Abstract
Background:
Inflammation in tumor microenvironment assists in both promotion and growth of tumor. Tumor-associated tissue eosinophilia (TATE) is the term used when eosinophils are observed in a tumor tissue with inflammatory infiltrate. Although carcinogenesis with inflammation is one of the important hallmarks, the exact role of eosinophils remains unclear. Various studies on oral squamous cell carcinoma (OSCC) that focused on eosinophils reported both favorable and unfavorable prognosis in cancer tissue, because of which the exact function of eosinophils still remains uncertain.
Aims and Objectives:
The present study aims at identifying the role of TATE in OSCC and in malignant transformation of oral epithelial dysplasia (OED).
Materials and Methods:
The study includes 70 samples that divided into two groups, of which 50 histopathologically proven cases of different grades of OSCC and 20 cases of OED (oral leukoplakia). Congo red stain was used to stain the tissue sections. Each slide was viewed under high power in 10 consecutive microscopic fields for counting of eosinophils.
Results:
Statistical analysis of values obtained was done using ANOVA, unpaired t-test and Mann–Whitney test. The results were statistically significant (P < 0.05) with a mean total eosinophil count of 2.12 in OED and 4.31 in OSCC.
Conclusion:
The present study showed higher eosinophil counts in OSCC when compared to dysplasia which should prompt for a thorough evaluation of tumor front for invasiveness. Therefore, tissue eosinophil count may be used as an adjunct to predict the malignant transformation of dysplastic lesions to OSCC.
Keywords: Congo red stain, malignant transformation, oral leukoplakia, oral squamous cell carcinoma, tumor-associated tissue eosinophilia
INTRODUCTION
Head-and-neck squamous cell carcinoma (HNSCC) constitutes the sixth most common cancer worldwide, with squamous cell carcinoma of the upper aerodigestive tract ranging above 90% of all HNSCC.[1] HNSCC comprises squamous cell carcinoma of the oral cavity, oropharynx and hypopharynx. In India, oral squamous cell carcinoma (OSCC) is the most common cancer in males and is the third most common cancer in females.[2] OSCC pathogenesis results from the multiple molecular events that develop from the combination of an individual's genetic predisposition and exposure to carcinogens, such as tobacco, oncogenic viruses and inflammation which can damage individual genes. Accumulation of these genetic changes leads to OSCC in some instances via clinically evident oral potentially malignant disorder (OPMD), which may undergo sequential pathological changes from dysplasia to invasive carcinoma.[3,4] The most common OPMD is oral leukoplakia (OL), with a risk of malignant transformation ranging between 0.13% and 34%, which was revealed in a systemic review of 24 studies.[5]
OSCC comprises the tumor epithelium and the surrounding connective tissue stroma. The connective tissue stroma constitutes the tumor microenvironment (TME) within which varying populations of mesenchymal cells, extracellular matrix and inflammatory cells are present.[6] TME provides the cross-talk between the tumor cells and the stromal elements such as inflammatory cells and cancer-associated fibroblasts, contributing to the development, growth, invasion and metastasis of the tumor.[7] Literature suggests six hallmarks in the development of cancer. Epidemiological studies, from the beginning of the 19th century, are done to propose the role of inflammation as the seventh hallmark of cancer.[8] Very few studies are conducted to elicit the role of the inflammatory environment in relation to OPMDs and in progression of OPMDs to cancer. However, these studies have provided substantial evidence that a higher level of immune cell infiltration is indicative of ongoing immune reactivity in premalignant lesions and also in cancers.[9]
Eosinophils are granulocytic cells which have a role both in health and in disease. Initially known to be implicated in the pathogenesis of helminthic, bacterial and viral infections; tissue injury and allergic diseases, but recent advances show its role in tumour growth too.[10] They were first observed by Wharton Jones in 1846 and later described by Paul Ehrlich in 1879.[11] Eosinophil degranulation is the prominent function of eosinophils, by which the various secretory mediators are released into the microenvironment. These mediators may cause cell death and inflammatory symptoms and play a role in tumor regulation and tumor progression. There is a consistent observation of the eosinophilic infiltrate-associated various carcinomas such as OSCC, colon cancer, nasopharyngeal cancers and prostate cancer.[12] In HNSCC, the presence of eosinophils in the stromal infiltrate ranges between 22% and 89%.[13] This stromal eosinophilic infiltrate in tumors is termed as tumor-associated tissue eosinophilia (TATE). TATE was first described by Prezewoski in 1896 in carcinoma of the uterine cervix as “eosinophilic stromal infiltration of a tumor not associated with tumor necrosis or ulceration.”[14] TATE is characterized by the presence of eosinophils as a component of peritumoral and intratumoral inflammatory infiltrate.[15]
Various studies on TATE and its influence on the tumor prognosis had shown variable results, few of which associated with good prognosis and few with poorer prognosis. This is attributed to its influence on the release of cytotoxic proteins and the production of angiogenic factors.[12] However, the exact role of eosinophils in OL and OSCC is still unknown. Sometimes, these eosinophils are difficult to identify in the routine hematoxylin and eosin-stained sections as they can assume an uncommon morphology. Hence, special stains such as Congo red which stains the granules in the eosinophils can be used. Based on these facts, the aim of the present study is to evaluate the relationship between TATE and histological grading of OSCC and dysplasia using Congo red stain. This study is one of its kinds to evaluate the differences in the eosinophil count in oral epithelial dysplasia (OED) and with varying grades of OSCC based on which evaluation for invasiveness can be assessed.
MATERIALS AND METHODS
The samples of tissue section were obtained from archives of the Department of Oral and Maxillofacial Pathology from Kamineni Institute of Dental Sciences, Nalgonda, which presented during 2014–2018. The sample size of 50 histopathologically diagnosed cases of various grades of OSCC and 20 cases of histopathologically diagnosed OED was obtained during that period. The samples were distributed into four groups with 20 cases of well-differentiated squamous cell carcinoma (WDSCC), 20 cases of moderately differentiated squamous cell carcinoma (MDSCC), 10 cases of poorly differentiated squamous cell carcinoma (PDSCC) and 20 cases of OED [Table 1]. Microtome cut-sections measuring 4-μm thickness were prepared from the tissue archives.
Table 1.
Distribution of study sample
| Groups | Sample size (n) | Description of groups |
|---|---|---|
| Group 1 | 20 | WDSCC |
| Group 2 | 20 | MDSCC |
| Group 3 | 10 | PDSCC |
| Group 4 | 20 | OED |
WDSCC: Well-differentiated squamous cell carcinoma, MDSCC: Moderately differentiated squamous cell carcinoma, PDSCC: Poorly differentiated squamous cell carcinoma, OED: Oral epithelial dysplasia
Inclusion criteria
All histopathologically diagnosed cases of OSCC and OED who have not undergone any prior treatment for the lesion.
Exclusion criteria
Patients with other systemic diseases, allergic conditions and parasitic infections
Patients on corticosteroids
Tissue sections with areas of red blood cells with superimposed mononuclear and polymorphonuclear leukocytes, eosinophils in vascular channels, areas of tumor necrosis and degenerated muscle tissues were excluded.
The sections were deparaffinized, hydrated through graded alcohols to water and then placed in 1% Congo red solution for 10 min, followed by dips in water. Later, differentiation was carried out with 2.5% potassium hydroxide solution. Then, the sections were counterstained with hematoxylin for 10 min. Differentiation was done with 1% acid alcohol. This is followed by dips in water. Finally, the sections were dehydrated through alcohol and cleared in xylene and mounted with diphenyl xanthene. Granules contained in the eosinophils take up the Congo red stain and appear as bright red-to-orange granules with basophilic-lobed nucleus.
Each tissue specimen slide was viewed under high power (×40) in 10 consecutive microscopic fields using modified zig-zag pattern, without overlap. The slides were examined by two observers independently to prevent interobserver bias. Intraclass correlation coefficient (ICC) analysis reveals a good agreement between two observers (ICC = 0.8). The number of eosinophils per 10 high power fields is recorded. In OEDs (OL), the inflammatory area adjacent to the epithelium is chosen. In OSCC, the invasive tumor front region was chosen for counting of eosinophils.
The results obtained were analyzed statistically using Mann–Whitney test and ANOVA test using SPSS 19 software (IBM Corp., IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corp). A P < 0.05 was considered statistically significant.
The tissue sections stained with Congo red stain are illustrated in Figures 1-3.
Figure 1.
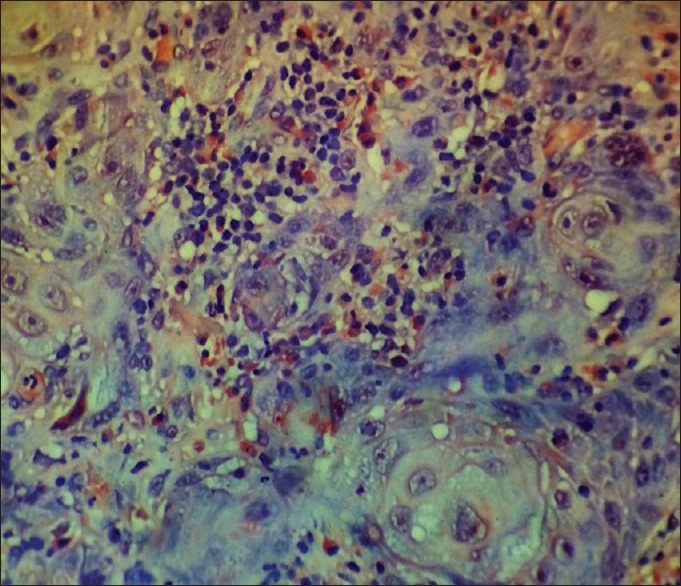
The section showing tissue eosinophils in a case of well-differentiated squamous cell carcinoma (Congo red stain, ×400)
Figure 3.
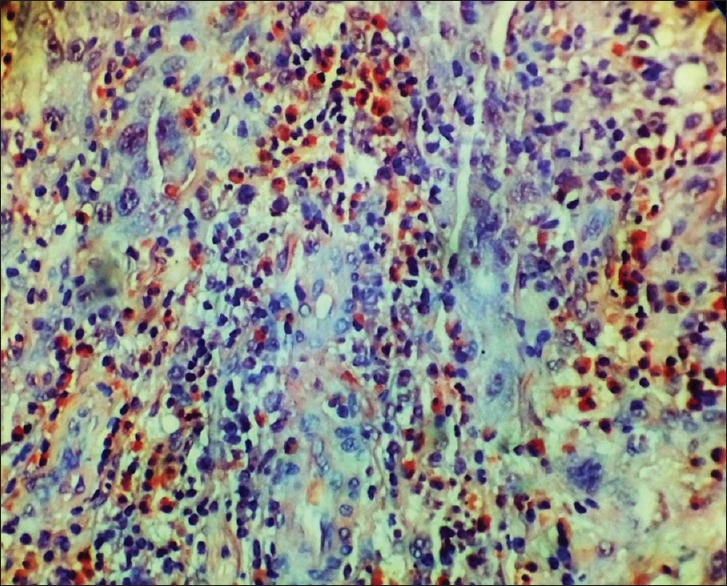
The section showing tissue eosinophils in a case of poorly differentiated squamous cell carcinoma (Congo red stain, ×400)
Figure 2.
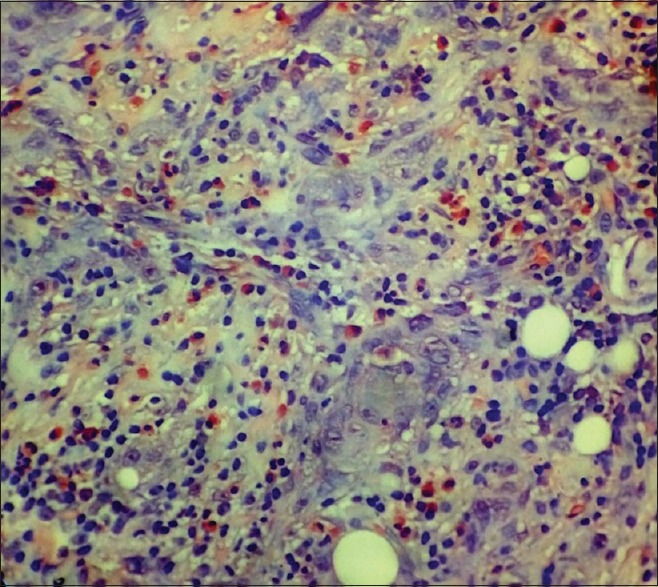
The section showing tissue eosinophils in a case of moderately differentiated squamous cell carcinoma (Congo red stain, ×400)
RESULTS
The mean and standard deviation (SD) of total eosinophil counts (TECs) among different groups are illustrated in Table 2. The box plot analysis of TEC in different grades of OSCC and OED is represented in Graph 1. The TEC in all the groups was compared using one-way ANOVA test, and the results obtained were significant with P = 0.00001.
Table 2.
The mean and standard deviation of total eosinophil counts among different groups
| WDSCC | MDSCC | PDSCC | OED | SS | Df | F | P | |
|---|---|---|---|---|---|---|---|---|
| n | 20 | 20 | 10 | 20 | 113.6412 | 3 | 12.36627 | 0.00001 |
| Mean | 3.36 | 4.43 | 5.96 | 2.12 | 202.1715 | 66 | ||
| SD | 1.402 | 2.1723 | 2.1996 | 1.29 | 315.8127 | 69 |
The result is significant at P<0.05. SD: Standard deviation, SS: Sum of squares, Df: Degrees of freedom. F- value: ANOVA value
Graph 1.
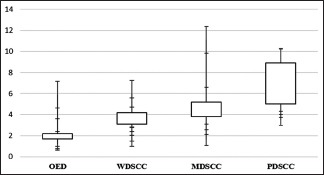
Box plot analysis of oral epithelial dysplasia and different grades of oral squamous cell carcinoma showing total eosinophil count. WDSCC: Well-differentiated squamous cell carcinoma, MDSCC: Moderately differentiated squamous cell carcinoma, PDSCC: Poorly differentiated squamous cell carcinoma, OED: Oral epithelial dysplasia
Comparison of TEC between OED and OSCC group was done using unpaired t-test, and the results obtained were statistically significant with a P = 0.000026. They are illustrated in Table 3. The mean values of TEC were found to be higher in OSCC (4.31) compared to OED (2.12).
Table 3.
Comparison of total eosinophil counts between oral epithelial dysplasia and oral squamous cell carcinoma
| Group | n | Mean | SD | t | P | Result |
|---|---|---|---|---|---|---|
| Oral epithelial dysplasia | 20 | 2.12 | 1.66 | −4.32281 | 0.000026 | <0.05 (significant) |
| OSCC | 50 | 4.31 | 4.41 |
OSCC: Oral squamous cell carcinoma, SD: Standard deviation
Comparison of eosinophil counts between WDSCC and MDSCC using unpaired t-test is illustrated in Table 4, and the results obtained are statistically significant. The mean and SD values of TEC in MDSCC (4.43) are comparatively higher than the WDSCC (3.36).
Table 4.
Comparison of total eosinophil counts between three groups using unpaired t-test
| Group | n | Mean | SD | t | P | Result |
|---|---|---|---|---|---|---|
| WDSCC | 20 | 3.36 | 1.97 | −1.85079 | 0.035 | <0.05 (significant) |
| MDSCC | 20 | 4.43 | 4.72 | |||
| MDSCC | 20 | 4.43 | 4.72 | −1.81119 | 0.040427 | <0.05 (significant) |
| PDSCC | 10 | 5.96 | 4.84 | |||
| WDSCC | 20 | 3.36 | 1.97 | −3.94961 | 0.00024 | <0.05 (significant) |
| PDSCC | 10 | 5.96 | 4.84 |
SD: Standard deviation, WDSCC: Well-differentiated squamous cell carcinoma, MDSCC: Moderately differentiated squamous cell carcinoma, PDSCC: Poorly differentiated squamous cell carcinoma
Comparison of TEC between MDSCC and PDSCC using unpaired t-test is illustrated in Table 4, and the results obtained are statistically significant. The mean and SD values of PDSCC (5.96) are comparatively higher than MDSCC (4.43).
The TEC values between WDSCC and PDSCC are compared using unpaired t-test, and the results obtained are statistically significant. The values are illustrated in Table 4. The mean and SD values of PDSCC (5.96) are higher compared to WDSCC (3.36).
DISCUSSION
The pathogenesis of invasive oral cancer is not only based on the genetic alterations in the tumor cells but also based on the intimate communication between the tumor cells, inflammatory cells, endothelial cells, fibroblasts and other stromal cells.[3] In view of inflammation-associated carcinogenesis, the role of eosinophils in tumor cytotoxicity is not well understood but has been characterized as more potent than other inflammatory cells in tumor-associated cytotoxic reaction.[15,16] Based on the above facts, this study is based on the ambiguity in functional role of eosinophils in oral cancers as they are hypothesized as being immunologically directed against tumor cells as well as in lowering the immune response facilitating the tumor growth.
The present study is one such study to compare the tumor eosinophilia in OED and different grades of OSCC. Most of the cases showed diffuse eosinophilic infiltration and are intimately associated with tumor cells. The results found a statistically significant increase in the TEC in OSCC compared to OED and a statistical increase in the mean values of TEC from WDSCC to PDSCC, suggesting a role in poorer prognosis of eosinophils in tumor front. Various studies reported the evidence of eosinophils in HNSCC, with a range between 22% and 89%.[17]
The studies which are in favor of the results obtained in the present study are as follows; Alrawi et al., in their study on tumor eosinophilia in patients with invasive and noninvasive HNSCC and correlated with tumor staging, concluded that there is an increased eosinophil count in invasive squamous cell carcinoma compared to noninvasive one.[18] Wong et al., in a study conducted on carcinogeninduced hamster oral cancer models, where the role of eosinophils in tumorigenesis is elicited using antiinterleukin (IL) 5 monoclonal antibody which acts against TATE providing a delay/inhibition of the tumor development. IL-5 is an eosinophil colony stimulating factor which results in proliferation and maturation of eosinophils. When anti IL-5 was used, it resulted in suppression of TATE and further also tumour growth. This states that eosinophils have a role in tumorigenesis. Based on the results obtained, this study concluded stating that TATE is protumorigenic.[19] Falconieri et al., in their study on OSCC to compare the clusters of eosinophilic infiltration in the tumor front, concluded that most OSCC with eosinophilic-rich inflammatory infiltrate is associated with stromal invasion and poorer prognosis.[20]
On the contrary, studies by Lowe and Fletcher, Goldsmith et al., Thompson et al., Ohashi et al., Debta et al., Dorta et al., Jain et al. and Yellapurkar et al. concluded that TATE is associated with favorable prognosis.[21,22,23,24,25,26,27,28]
Increase in TEC from OED to OSCC in the present study is in accordance with the study conducted by Jain et al., which includes the comparison of TEC between dysplasia and metastatic and nonmetastatic OSCC. They concluded that TATE might play a role in stromal invasion.[25]
Eosinophils being inflammatory cells release various substances in response to different stimuli. They contain substances which are released as an effector immune activation and also immune inhibition.[29] During an immune response, an antigenic stimulus is required for the activation of naïve CD4+ T-cells and CD8+ T-cells, which further release cytokines to provide humoral and cytotoxic responses, respectively.[30] Humoral response is by Th1 and Th2 cell stimulation. Th1 response generally helps in the production of factors, such as interferon-γ, interleukin (IL)-4 and IL-5, which provide environment favorable for tumor necrosis or eradication. The initial recruitment and activation of eosinophils are mainly related to Th2 response.[11]
At the tumor front, the cytokines released by tumor cells and inflammatory cells that facilitate recruitment of eosinophils are IL-4 and IL-13 and eosinophilic chemotactic factor, C5a and eotaxin help in activation. The downregulation of antitumor immunity by eosinophils is mediated by cytokines such as IL-10 and indoleamine oxidase. The mechanism of antitumor immunity is represented in Figure 4. The other factors produced by eosinophils in response to tumor cells are vascular endothelial growth factor, fibroblast growth factor, tumor necrosis factor-α, granulocyte-macrophage colony-stimulating factor, transforming growth factor-β and IL-8, which promote fibroblast activation and angiogenesis facilitating tumor progression.[29,31]
Figure 4.
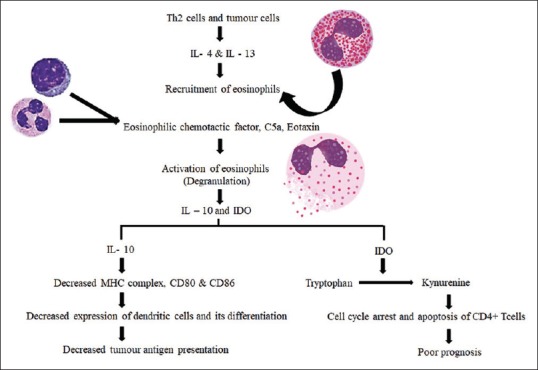
The mechanism of immune evasion by eosinophils in tumor microenvironment
Usually, oral precancer and oral cancer are commonly associated with a habit history of tobacco in the form of either chewing or smoking. The product of tobacco, i.e., 4-nitroquinoline-1-oxide, upon increased uptake decreases the Th1 response and increases the Th2 response, correlating to tumor growth.[32]
CONCLUSION
To the best of our knowledge, the present study is the first of its kind to compare the TEC in OED and different grades of OSCC using Congo red stain. It showed an increased level of eosinophilia and is associated with invasion and poor prognosis of OSCC, which mandates that the TEC in the routine histopathologic examination provides an insight into the aggressiveness of the lesion. The associated eosinophil infiltration in OED could suggest an invasive lesion and also prompts its role in increased malignant transformation potential of the lesion. Hence, the histopathologists ought to examine for eosinophilic infiltration and provide the same in the report to clinicians, which should provide for an attempt in prompt treatment and follow-up of the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Duvvuri U, Myers JN. Cancer of the head and neck is the sixth most common cancer worldwide. Curr Probl Surg. 2009;46:114–7. doi: 10.1067/j.cpsurg.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45:309–16. doi: 10.1016/j.oraloncology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: Implications for therapy. J Dent Res. 2008;87:14–32. doi: 10.1177/154405910808700104. [DOI] [PubMed] [Google Scholar]
- 4.Sharma M, Bairy I, Pai K, Satyamoorthy K, Prasad S, Berkovitz B, et al. Salivary IL-6 levels in oral leukoplakia with dysplasia and its clinical relevance to tobacco habits and periodontitis. Clin Oral Investig. 2011;15:705–14. doi: 10.1007/s00784-010-0435-5. [DOI] [PubMed] [Google Scholar]
- 5.Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: A systematic review of observational studies. J Oral Pathol Med. 2016;45:155–66. doi: 10.1111/jop.12339. [DOI] [PubMed] [Google Scholar]
- 6.Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, et al. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41:217–34. doi: 10.1053/j.seminoncol.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4:66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 9.Young MR. Redirecting the focus of cancer immunotherapy to premalignant conditions. Cancer Lett. 2017;391:83–8. doi: 10.1016/j.canlet.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 11.Liao W, Long H, Chang CC, Lu Q. The eosinophil in health and disease: From bench to bedside and back. Clin Rev Allergy Immunol. 2016;50:125–39. doi: 10.1007/s12016-015-8507-6. [DOI] [PubMed] [Google Scholar]
- 12.Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer Immunol Res. 2014;2:1–8. doi: 10.1158/2326-6066.CIR-13-0196. [DOI] [PubMed] [Google Scholar]
- 13.Peter CD, Shashidara R, Haragannavar VC, Samuel P, Sridhara SU, Gopalkrishna AH, Poojary S, Nayak SR, Sushanth A. Assessment of Tumor Associated Tissue Eosinophilia (TATE) in Oral Squamous Cell Carcinoma Using Carbol Chromotrope Stain. Int J Odontostomat. 2015;9:91–5. [Google Scholar]
- 14.Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA. Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer. 1996;77:436–40. doi: 10.1002/(SICI)1097-0142(19960201)77:3<436::AID-CNCR3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Tadbir AA, Ashraf MJ, Sardari Y. Prognostic significance of stromal eosinophilic infiltration in oral squamous cell carcinoma. J Craniofac Surg. 2009;20:287–9. doi: 10.1097/SCS.0b013e318199219b. [DOI] [PubMed] [Google Scholar]
- 16.Lorena SC, Dorta RG, Landman G, Nonogaki S, Oliveira DT. Morphometric analysis of the tumor associated tissue eosinophilia in the oral squamous cell carcinoma using different staining techniques. Histol Histopathol. 2003;18:709–13. doi: 10.14670/HH-18.709. [DOI] [PubMed] [Google Scholar]
- 17.Said M, Wiseman S, Yang J, Alrawi S, Douglas W, Cheney R, et al. Tissue eosinophilia: A morphologic marker for assessing stromal invasion in laryngeal squamous neoplasms. BMC Clin Pathol. 2005;5:1. doi: 10.1186/1472-6890-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alrawi SJ, Tan D, Stoler DL, Dayton M, Anderson GR, Mojica P, et al. Tissue eosinophilic infiltration: A useful marker for assessing stromal invasion, survival and locoregional recurrence in head and neck squamous neoplasia. Cancer J. 2005;11:217–25. doi: 10.1097/00130404-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Wong DT, Bowen SM, Elovic A, Gallagher GT, Weller PF. Eosinophil ablation and tumor development. Oral Oncol. 1999;35:496–501. doi: 10.1016/s1368-8375(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 20.Falconieri G, Luna MA, Pizzolitto S, DeMaglio G, Angione V, Rocco M. Eosinophil-rich squamous carcinoma of the oral cavity: A study of 13 cases and delineation of a possible new microscopic entity. Ann Diagn Pathol. 2008;12:322–7. doi: 10.1016/j.anndiagpath.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Debta P, Debta FM, Chaudhary M, Wadhawan V. Evaluation of prognostic significance of immunological cells infiltration in oral squamous cell carcinoma. J Cancer Sci Ther. 2011;3:201–4. [Google Scholar]
- 22.Lowe D, Fletcher CD. Eosinophilia in squamous cell carcinoma of the oral cavity, external genitalia and anus – Clinical correlations. Histopathology. 1984;8:627–32. doi: 10.1111/j.1365-2559.1984.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith MM, Belchis DA, Cresson DH, Merritt WD, 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27–33. doi: 10.1177/019459989210600124. [DOI] [PubMed] [Google Scholar]
- 24.Dorta RG, Landman G, Kowalski LP, Lauris JR, Latorre MR, Oliveira DT. Tumour-associated tissue eosinophilia as a prognostic factor in oral squamous cell carcinomas. Histopathology. 2002;41:152–7. doi: 10.1046/j.1365-2559.2002.01437.x. [DOI] [PubMed] [Google Scholar]
- 25.Jain M, Kasetty S, Sudheendra US, Tijare M, Khan S, Desai A. Assessment of tissue eosinophilia as a prognosticator in oral epithelial dysplasia and oral squamous cell carcinoma-an image analysis study. Patholog Res Int. 2014;2014:507512. doi: 10.1155/2014/507512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yellapurkar S, Natarajan S, Boaz K, Baliga M, Shetty P, Manaktala N, et al. Tumour-associated tissue eosinophilia in oral squamous cell carcinoma – A boon or a bane? J Clin Diagn Res. 2016;10:ZC65–8. doi: 10.7860/JCDR/2016/16440.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson AC, Bradley PJ, Griffin NR. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am J Surg. 1994;168:469–71. doi: 10.1016/s0002-9610(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi Y, Ishibashi S, Suzuki T, Shineha R, Moriya T, Satomi S, et al. Significance of tumor associated tissue eosinophilia and other inflammatory cell infiltrate in early esophageal squamous cell carcinoma. Anticancer Res. 2000;20:3025–30. [PubMed] [Google Scholar]
- 29.Martinelli-Kläy CP, Mendis BR, Lombardi T. Eosinophils and oral squamous cell carcinoma: A short review. J Oncol. 2009;2009:310132. doi: 10.1155/2009/310132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ananthanarayan R. Textbook of Microbiology. India: University Press; 2013. [Google Scholar]
- 31.Joshi PS, Kaijkar MS. A histochemical study of tissue eosinophilia in oral squamous cell carcinoma using Congo red staining. Dent Res J (Isfahan) 2013;10:784–9. [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang C, Ye D, Qiu W, Zhang X, Zhang Z, He D, et al. Response of lymphocyte subsets and cytokines to Shenyang prescription in Sprague-Dawley rats with tongue squamous cell carcinomas induced by 4NQO. BMC Cancer. 2007;7:40. doi: 10.1186/1471-2407-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]


