Abstract
Introduction:
Telomerase is a ribonucleoprotein complex responsible for de novo telomere synthesis and addition of telomeric repeats to existing telomeres. Telomerase activity is generally found to be absent in normal tissues. Telomerase is known to be induced upon malignant transformation of human cells.
Method:
In the present study, we analyzed both telomere length and telomerase activity in saliva samples from oral carcinoma patients. The study was done to investigate the presence of telomerase activity in oral squamous cell carcinoma by TRAP assay.
Result:
Telomerase activity was detectable in 79 of 100 human OSCC and 51 of 100 premalignant cases and 8 of 100 normal patients.
Conclusion:
These results indicate that telomerase is activated frequently during the late stage of oral premalignancy and may play a crucial role in OSCC.
Keywords: Oral squamous cell carcinoma, saliva, telomerase, telomere
INTRODUCTION
Oral cancer continues to be the most prevalent cancer, resulting from the consumption of tobacco, alcohol and other carcinogenic products. In nature, more than 95% of the carcinomas of the oral cavity represent squamous cell type.
A large part of cancer load in parts of India is formed from oral cancer.[1] They constitute a major health problem in developing countries including India, representing an increase in mortality rate leading to the cause of death. The survival index continues to be small (50%), as compared to the progress in treatment and diagnosis of other malignant tumors. According to the World Health Organization, in developing countries, oral carcinoma in males is the sixth most common cancer after lung, prostate, colorectal, stomach and bladder cancer, while in females, it is the tenth most common site of cancer after the breast, colorectal, lung, stomach, uterus, cervix, ovary, bladder and liver.[2]
Oral cancer is categorized into precancerous and cancerous levels. Precancerous stage includes leukoplakia, erythroplakia and lichen planus, while cancerous or malignant stage is squamous cell carcinoma which occurs from preexisting lesions, 85% of precancer cases are found to be lesions of leukoplakia[3] and 95% of oral cancers are squamous cell carcinomas.[4] In India, the vast majority of oral squamous cell carcinomas (OSCCs) arises from precancer, leukoplakia.[5] Oral carcinogenesis is a highly complex, multistep process which involves accumulation of genetic alterations that lead to the induction of proteins promoting cell growth (encoded by oncogenes), increased enzymatic (telomerase) activity promoting cell proliferation, as well as the loss of proteins which restrain cell proliferation (encoded by tumor suppressor genes).[6]
The molecules (telomerase) involved in these processes may, therefore, provide markers for the early detection of malignant transformation.
Maintenance of telomeres plays an essential role during the transformation of cancer to malignant stage. Mammalian telomeres, specialized nucleoprotein structures are composed of large concatemers of the guanine-rich sequence 5_-TTAGGG-3_, constitute the ends of eukaryotic chromosomes which serve to protect linear chromosomes from damage and also promote genomic stability to the chromosomes. In addition to this protective function, the regulation of telomere length as an important factor in regulating cellular life span. The roles of telomeres in regulating both stability of genome and replicative immortality seem to contribute in essential ways in cancer initiation and progression. Telomeres, the end sequence of DNA are maintained by a multisubunit enzyme called as Telomerase which is comprised of an RNA component, hTR (it contains a template strand of RNA sequence for the synthesis of complementary sequence of telomeric repeats) and a protein component which is a reverse transcriptase component (hTERT), it catalyzes the synthesis of DNA end or telomeric repeat sequences.
Activity of telomerase is generally absent in normal tissues. It is known to be induced upon immortalization or malignant transformation of human cells such as in oral cancer cells. Telomerase activity is absent in normal tissues of the oral mucosa.[7]
Telomerase is an enzyme with polymerase activity formed from a protein-RNA complex. It is produced in embryonic germ line cells and its function is to lengthen the telomeres by copying the TTAGGG sequence. It is suppressed by mature somatic cells after birth, allowing telomere shortening after each cell division. Telomerase plays an important role in the formation, maintenance and renovation of telomeres, preventing cell apoptosis. Activity of telomerase is generally absent in normal tissues. It is known to be induced upon immortalization or malignant transformation of human cells such as in oral cancer cells. Telomerase activity has been detected in human immortalized cell lines and tumor tissues, saliva.[8] Telomerase enhanced activity has been demonstrated in a high percent of extracts from most tumor types.[9] For example, telomerase has been demonstrated in oral carcinomas.[7] lung cancers,[10] prostate cancers,[11] liver cancers,[12] breast cancers,[13] neuroblastomas,[14] colorectal cancers,[15] and bladder cancers.[16,17]
In humans, activity of telomerase is absent in most of the normal cells but present in the majority of cancer cells.[8]
The blood and saliva are the body fluids which have the most attention for the study, as it may contain reliable biomarkers for detection of carcinoma. Saliva is an informative body fluid which contains an array of analytes (protein such as enzymes, mRNA and DNA) that is used as biomarkers for translation and clinical applications.[18] Saliva as a diagnostic tool can be used identification of high-risk group, patients with premalignant lesions and patients of cancer.[19] The most important point for selecting saliva as a diagnostic tool is that it also contains the fallen cells in the oral cavity which allow saliva to be the first choice of screening and identification of potential biomarkers in oral cancer.[9] In this study, we attempted to investigate the presence and level of telomerase enzyme in saliva of oral precancer and cancer patients.
Normal cells do have the capability to express telomerase activity given proliferative conditions. Telomerase activity is present in highly proliferative normal tissues in the oral mucosa.[7] Telomerase activity, therefore, supports the use of telomerase as a biomarker for detection of tumors. Over expression of leukoplakia, shows increased telomerase activity, an initial process of oral carcinogenesis, a precancerous stage. It is concluded that activity of telomerase can be used as a biomarker for diagnosis of malignant oral cancer and a target for inactivation in chemotherapy or gene therapy. Its expression will also prove to be an important diagnostic tool as well as a novel target for cancer therapy. The activation of telomerase may be an important stepin carcinogenesis which can be controlled by blocking its activity at an early stage.[20]
METHODOLOGY
The study was conducted in the Department Oral Pathology and Department of Biochemistry of King George's Medical University, Lucknow. Cancer patients undergoing, radiotherapy, chemotherapy and/or surgery were excluded in this study. Patients were examined for signs or symptoms of oral cancer.
Detailed information of each patient was noted in a pretested pro forma. Information regarding the patient's name, age, sex, occupation, background, dietary habits, dental hygiene, personal habits and present complaints were gathered. Emphasis was given to addictions such as areca nut, tobacco (smoke and smokeless) and alcohol. Detailed clinical examination of each patient was done to assess the site, size and type of lesion [Figure 1]. Epidemiological information was collected as per the preset pro forma.
Figure 1.
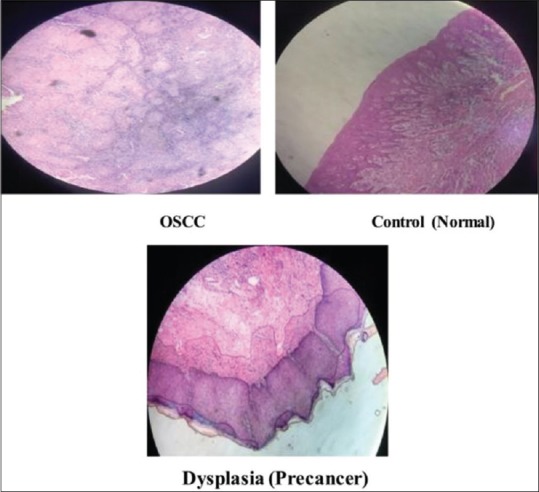
The histopathological result of the tissue samples of oral squamous cell carcinoma, precancer and control (normal) patients
Study population
In the study, we have collected a total of 100 samples of saliva from OSCC, 100 samples from premalignant cases and 100 samples of oral normal mucosa (control) patients without any lesion visiting to the Department of Oral Pathology and Microbiology, K. G. M. U, Lucknow. A detailed history of all the participants was taken. Individuals were excluded if they had received antibiotic, steroid or antifungal therapy during the previous 3 months, if they had a history of underlying systemic disease or if they were HIV-seropositive or had any other condition that could potentially decrease their immunity. We also excluded oral cancer patients who were undergoing or had undergone radiation therapy or surgical treatment for an oral lesion.
The staining procedure for tissue sections with hematoxylin and eosin stain was as follows
The sections were:
Deparaffinized on slide warming table
Cleared in xylene
Hydrated through descending grades of alcohol: 100%, 90%, 70% and 50% (5 min each)
Stained with Harris hematoxylin for 5 min
Differentiated in 1% acid alcohol (one dip)
Brought to running water for about 5 min
Washed in water (blueing)
Stained in eosin Y for 2 min
Dehydrated in ascending grades of alcohol (50%, 60%, 70%, 80%, 96% and 100%)
Cleared in xylene
Mounted with DPX.
Sample collection
The samples were obtained by 25 ml of sterile saline were rinsed and gargled briefly (15 s) and spit into a sterile 50-ml conical tube. Cells were pelleted at 2500 ×g for 15 min at 4°C, the supernatant was discarded, and pelleted cells were resuspended in 1 ml of saline and snap frozen in liquid nitrogen for storage.
Prior to testing, lysates were thawed, and a 5 μl aliquot was placed in 20 μl of CHAPS ([(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate) detergent buffer,[21] mixed and incubated at 4°C for 1 h. Care was taken so that sample to sample contamination, PCR contamination, and RNase contamination did not occur, including handling all specimens with gloves, changing the cryostat knife, washing the cryostat apparatus with water, 100% ethanol, and xylene between sample cutting, and strict segregation of samples, oral rinses, and positive and negative controls [Figures 2,3].
Figure 2.
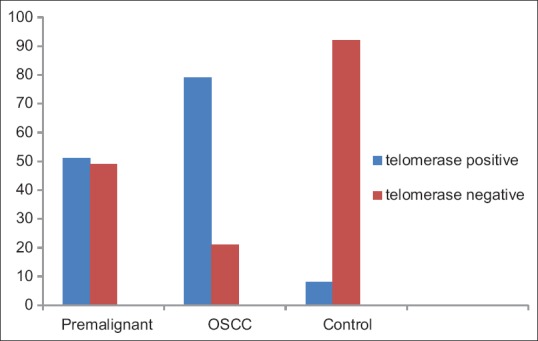
Graph represents the telomerase activity in saliva of oral squamous cell carcinoma, precancer and normal patients
Figure 3.
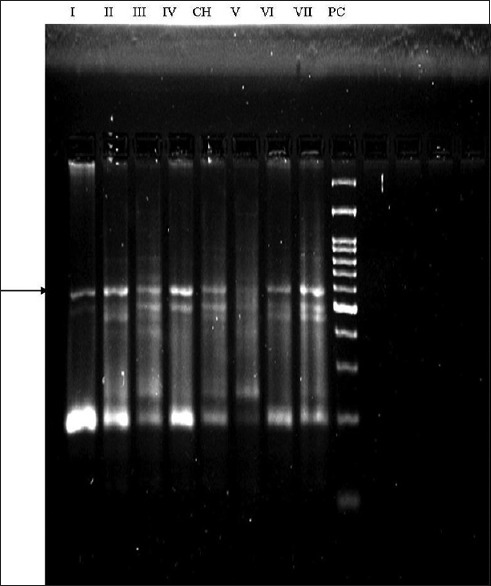
Telomerase activity in primary tumors and saliva. Representative samples of telomerase-positive tumors, non-heat treated telomerase-positive saliva samples, and non- heat treated telomerase-negative saliva samples. Arrow indicates the position of internal PCR control band. CH, CHAPS([(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate) buffer alone without heat treatment; PC(positive control) cell extract-positive control; V- telomerase-negative saliva; VI and VII, telomerase positive in tissue; I, II, III and IV, telomerase-positive saliva (I and IV-Premalignant saliva samples, II and III OSCC saliva samples). This figure is a composite of four different autoradiographs displaying variations of some banding patterns due to a slight variation in electrophoresis conditions
The digests were then centrifuged at 13,000 ×g for 10 min, and the supernatant was divided so that a heat-treated (85°C for 15 min) negative control could be run with each sample. Four microliters aliquots of each supernatant were placed in 21 μl of Telomeric Repeat Amplification Protocol Master Mix to yield a solution containing a final concentration of 20 mM Tris-HCl (pH 8.3), 1.5 mM MgCI2, 63 mM KC1,1 mM EGTA, 0.005% tween 20, 0.5 mM each of deoxynucleotide triphosphate and 0.25 μg of T4 gene 32 protein following incubation at 30°C for 1 h. Negative samples were then rerun in dilutions of 1:10 and 1:100 to attempt recovery of telomerase activity in the event that telomerase (or PCR) inhibitors prevented adequate extension. This was found to be true for a small minority of samples (<10%). DNA extension products were then phenol–chloroform extracted and ethanol precipitated in a standard fashion to eliminate any PCR inhibitors and resuspended in 25 μl of ddH2O. Twenty-five microliters of master mix, including 10 units of taq polymerase and 4 μCi of (γ32 P) dCTP, were added to the resuspended DNA extension product mixture to yield a final concentration of 20 mM Tris-HC1 (pH 8.3), 1.5 mM MgCl2, 63 mM KCI, 1 mM EGTA, 0.005% Tween 20, 0.25 mM each of deoxynucleotide triphosphate and 1.5 × 10−17 g of an internal positive control for PCR. This internal control was made by amplifying microsatellite-repeat D4S243 using modified primers such that each primer consists of a forward or reverse D4S243 primer on the 3’ end and either CX or TS primer sequences on the 5’ end of each primer, respectively. Primer sequences used to amplify this control amplicon are 5’-AATCCTTCGAGCAGAGTTT AGGAGCCTGTGGTCCTGTT-3’ and 5’-CCCTTACCCTTACCCTTACCCTAATCAGTCTC TCTTTCTCCTTGCA-3’. The resulting approximately 200-bp PCR amplified product was then gel purified and quantitated, and 1.5 × l0−17 g were added to each reaction mixture. This mixture was separated from 100 ng of CX primer by a wax barrier as described. Reactions were then incubated at 90°C for 1 min and then cycled at 95°C for 30 s and 50°C for 30 s and at 70°C for 1 min for 35 cycles with a final extension step of 70°C for 5 min. Five microliters of lOX gel loading buffer were added and 25 μl of this mixture were run on a 10% polyacrylamide gel, dried and exposed to autoradiographs as described for approximately 8–10 h. Frozen sections of tumor samples were checked to assure the presence of tumor cells and absence of necrosis.
Statistical analysis
Student's t-test and ANOVA test were applied to validate the significance of the difference between groups.
RESULTS
In all of the oral rinses from patients with OSCC were found to possess telomerase activity [Table 1]. It was detected positively in 79.0% (79/100) of patients with OSCC, while it was positive in 51% (51/100) of premalignant patients and positive in 6.67% (8/100) of normal persons [Figure 2]; the statistical difference was significant with P < 0.001. However, the difference of expression of telomerase activity between the patients in the clinical early and late stage was not significant with P > 0.05, the same to that between the patients with and without lymph nodes metastasis with P > 0.05. The results suggest that the telomerase in saliva could be used as an assistant marker for OSCC.
Table 1.
Telomerase activity in oral rinses of oral squamous cell carcinoma, premalignant and control (normal) patients
| Cases | Total | Telomerase positive (%) | Telomerase negative (%) |
|---|---|---|---|
| Premalignant | 100 | 51 (51/100) | 49 (49/100) |
| OSCC | 100 | 79 (79/100) | 21 (21/100) |
| Control | 100 | 8 (8/100) | 92 (92/100) |
OSCC: Oral squamous cell carcinoma
DISCUSSION
As percentage of cancer populations is increasing sharply, the incidence of OSCC has also been expected to increase as well. Cancer prevention is more important than treatment for overcoming increased cancer death in the future. A large part of cancer load in parts of India is formed from oral cancer. Oral cancer is categorized into precancerous and cancerous stages. Precancerous stage includes leukoplakia, erythroplakia, oral submucous fibrosis and lichen planus, while cancerous or malignant stage is squamous cell carcinoma. Oral cancer development is a multistep process which arises from preexisting malignant lesions. Oral carcinogenesis is a highly complex, multistep process which involves accumulation of genetic alterations that lead to the induction of proteins promoting cell growth (encoded by oncogenes), increased enzymatic (telomerase) activity promoting cell proliferation. Maintenance of telomeres plays an essential role during transformation of cancer to malignant stage.
It was concluded that telomerase, a specialized reverse transcriptase enzyme, had a salient role in the process of tumorgenesis. Telomerase activity has been readily found in most cancer biopsies, in premalignant lesions or in germ cells. Activity of telomerase is generally absent in normal tissues. It is known to be induced upon immortalization or malignant transformation of human cells such as in oral cancer cells. Maintenance of telomeres plays an essential role during transformation of precancer to malignant stage. The roles of telomeres in regulating both stability of genome and replicative immortality seem to contribute in essential ways in cancer initiation and progression. Its expression will also prove to be an important diagnostic tool as well as a novel target for cancer therapy. The activation of telomerase may be an important step in tumorgenesis which can be controlled by inactivating its activity during chemotherapy. It is concluded that activity of telomerase can be used as a biomarker for diagnosis of malignant oral cancer and a target for inactivation in chemotherapy or gene therapy. Its expression will also prove to be an important diagnostic tool as well as a novel target for cancer therapy. The activation of telomerase may be an important step in carcinogenesis which can be controlled by blocking its activity at an early stage.
CONCLUSION
It was concluded that telomerase, a specialized reverse transcriptase enzyme, had a salient role in the process of tumorgenesis. Telomerase activity has been readily found in most cancer biopsies, in premalignant lesions or in germ cells.
Financial support and sponsorship
ICMR, New Delhi.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors sincerely acknowledge the contribution of Dr. Shadab Mohammad, Department of Oral and Maxillofacial Surgery, King George's Medical University, Lucknow, Uttar Pradesh, India and Dr. Vijay Kumar, Department of Surgical Oncology, Surgery, King George's Medical University, Lucknow, Uttar Pradesh, India in the study.
REFERENCES
- 1.Sunny L, Yeole BB, Hakama M, Shiri R, Sastry PS, Mathews S, et al. Oral cancers in Mumbai, India: A fifteen years perspective with respect to incidence trend and cumulative risk. Asian Pac J Cancer Prev. 2004;5:294–300. [PubMed] [Google Scholar]
- 2.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31, 1. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Bouquot JE, Whitaker SB. Oral leukoplakia – Rationale for diagnosis and prognosis of its clinical subtypes or “phases”. Quintessence Int. 1994;25:133–40. [PubMed] [Google Scholar]
- 4.Chen JK, Eisenberg E, Krutchkoff DJ, Katz RV. Changing trends in oral cancer in the United States, 1935 to 1985: A Connecticut study. J Oral Maxillofac Surg. 1991;49:1152–8. doi: 10.1016/0278-2391(91)90406-c. [DOI] [PubMed] [Google Scholar]
- 5.Gupta PC. Leukoplakia and incidence of oral cancer. J Oral Pathol Med. 1989;18:17. doi: 10.1111/j.1600-0714.1989.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsantoulis PK, Kastrinakis NG, Tourvas AD, Laskaris G, Gorgoulis VG. Advances in the biology of oral cancer. Oral Oncol. 2007;43:523–34. doi: 10.1016/j.oraloncology.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Kannan S, Tahara H, Yokozaki H, Mathew B, Nalinakumari KR, Nair MK, et al. Telomerase activity in premalignant and malignant lesions of human oral mucosa. Cancer Epidemiol Biomarkers Prev. 1997;6:413–20. [PubMed] [Google Scholar]
- 8.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–5. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 9.Shah FD, Begum R, Vajaria BN, Patel KR, Patel JB, Shukla SN, et al. A review on salivary genomics and proteomics biomarkers in oral cancer. Indian J Clin Biochem. 2011;26:326–34. doi: 10.1007/s12291-011-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiyama K, Ishioka S, Shirotani Y, Inai K, Hiyama E, Murakami I, et al. Alterations in telomeric repeat length in lung cancer are associated with loss of heterozygosity in p53 and rb. Oncogene. 1995;10:937–44. [PubMed] [Google Scholar]
- 11.Sommerfeld HJ, Meeker AK, Piatyszek MA, Bova GS, Shay JW, Coffey DS. Telomerase activity: A prevalent marker of malignant human prostate tissue. Cancer Res. 1996;56:218–22. [PubMed] [Google Scholar]
- 12.Tahara H, Nakanishi T, Kitamoto M, Nakashio R, Shay JW, Tahara E, et al. Telomerase activity in human liver tissues: Comparison between chronic liver disease and hepatocellular carcinomas. Cancer Res. 1995;55:2734–6. [PubMed] [Google Scholar]
- 13.Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, Gazdar AF, et al. Telomerase activity in human breast tumors. J Natl Cancer Inst. 1996;88:116–22. doi: 10.1093/jnci/88.2.116. [DOI] [PubMed] [Google Scholar]
- 14.Hiyama E, Hiyama K, Yokoyama T, Matsuura Y, Piatyszek MA, Shay JW, et al. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995;1:249–55. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 15.Tahara H, Kuniyasu H, Yokozaki H, Yasui W, Shay JW, Ide T, et al. Telomerase activity in preneoplastic and neoplastic gastric and colorectal lesions. Clin Cancer Res. 1995;1:1245–51. [PubMed] [Google Scholar]
- 16.Kyo S, Kunimi K, Uchibayashi T, Namiki M, Inoue M. Telomerase activity in human urothelial tumors. Am J Clin Pathol. 1997;107:555–60. doi: 10.1093/ajcp/107.5.555. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Miyamoto H, Fujinami K, Uemura H, Hosaka M, Iwasaki Y, et al. Telomerase activity in human bladder cancer. Clin Cancer Res. 1996;2:929–32. [PubMed] [Google Scholar]
- 18.Califano J, Ahrendt SA, Meininger G, Westra WH, Koch WM, Sidransky D, et al. Detection of telomerase activity in oral rinses from head and neck squamous cell carcinoma patients. Cancer Res. 1996;56:5720–2. [PubMed] [Google Scholar]
- 19.Jiang J, Park NJ, Hu S, Wong DT. A universal pre-analytic solution for concurrent stabilization of salivary proteins, RNA and DNA at ambient temperature. Arch Oral Biol. 2009;54:268–73. doi: 10.1016/j.archoralbio.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutirangura A, Supiyaphun P, Trirekapan S, Sriuranpong V, Sakuntabhai A, Yenrudi S, et al. Telomerase activity in oral leukoplakia and head and neck squamous cell carcinoma. Cancer Res. 1996;56:3530–3. [PubMed] [Google Scholar]
- 21.Wright WE, Shay JW, Piatyszek MA. Modifications of a telomeric repeat amplification protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic Acids Res. 1995;23:3794–5. doi: 10.1093/nar/23.18.3794. [DOI] [PMC free article] [PubMed] [Google Scholar]


