Abstract
Background:
Oral submucous fibrosis (OSMF) is a chronic debilitating condition of the oral mucosa that has been classified as a potentially malignant disorder with a malignant transformation rate of 2%–8%. Several in vitro and in vivo experiments have been performed to formulate a treatment modality for OSMF, yet no ideal in vitro primary oral fibroblast model has been developed.
Aim:
To establish an in vitro primary oral fibroblast model.
Setting and Design:
In vitro laboratory setting.
Materials and Methodology:
Primary cell culture protocol was performed after obtaining normal oral tissue. Karyotyping was performed to rule out chromosomal abnormalities. Immunofluorescence staining was carried with a panel of fibroblast-specific markers (vimentin, phalloidin, transforming growth factor-β receptor 1 [TGFβR1] and s100a4) and Masson trichrome staining (MTS) to demonstrate the presence of extracellular matrix (ECM) qualitatively.
Results:
A monolayer of oral fibroblasts was observed on the 9th-day postseeding. No chromosomal abnormality was observed in the patient samples. Positive staining was observed with vimentin, phalloidin, TGFβR1 and s100a4, thereby confirming the cell type. MTS revealed fibroblasts with spindle morphology and scanty ECM.
Conclusion:
The present study lays down a protocol to design and characterize primary buccal fibroblast cell culture model that would aid researchers in performing in vitro preliminary experiments in areas concerning fibrosis.
Keywords: Fibrosis, oral fibroblasts, phalloidin, transforming growth factor
INTRODUCTION
Oral submucous fibrosis (OSMF) is defined as a chronic, progressive, scarring disease that predominantly affects people of Southeast Asian origin. The initial stages of OSMF commence with fibrotic bands at the retromolar region that further progresses to the buccal mucosa gradually involving the soft palate, uvula, faucial pillars and sometimes the pharynx.[1,2] Induction of fibrosis in buccal cells during areca nut chewing and arecoline association (17.6%) was clearly demonstrated in the Taiwanese population.[3] In an in vitro study, on performing real-time polymerase chain reaction, upregulation of transforming growth factor β1 (TGFβ1) and downregulation of bone morphogenetic protein 7 were observed, signifying increased collagen deposition.[4] Processed fibroblast cell lines (gingival, dermal, uterine, bladder, foreskin and 3T3 [Swiss albino mouse embryo]) (ATCC cell lines) are available for carrying out in vitro experiments, but they are not suitable for studying mechanisms involved in OSMF. In vitro studies on human buccal cell line-based model would be alongside the in vivo response of arecoline-induced fibrosis that would help in identifying morphological changes during the early stages of fibrogenesis. Hence, we attempted to isolate and seed primary cell cultures from human buccal cells.
Primary fibroblast cultures were assessed for morphology using microscopy (shape and numbers) and for chromosomal abnormalities by performing karyotyping, to determine the cell type and origin, to demonstrate in vitro fibroblast differentiation using immunohistochemistry and finally to demonstrate collagen production in the extracellular matrix (ECM); Masson trichrome staining (MTS) was also performed.
MATERIALS AND METHODOLOGY
The study was carried out after obtaining institutional ethical committee approval (IEC-NI/II/APR/22/19). The present study was an in-vitro study; therefore, an average of three samples was obtained from three patients. Patients were duly informed about the study design. After obtaining approval, approximately 4 mm of tissue was collected from normal buccal mucosa. Tissue samples were collected from patients who underwent third molar impaction surgery at the Department of Oral and Maxillofacial Surgery, Faculty of Dental Sciences, Sri Ramachandra Medical College and Research Institute, Chennai.
The inclusion criteria involved healthy subjects aged above 21 years undergoing third molar impaction surgery, with no habits (tobacco chewing, smoking and alcohol consumption) and with normal mucosa.
The exclusion criteria were subjects with habits such as tobacco chewing, smoking and alcohol consumption; those taking drugs that could affect fibroblastic activity, e.g., calcium channel blockers, phenytoin sodium, cyclosporine and steroids; patients having diabetes mellitus, hypertension and ischemic heart disease and finally children below 12 years.
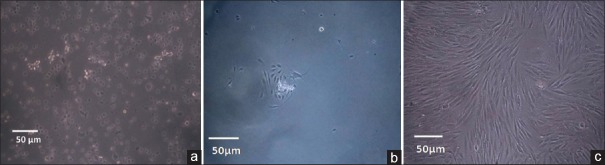
Standardization procedure for attaining primary fibroblast cell culture from buccal tissue samples was performed according to a protocol published earlier by Adtani et al. in 2017.[5] Dulbecco's modified eagle's medium (DMEM) optimized the multiplication and expansion of the fibroblast population. Of the three samples, the yield of viable cells ranged from 105 to 106/mL requiring seeding with adjusted appropriate volumes of harvested cells at the rate of 103/mL. The morphology of established cultures is shown in Figure 1a-c.
Figure 1.
Establishment and morphology of primary cell cultures of human oral buccal fibroblasts showing (a) seeded cells, (b) 3 days postinoculation and (c) confluency on the 9th day. Images taken using confocal microscopy
Cultured human buccal fibroblasts (HBFs) for the first in mitotic phase were harvested into T25 flasks for karyotyping. Seventy-five microliters of colcemid was added to arrest cells in metaphase and to separate the chromosomes for cytogenetic studies. Four milliliters of trypsin-ethylenediaminetetraacetate was added for detachment of cells. The detached cells were subjected to centrifugation at 1000 rpm for 10 min. To the pellet of cells formed, 1 mL of prewarmed hypotonic solution (potassium chloride) was added and subjected to centrifugation. The second pellet of cells thus formed was fixed with prechilled fixative (Carnoy's fixative). A few drops of cells were cast on a prechilled, clean glass slide from a height of 10–12 cm with the slide at an angle of 45°. The slides were then dried and visualized under a microscope to check spreading of metaphases and mitotic index followed by aging overnight at 60°C for staining with Giemsa and GTG banding (Giemsa banding). Metaphases were karyotyped using the CytoVision software, Leica Biosystems, Bengaluru, Karnataka, India and analyzed for numerical or structural chromosomal abnormalities under 100× oil immersion objective. A minimum of 20–30 banded metaphases were captured using image analysis through a charged-coupled device camera.[6]
1 × 104 cells suspended in the growth media (DMEM low glucose, prostate-specific antigen [PSA], 500 μl Gluta XL and 10% fetal bovine serum [FBS]) were seeded onto a coverslip placed in 12-well plates to carry out immunofluorescence staining with a specific panel of markers. On attaining 40%–50% confluency, growth media (DMEM low glucose, PSA, 500 μl Gluta XL and 10% FBS) were aspirated using serological pipettes and discarded in sterile test tubes. The cells on the coverslip were washed thrice in 1× phosphate-buffered saline (PBS) at room temperature. Fixation was carried out using methanol (−20°C) and incubated for 10 min. 0.05% Triton X was used for permeabilization. Postpermeabiliazation blocking was performed using blocking solution (3% goat serum, 1% bovine serum antigen in PBS and 16.3 M glycine) for 60 min. Later, the blocking solution was discarded and the primary antibodies (TGFβR1, s100a4, vimentin and phalloidin in blocking solution) were added.[7,8,9,10] The cells were incubated overnight in a humidified chamber at 4°C. The next day, primary antibodies were aspirated using serological pipets and discarded in sterile test tubes. Secondary antibodies (goat anti-rabbit IgG monoclonal fluorescein isothiocyanate tagged and goat anti-mouse IgG monoclonal tetramethylrhodamine [TRITC] tagged) were added and incubated for 60 min. Counterstaining was performed using 4’,6-diamidino-2-phenylindole (DAPI) with an incubation time of 20 min. Mounting was performed using an antifading agent 1,4-diazobicyclo-2, 2,2-octane medium. The mounted cells on the coverslip were then observed using confocal microscope.
MTS was performed, wherein HBFs were seeded onto 12-well culture plates and allowed to grow to 50% confluency. On attaining the desired confluency, fixation was carried out using methanol at −20°C. Staining was carried out stepwise with freshly prepared MTS according to the protocol earlier published by Adtani et al. (2017).[5]
RESULTS
On initial microscopic evaluation, live cells at the time of seeding appeared round [Figure 1a]. Microscopy of the cultures on incubation for 72 h showed attached monolayer of spindle-shaped fibroblasts in FBS [Figure 1b]. Fibroblasts showed complete (80%) confluency on day 9 [Figure 1c].
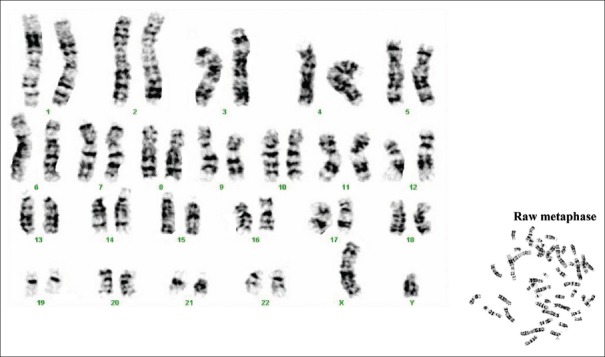
On genetically characterizing the attached cells, G-banding of chromosomes of cultured buccal fibroblast cells showed normal chromosomal morphologies with no chromosomal abnormalities [Figure 2].
Figure 2.
Karyogram from cultured human buccal fibroblasts using Giemsa staining
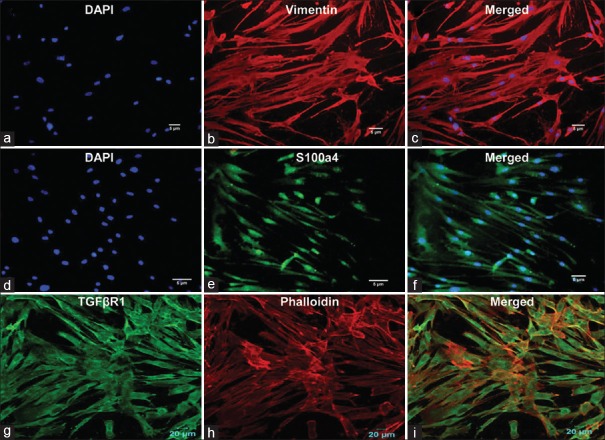
Microscopic analysis of cultured cells showed normal fibroblast growth in cultures. Immunofluorescence staining was performed to ensure and confirm the cell type and origin. The mesenchymal origin of the cultured cells was confirmed by positive staining with vimentin [Figure 3b and c].[7] Fibroblastic phenotyping was performed using fibroblastic-specific protein-1 (Fsp1, s100a4), a characteristic fibroblastic filament-associated calcium-binding protein [Figure 3e and f]. The nucleus of fibroblast cells was counterstained with DAPI [Figure 3a and d].[8]
Figure 3.
Immunofluorescence staining of cultured human buccal cells leading to the formation of homogenous fibroblast cells. Vimentin (b and c) staining indicated mesenchymal origin, while s100a4 (e and f) detected the intracellular filaments in fibroblasts. Surface protein receptor (g) transforming growth factor β receptor 1 indicated the fibrotic nature of the differentiated cells, while phalloidin (h) was used to show the presence of actin filaments in such cells. 4’,6-diamidino-2-phenylindole staining of nucleated cells confirmed the viability of the growing cells (a and d), (i) Merged image (TGFβR1 and phalloidin)
Cells stained positive for TGFβR1, a potent fibrotic mediator that indicates its presence on the cell surface of differentiated cells.[9] The cytoplasm of cultured cells exhibited positive phalloidin staining, indicating the contractile property of fibroblasts [Figure 3g-i]. Thus, using these immunomarkers, we established a protocol to culture normal primary fibroblasts in vitro.
Morphological assessment of human buccal fibroblasts and qualitative assessment of collagen
Masson trichrome is a connective tissue stain, used to differentiate between collagen and smooth muscle in normal and pathological tissue. It helps to qualitatively assess the effects of antifibrotic agents on ECM deposition.[11] MTS [Figure 4] revealed fibroblasts with spindle morphology and faintly stained collagen fibers, thereby demonstrating no fibrotic activity in the ECM.
Figure 4.

Qualitative assessment of collagen deposition and fibroblast morphology in cultured cells (MTS, ×40). Adtani et al. Translational Research in Oral Oncology
DISCUSSION
In the present study, primary adherent fibroblast cell culture model using buccal tissue samples from healthy volunteers was established. Confluent fibroblast monolayer obtained on the 3rd day of seeding [Figure 1a-c] showed normal karyotype [Figure 2].
The immunohistological pattern of fibroblast cell differentiation was established using specific markers such as vimentin, s100a4, TGFβR1 and phalloidin. Spindle-shaped fibroblasts and mesenchymal origin of the cultured cells were confirmed by positive vimentin staining [Figure 3]. Our results were concurrent with studies performed by Goodpaster et al., who reported maximum (100%) staining intensity with vimentin in dermal and lung fibroblasts when compared to monocyte/macrophage, glioma and osteosarcoma cells explaining its specificity for fibroblasts.[7]
Mathew et al. reported mitotic (FI, FII and FIII) population of fibroblasts cultured from tissues of patients with normal oral mucosa; they observed that FI population of cells have a spindled morphology which stained positive for vimentin.[12] Similar fibroblast morphology was also observed and reported by Bayreuther et al. in skin fibroblasts.[13] Studies done by Waal et al. reported that fibroblasts cultured from patients with normal mucosa and no areca nut chewing habit were of the FI (spindle) type when compared to the FIII type (large pleomorphic and epithelioid) in OSMF patients.[14] In the present study, FI type of fibroblast population was observed.
Fsp1 also called as s100a4 (filament-associated calcium-binding protein) is associated with cells of mesenchymal origin or of fibroblastic phenotype. Cultured HBFs demonstrated s100a4 staining, confirming their fibroblastic phenotype [Figure 3d-f]. Nishitani et al. also demonstrated Fsp1 expression in fibroblasts accumulating in areas with severe renal interstitial fibrosis.[8]
TGFβ, a potent fibrotic mediator and a scar-inducing factor, is known to be upregulated in many fibrotic conditions; its receptors type I and II are present as cell membrane receptors on cells such as fibroblasts. In the present study [Figure 3g-i], accumulation of TGFβR1 present on cultured fibroblasts was demonstrated. A similar study by Komuta et al. in 2010 demonstrated TGFβR1 via in situ hybridization (immunofluorescence staining) on meningeal fibroblasts in mouse brain tissue.[9]
The cultured human fibroblasts showed intracellular cytoplasmic staining with phalloidin, a dye specific for actin filaments [Figure 3g-i]. Verderame et al. also demonstrated thick actin filaments stained with phalloidin, well distributed throughout the cytoplasm in a 3T3 rat fibroblast cell line.[10] Thus, a primary confluent buccal fibroblast culture which demonstrated mesenchymal origin and fibroblast cell-type properties was obtained that can be used for testing natural or synthetic compounds for their antifibrotic activity.
CONCLUSION
The present study lays down a protocol to design and characterize primary buccal fibroblast cell culture model that would aid researchers in performing in vitro preliminary experiments in areas concerning fibrosis.
Financial support and sponsorship
One of the authors received funding under the ICMR - TALENT SEARCH SCHEME from the Indian Council of Medical Research, New Delhi, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to thank Dr. Solomon FD Paul and Dr. Teena Koshy, Department of Human Genetics, Sri Ramachandra Institute of Higher Education and Research for their extended help in performing karyotyping.
REFERENCES
- 1.Chole RH, Gondivkar SM, Gadbail AR, Balsaraf S, Chaudhary S, Dhore SV, et al. Review of drug treatment of oral submucous fibrosis. Oral Oncol. 2012;48:393–8. doi: 10.1016/j.oraloncology.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran A, Sivapathasudharam B. 6th ed. Delhi, India: Mosby Elsevier; 2009. Shafer's Textbook of Oral Pathology; pp. 96–100. [Google Scholar]
- 3.Yang YH, Lee HY, Tung S, Shieh TY. Epidemiological survey of oral submucous fibrosis and leukoplakia in aborigines of Taiwan. J Oral Pathol Med. 2001;30:213–9. doi: 10.1034/j.1600-0714.2001.300404.x. [DOI] [PubMed] [Google Scholar]
- 4.Khan I, Agarwal P, Thangjam GS, Radhesh R, Rao SG, Kondaiah P, et al. Role of TGF-β and BMP7 in the pathogenesis of oral submucous fibrosis. Growth Factors. 2011;29:119–27. doi: 10.3109/08977194.2011.582839. [DOI] [PubMed] [Google Scholar]
- 5.Adtani PN, Narasimhan M, Punnoose AM, Kambalachenu HR. Antifibrotic effect of Centella asiatica Linn and asiatic acid on arecoline-induced fibrosis in human buccal fibroblasts. J Investig Clin Dent. 2017;8:1–9. doi: 10.1111/jicd.12208. [DOI] [PubMed] [Google Scholar]
- 6.Howe B, Umrigar A, Tsien F. Chromosome preparation from cultured cells. J Vis Exp. 2014;28:e50203. doi: 10.3791/50203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodpaster T, Legesse-Miller A, Hameed MR, Aisner SC, Randolph-Habecker J, Coller HA, et al. An immunohistochemical method for identifying fibroblasts in formalin-fixed, paraffin-embedded tissue. J Histochem Cytochem. 2008;56:347–58. doi: 10.1369/jhc.7A7287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishitani Y, Iwano M, Yamaguchi Y, Harada K, Nakatani K, Akai Y, et al. Fibroblast-specific protein 1 is a specific prognostic marker for renal survival in patients with IgAN. Kidney Int. 2005;68:1078–85. doi: 10.1111/j.1523-1755.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 9.Komuta Y, Teng X, Yanagisawa H, Sango K, Kawamura K, Kawano H, et al. Expression of transforming growth factor-beta receptors in meningeal fibroblasts of the injured mouse brain. Cell Mol Neurobiol. 2010;30:101–11. doi: 10.1007/s10571-009-9435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verderame M, Alcorta D, Egnor M, Smith K, Pollack R. Cytoskeletal F-actin patterns quantitated with fluorescein isothiocyanate-phalloidin in normal and transformed cells. Proc Natl Acad Sci U S A. 1980;77:6624–8. doi: 10.1073/pnas.77.11.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu C, Wang W, Xu M, Zhang J. Asiatic acid ameliorates tubulointerstitial fibrosis in mice with ureteral obstruction. Exp Ther Med. 2013;6:731–6. doi: 10.3892/etm.2013.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew DG, Skariah KS, Ranganathan K. Proliferative and morphologic characterization of buccal mucosal fibroblasts in areca nut chewers: A cell culture study. Indian J Dent Res. 2011;22:879. doi: 10.4103/0970-9290.94693. [DOI] [PubMed] [Google Scholar]
- 13.Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI, et al. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988;85:5112–6. doi: 10.1073/pnas.85.14.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Waal J, Olivier A, van Wyk CW, Maritz JS. The fibroblast population in oral submucous fibrosis. J Oral Pathol Med. 1997;26:69–74. doi: 10.1111/j.1600-0714.1997.tb00024.x. [DOI] [PubMed] [Google Scholar]