Abstract
Background:
Candidal infections have increased significantly in denture wearers, especially in immunocompromised patients. The increase in resistance to existing antifungal drugs and number of patients at risk, in conjunction with the restricted number of commercially available antifungal drugs that still present many side effects, are the cause for this problem. These limitations emphasize the need to develop new and more effective antifungal agents with lesser side effects.
Materials and Methods:
The present study was undertaken to investigate the possible antifungal action of the alcoholic extract of different concentrations of Curcuma longa on four dilutions of Candida albicans (1:10, 1:20, 1:40 and 1:80) and to determine its minimum inhibitory concentration (MIC) and minimum fungicidal concentration using Sabouraud's agar medium.
Results:
There was complete inhibition of the growth of all four dilutions of Candida at a concentration of 800 μl which is considered as the MIC of alcoholic extract of turmeric on C. albicans, and the minimum fungicidal concentration was at 1600 μl.
Conclusion:
This study indicates a potent antifungal action of C. longa against C. albicans.
Keywords: Candida albicans, curcuma, in vitro techniques
INTRODUCTION
Candida species are human fungal pathogens capable of initiating various recurring superficial mycoses, especially in the oral mucosa.[1] Particularly, Candida albicans is a major cause of oral and oropharyngeal infections in patients with various local and systemic causes such as hyposalivation, denture wearers, diabetes mellitus, prolonged use of antibiotics and immunocompromised status. It is also known that up to 90% of patients with HIV infection or acquired immunodeficiency syndrome (AIDS) suffer from oropharyngeal candidiasis.[2] The inadvertent use of antifungals in these patients has made them resistant to current antifungal therapy due to their inherent adaptive qualities such as formation of biofilms, expression of different genes, development of efflux pumps or persister cells.[3] Added to this, the high cost and long duration of antifungal therapy have led to search for alternative antifungal agents with better efficacy and lesser side effects.[4,5] Various studies have been carried out with the aim of examining the activity of natural products against fungi that cause infections.
Turmeric is a well-known indigenous herbal medicine belonging to the family Zingiberaceae.[6] This tuberous rhizome has long held a place of honor in India's traditional Ayurvedic medicine. The significance of turmeric in medicine has changed considerably since the discovery of the antioxidant and antimicrobial properties of its naturally occurring phenolic compounds. The mode of action by which the turmeric extract inhibits the fungal growth is by alteration in the morphology of the hyphae which may appear severely collapsed, plasma membrane disruption, mitochondrial destruction, lack of cytoplasm, folding of the nuclear membrane and thickened cell wall caused by chemical components of spice extract.[6] They also have anti-inflammatory,[7,8,9] anticancer,[7,8,10] hepatoprotective,[11] antiallergic,[11] wound healing,[11] antispasmodic,[11] and anti-HIV properties.[2,11]
The present study has been undertaken to evaluate the in vitro action of turmeric (C. longa species) against C. albicans, the most common fungal species isolated from oral infection. The minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of the herb were determined according to the Clinical and Laboratory Standards Institute guidelines.
MATERIALS AND METHODS
This is a prospective laboratory trial study with no ethical concerns. Rhizomes of turmeric (C. longa Linn.) were purchased from a local market in Sullia, Karnataka. Fresh rhizomes were cleaned and cut into small pieces and air-dried for 2 days. The dried sample was again dried in a hot air oven at 50°C for 24 h, then ground into powder and passed through a sieve with nominal mesh size of 2 mm in diameter. Ground spices were stored in a plastic zip bag until use. The alcoholic extract of C. longa was prepared in a way that is described below, and a pure standard specimen of C. Albicans (PTCC5027, Merck, Germany) was used for culture.
Alcoholic extract of Curcuma longa
A slightly modified method from Singh and Jain was used for the alcoholic extract of C. longa. 5 g of dry powder of C. longa rhizome (turmeric) was soaked in 10 ml of absolute alcohol overnight in a refrigerator to avoid evaporation of alcohol. On the next day, it was centrifuged at a temperature of 22°C, speed of 3870 rotations per minute and timed for 12 min. At this point, a clear solution was visible at the top of the test tube and debris settled at the bottom. The clear solution at the top was carefully poured into a new test tube so that all debris remained in the original test tubes. This extract of turmeric in ethanol served as a stock for experimentation. This solution was stored at four degrees centigrade to avoid evaporation of the ethanol. The total mass of the debris was determined to be 3.37 g. The net turmeric in the 10 ml of solution was determined to be 1.73 g[12] Table 1 for the amount of turmeric in different concentrations of extract].
Table 1.
The calculation for turmeric concentration measured in grams of turmeric/ml of Sabouraud’s agar
| Turmeric Solution (μl) | Mass of Turmeric in Solution (g) |
|---|---|
| 0.000 ( Alcohol only) | 0.000 |
| 50.000 | 0.00865 |
| 100.000 | 0.0173 |
| 200.000 | 0.0346 |
| 400.000 | 0.0692 |
| 800.000 | 0.1384 |
| 1600.000 | 0.2768 |
| 3200.000 | 0.5536 |
Culture of Candida albicans
Surface of vial-containing fungi was disinfected by alcohol and broken within cotton dipped in alcohol near the heat. Sabouraud's dextrose agar (SDA) slope was used to subculture from a stock culture of C. albicans (PTCC5027, Merck, Germany) prepared using the necessary sterile precautions. This growth was used to prepare an inoculum in sterile saline, and four different concentrations of the fungus were prepared using doubling dilution method comprising of 1:10, 1:20, 1:40 and 1:80 concentrations.[13] An agar dilution assay was modified from the National Committee for Clinical Laboratory Standards (NCCLS, 2002) and used for determination of the MIC.[14] SDA plates mixed with 7 different concentrations of 50, 100, 200, 400, 800, 1600 and 3200 μl of alcoholic turmeric extract in 7 separate plates were prepared. Each plate was divided into four quadrants; different dilutions of fungal suspension were streaked onto the different quadrants of the culture plates 15 min after the preparation of the suspension so that the density does not change. The medium was inoculated by even streaking with the help of a sterile cotton wool swab and incubated for 48 h at 37°C. Following incubation, the number of colonies was visually counted and the relative size of the colonies was visually inspected and data were recorded.
Minimum inhibitory concentration and minimum fungicidal concentration of Curcuma longa
The culture plate that did not demonstrate visible growth corresponds with the MIC of the antimicrobial agent. The MIC endpoint is the lowest concentration of the C. longa extract at which there was no visible growth in the tubes. The culture plate demonstrating no visible growth was subcultured to Sabouraud agar plates, and MFC was determined by comparing the growth with the positive control. The MFC endpoint is defined as the lowest concentration of antimicrobial agent that kills >99.9% of the initial fungal population where no visible growth of the fungi was observed on the SDA plates.
RESULTS
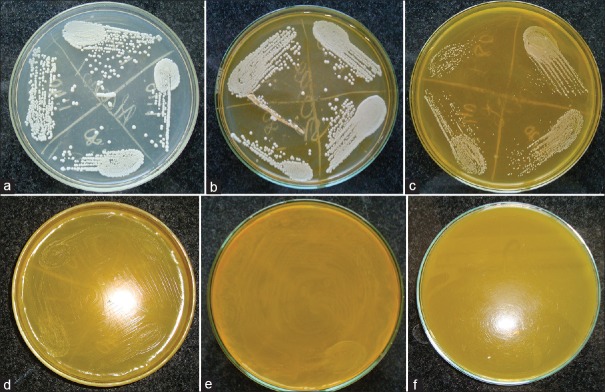
Antifungal activity of ethanolic turmeric extract was tested for C. albicans using agar dilution method. Alcoholic control was prepared to rule out the antifungal activity of ethanol [Figure 1a]. Evaluation of plates showed that there was absolutely no antifungal effect in a concentration as low as 50 μl [Figure 1b]. The size and number of the fungal colonies decreased with the increase in concentration of alcoholic extract of turmeric. As the concentration of the alcoholic extract of turmeric increased the size and the number of the fungal colonies decreased [Figure 1c and d]. The size and number of colonies were inversely proportional to the concentration of turmeric [Figure 2]. It was also noted that with increasing dilutions of C. albicans, the number of colonies decreased, thereby the number of colonies was inversely proportional to the amount of dilution of Candida [Figure 3]. The control plate with alcohol only showed maximum growth. Hence, alcohol was not responsible for the inhibition of the growth of Candida.
Figure 1.
(a-d) Denotes the decrease in diameter of the colonies as the concentration of turmeric increases. (e and f) The complete inhibition of candida colonies at a concentration of 800 μl onward
Figure 2.
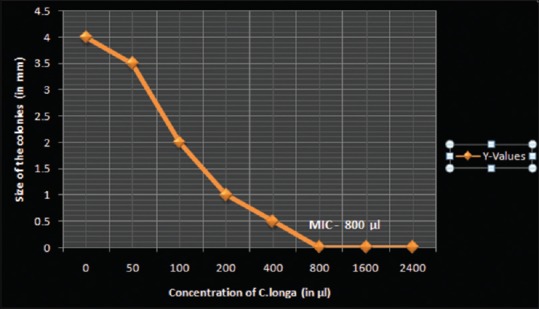
A scatter graph plotted to indicate the decrease in colony diameter with increasing concentrations of Curcuma longa
Figure 3.
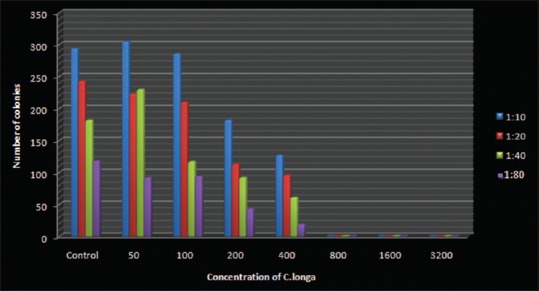
The graphical representation above shows the decrease in number of colonies with increase in concentration of turmeric extract at different dilutions of Candida
There was complete inhibition of visible growth of all four dilutions of Candida at a concentration of 800 μl and greater [Figure 1e and f]. Thus, 800 μl (0.1384 g of C. longa) was considered as the MIC of alcoholic extract of turmeric on C. albicans. On subculturing these plates, 800 μl showed some amount of growth, while 1600 and 3200 μl did not show any growth in subculture. Hence, the MFC of alcoholic extract of turmeric was 1600 μl (0.2768 g of C. longa).
DISCUSSION
The interest in oral candidiasis has waxed and waned from the period of Hippocrates. Over the years there has been a definite increase in candidal infections and these figures have bolstered up due to HIV and diabetes mellitus. However, the various side effects of drugs available for the management of candidiasis and the emergence of resistant isolates has made antifungal drug susceptibility testing an important component of current health care management.[14] In 1995, in a landmark publication in the field of antifungal susceptibility testing, the NCCLS published the reference method for agar dilution antifungal susceptibility testing of yeasts, which proposed a standard method to determine yeast susceptibilities. This method measures and compares colony sizes of individual strains at different drug concentrations on solid agar medium. The colony size method is simple, fast and inexpensive and requires no instrumentation. Even without using the microscope, simple comparisons of the colony sizes could usually determine the MICs for strains; multiple isolates could be streaked on a single petri dish. Most importantly, the colony size method could provide a statistical meaning to the MIC. In this study, the above method has been slightly modified and adapted.[14]
Oral candidiasis is frequently seen in both dental and general practice. It is mainly caused by C. albicans. Several factors, including iatrogenic, pathophysiological and behavioral, may promote oral candidiasis generating a disturbance in the oral microbial ecosystem and causing an increase in the “mycotic count.” Generally, treatment to control these episodes in small lesions is exclusively topical, employing nystatin in an oral suspension or other options such as clotrimazole tablets which dissolve in the mouth and miconazole. The increase in resistance to antifungals and the long duration of antifungal therapy have led to various studies being carried out with the aim of examining the activity of natural products against fungi that cause infections.[7]
In our study, we have used ethanol (absolute alcohol) as the solvent based on the methodology from Singh and Jain; a control plate was prepared to rule out its antifungal activity. The control plate showed maximum growth proving that there was no possible interference of alcohol in the antifungal activity. The ethanolic extract of turmeric was used because it contains many chemical components in their extracts including phenolic compounds and its derivatives, the esters of weak acid, fatty acid, terpenes and others. Since a large number of different chemical compounds are present in these crude extracts, they can affect multiple target sites against the fungus.[12] In a study by Neda and Shiva, methanol was used initially, but it was revealed that methanol has antifungal activity and it was replaced by 50% dimethyl sulfoxide.[15]
Neda and Shiva conducted a study using three laboratory methods which included cell count technique, cup bioassay technique and disk diffusion method to evaluate antifungal effect of curcumin against C. albicans, and this effect generally increases by increasing the dosage.[15]
In a study by Jianping et al., the MIC for fluconazole against C. albicans was determined by agar dilution assay. It was observed that when different concentrations of fluconazole were tested against Candida, the diameter of the colonies decreased as the drug concentration increased. This concept has been adapted in our study to determine the MIC of turmeric.[16]
A study was conducted by Garcia – Gomes to evaluate the capability of curcumin, a natural compound found in the Curcuma longa plant, to sensitize a clinical isolate of Candida albicans, which was found to have a high resistance to fluconazole. Curcumin was found to have a great capability to inhibit fluconazole resistance of the isolate of C. albicans. It was capable of restoring its sensitivity to flucanazole when used at 11 μM. This study suggested that curcumin is not only a natural compound capable of inhibiting C. albicans growth, but is also able to act synergistically with fluconazole.[17]
According to a study conducted by Martins et al. (2009), curcumin was a more potent antifungal than fluconazole. Curcumin intensely inhibited the adhesion of Candida species isolated from AIDS patients to buccal epithelial cells, indicating that curcumin is a promising lead compound that can be put to therapeutical use in immunocompromised patients.[18]
Alalwan et al. investigated the biological impact of curcumin on C. albicans and showed that curcumin preferentially affected immature morphological forms (yeast and germlings) and actively promoted aggregation of the cells. Transcriptional analyses showed that key adhesins were downregulated (ALS1 and ALS3) whereas aggregation-related genes (ALS5 and AAF1) were upregulated. Collectively, the above data established that curcumin produces antiadhesive effects and that induces transcription of genes integrally involved in the processes associated to biofilm formation. Curcumin and related polyphenols therefore have the capacity to be developed for usage in oral healthcare to amplify existing preventive strategies for candidal biofilms on the denture surface.[19]
In a study by Sharma et al., the use of curcumin alone or an antifungal drug (nystatin or amphotericin B) alone did not create any halo after 48 h. However, when curcumin was used simultaneously with each of two antifungal drugs, there was a 1–1.5 mm halo because of synergic effect of drugs. This may be because they used 23 μg/ml concentration of curcumin and also 0.078 μg/ml amphotericin B; at such low concentrations, these drugs may not have been effective alone.[20] In another study, curcumin was shown to enhance the activity of azole and polyene antifungals.[21]
From the results of our study, it appears that turmeric possesses definite antifungal properties. It shows static effects at lower concentration and fungicidal effects at higher concentrations. The extract used in our study was a crude extract from whole turmeric, the active ingredient that endows it with antifungal action was not isolated. It is possible that, after a purification step, the concentration required for an antifungal action would be lower than that obtained in the present work. An attempt can also be made to identify and isolate the active ingredient of C. longa responsible for the antifungal activity to obtain MIC and MFC at low concentrations of the extract.
CONCLUSION
In this study, effect of the antifungal agent C. longa was carried out on C. albicans. Characterization of C. longa was carried out with respect to its antifungal activity, extraction methods and MIC/MFC. Within the limitations of the study, it was concluded that:
C. longa is anticandidal in nature against C. albicans
Alcoholic extract of C. longa is effective against C. albicans
MIC and MFC of the alcoholic extract of C. longa for oral Candida are at 800 and 1600 μl, respectively.
Today, most pathogenic organisms are becoming resistant to drugs. To overcome this alarming problem, the discovery of novel active compounds against targets is a matter of urgency.[18] Most of the spices extract have biologically active compounds, which can be used in the synthesis of potent drugs. Thus, spices, which are normal ingredients of our routine food preparations, can provide protection to a certain extent against infections. There should be encouragement for the assessment of phytomedicinal products like Curcuma Longa. The results of our study suggest that the extract of C. longa can be considered a good candidate for topical use in the form of mouthwashes, lozenges etc, bearing in mind the antifungal performance demonstrated in vitro. Furthermore, the lesser side effects and low cost are important points in favor of this drug in developing countries like India.[6] However, further clinical studies are required to prove its efficacy in oral candidal infections.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Dr. Yellappa G. K, Professor, Ramakrishna Ayurvedic Medical College for his guidance in selection of the turmeric rhizome
KVG Ayurveda Medical College, Sullia for their help in preparing the turmeric rhizome powder.
REFERENCES
- 1.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, et al. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol. 2003;47:321–6. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 2.Pelletier R, Peter J, Antin C, Gonzalez C, Wood L, Walsh TJ, et al. Emergence of resistance of Candida albicans to clotrimazole in human immunodeficiency virus-infected children: In vitro and clinical correlations. J Clin Microbiol. 2000;38:1563–8. doi: 10.1128/jcm.38.4.1563-1568.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat V, Sharma SM, Shetty V, Shastry CS, Rao CV, Shenoy S, et al. Characterization of herbal antifungal agent, Origanum vulgare against oral Candida spp. Isolated from patients with Candida-associated denture stomatitis: An In vitro study. Contemp Clin Dent. 2018;9:S3–10. doi: 10.4103/ccd.ccd_537_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadeem SG, Shafiq A, Hakim ST, Anjum Y, Kazm SU. Effect of growth media, pH and temperature on yeast to Hyphal transition in Candida albicans. Open J Med Microbiol. 2013;3:185–92. [Google Scholar]
- 5.Shinobu Mesquita CS, Bertoni TA, Guilhermetti E, Svidzinki TI. Antifungal activity of the extract of Curcuma zedoaria (Christm.) Roscoe, Zingiberaceae, against yeasts of the genus Candida isolated from the oral cavity of patients infected with HIV. Braz J Pharmacogn. 2011;21:128–32. [Google Scholar]
- 6.Chandarana H, Shipra B, Chanda SV. Comparison of antibacterial activities of selected species of Zingiberaceae family and some synthetic compounds. Turk J Biol. 2005;29:83–97. [Google Scholar]
- 7.Sa G, Das T, Banerjee S. Curcumin: From modern spice to anticancer drug. Al Ameen J Med Sci. 2010;3:21–37. [Google Scholar]
- 8.Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK. Turmeric and curcumin: Biological actions and medicinal applications. Curr Sci. 2004;87:44–53. [Google Scholar]
- 9.Jacob A, Wu R, Zhou M, Wang P. Mechanism of the anti-inflammatory effect of curcumin: PPAR-gamma activation. PPAR Res. 2007;2007:89369. doi: 10.1155/2007/89369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu WD, Qin Y, Yang C, Li L, Fu ZX. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics (Sao Paulo) 2013;68:694–701. doi: 10.6061/clinics/2013(05)18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Mahajan S, Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol Rep (Amst) 2015;6:51–5. doi: 10.1016/j.btre.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh RP, Jain DA. Evaluation of antimicrobial activity of volatile oil and total curcuminoids extracted from turmeric. Int J Chem Tech Res. 2011;3:1172–7. [Google Scholar]
- 13.Rodriguez-Tudela JL, Barchiesi F, Bille J, Chryssanthou E, Cuenca-Estrella M, Jenning D, et al. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin Microbiol Infect. 2003;9:1–8. [Google Scholar]
- 14.Kumar RS, Ganvir S, Hazarey V. Candida and calcofluor white: Study in precancer and cancer. J Oral Maxillofac Pathol. 2009;13:2–8. doi: 10.4103/0973-029X.44575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neda B, Shiva Z. Inhibitory effect of curcumin on Candida albicans compared with nystatin: An in vitro study. J Dent Mater Tech. 2016;5:196–201. [Google Scholar]
- 16.Xu J, Vilgalys R, Mitchell TG. Colony size can be used to determine the MIC of fluconazole for pathogenic yeasts. J Clin Microbiol. 1998;36:2383–5. doi: 10.1128/jcm.36.8.2383-2385.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Gomes S, Curvelo JA, Soares RM, Ferreira-Pereira A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Medical Mycology. 2012;50:26–32. doi: 10.3109/13693786.2011.578156. [DOI] [PubMed] [Google Scholar]
- 18.Martins CV, da Silva DL, Neres AT, Magalhães TF, Watanabe GA, Modolo LV, et al. Curcumin as a promising antifungal of clinical interest. J Antimicrob Chemother. 2009;63:337–9. doi: 10.1093/jac/dkn488. [DOI] [PubMed] [Google Scholar]
- 19.Alalwan H, Rajendran R, Lappin DF, Combet E, Shahzad M, Robertson D, et al. The anti-adhesive effect of curcumin on Candida albicans biofilms on denture materials. Front Microbiol. 2017;8:659. doi: 10.3389/fmicb.2017.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma M, Manoharlal R, Negi AS, Prasad R. Synergistic anticandidal activity of pure polyphenol curcumin I in combination with azoles and polyenes generates reactive oxygen species leading to apoptosis. FEMS Yeast Res. 2010;10:570–8. doi: 10.1111/j.1567-1364.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- 21.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–99. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]