic antimicrobials: Spectrum and susceptibility testing. Clin Microbiol Rev. 2013;26:526–46. doi: 10.1128/CMR.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alsina M, Olle E, Frias J. Improved, low-cost selective culture medium for Actinobacillus actinomycetemcomitans. J Clin Microbiol. 2001;39:509–13. doi: 10.1128/JCM.39.2.509-513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slots J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J Clin Microbiol. 1982;15:606–9. doi: 10.1128/jcm.15.4.606-609.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zbinden R. Aggregatibacter, Capnocyttophaga, Eiknella, Kingella, Pasturella and other fastidious rarely encountered gram negative rods. In: Jorgensen JH, Pfaller MA, Carroll KC, Landry ML, Funke G, Richter SS, editors. Manual of Clinical Microbiology. 11th ed. Vol. 1. Washington, DC, USA: ASM Press; 2015. pp. 652–84. [Google Scholar]
- 13.CLSI Document M100-S22. Wayne, PA: Clinical and Laboratory Standard Institute; 2012. Clinical and Laboratory Standard Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty Second Informational Supplement. [Google Scholar]
- 14.Mombelli A, Gmür R, Gobbi C, Lang NP. Actinobacillus actinomycetemcomitans in adult periodontitis. II. Characterization of isolated strains and effect of mechanical periodontal treatment. J Periodontol. 1994;65:827–34. doi: 10.1902/jop.1994.65.9.827. [DOI] [PubMed] [Google Scholar]
- 15.Oettinger-Barak O, Dashper SG, Catmull DV, Adams GG, Sela MN, Machtei EE, et al. Antibiotic susceptibility of Aggregatibacter actinomycetemcomitans JP2 in a biofilm. J Oral Microbiol. 2013;5:1–8. doi: 10.3402/jom.v5i0.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardila CM, Granada MI, Guzmán IC. Antibiotic resistance of subgingival species in chronic periodontitis patients. J Periodontal Res. 2010;45:557–63. doi: 10.1111/j.1600-0765.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 17.Kulik EM, Lenkeit K, Chenaux S, Meyer J. Antimicrobial susceptibility of periodontopathogenic bacteria. J Antimicrob Chemother. 2008;61:1087–91. doi: 10.1093/jac/dkn079. [DOI] [PubMed] [Google Scholar]
- 18.Müller HP, Holderrieth S, Burkhardt U, Höffler U. In vitro antimicrobial susceptibility of oral strains of Actinobacillus actinomycetemcomitans to seven antibiotics. J Clin Periodontol. 2002;29:736–42. doi: 10.1034/j.1600-051x.2002.290810.x. [DOI] [PubMed] [Google Scholar]
- 19.Van Winkelhoff AJ, Herrera D, Winkel EG, Dellemijn-Kippuw N, Van-denbroucke-Grauls CM, Sanz M. Anti-microbial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol. 2000;27:79–86. doi: 10.1034/j.1600-051x.2000.027002079.x. [DOI] [PubMed] [Google Scholar]
- 20.van Winkelhoff AJ, Herrera D, Oteo A, Sanz M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in the Netherlands and Spain. J Clin Periodontol. 2005;32:893–8. doi: 10.1111/j.1600-051X.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- 21.Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand J Dent Res. 1993;101:299–303. doi: 10.1111/j.1600-0722.1993.tb01124.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues RM, Gonçalves C, Souto R, Feres-Filho EJ, Uzeda M, Colombo AP. Antibiotic resistance profile of the subgingival microbiota following systemic or local tetracycline therapy. J Clin Periodontol. 2004;31:420–7. doi: 10.1111/j.1600-051X.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Ishihara K, Kato T, Okuda K. Susceptibility of Actinobacillus actinomycetemcomitans to six antibiotics decreases as biofilm matures. J Antimicrob Chemother. 2007;59:59–65. doi: 10.1093/jac/dkl452. [DOI] [PubMed] [Google Scholar]
- 24.Lewis JS, Bush K. Antibacterial agents. In: Jorgensen JH, Pfaller MA, Carroll KC, Landry ML, Funke G, Richter SS, editors. Manual of Clinical Microbiology. 11th ed. Vol. 1. Washington, DC, USA: ASM Press; 2015. pp. 652–84. [Google Scholar]


