Abstract
The occurrence of the most important mycotoxins (deoxynivalenol, fumonisin B1 and B2, aflatoxins B1, B2, G1, and G2, ochratoxin A, zearalenone, T-2, and HT-2 toxins) was determined in 64 extruded cat foods purchased in Italy through ultra-performance liquid chromatography coupled with tandem mass spectrometry. Deoxynivalenol and fumonisins were the most common contaminants (quantified in 80 and 95% of the samples, respectively). Conversely, aflatoxins B2, G1, and G2 were not identified in any sample. Some cat foods exceeded the regulatory limit for aflatoxin B1 (n = 3) or the guidance values for zearalenone (n = 3), fumonisins (n = 2), ochratoxin A (n = 1), and T-2 (n = 1) recently established for pets in the European Union. A widespread co-occurrence of mycotoxins was observed (28, 42, and 8% of the samples contained quantifiable amounts of two, three, and four mycotoxins, respectively). This study describes criticisms regarding the mycotoxin issue in pet food and suggests an improvement of the monitoring of the pet food chain.
Introduction
Nowadays, feeding practice based on commercially prepared pet food is extremely widespread throughout the world since it represents an easy and economical way to meet the nutrient requirements of dogs and cats at the various stages of their life both in healthy and pathological conditions.
In consideration of the current strict human–animal bond and the importance of the feeding time perceived by pet owners as an opportunity of interaction and gratification,1 pet food quality is considered of great importance given its recognized connection to pet health. In this regard, among recent concerns about pet food safety, mycotoxin contamination represents a well-known problem.2 Mycotoxins are chemical compounds derived from the secondary metabolism of various genera of fungi such as Aspergillus, Fusarium, and Penicillium that can grow on agricultural crops. Consequently, these toxins are frequently detected in plant-based foods as well as animal feed.3
Although dogs and cats belong to the Carnivora order, extruded pet food typically contains relatively high amounts of cereals and cereal byproducts since the extrusion process, by favoring starch gelatinization, makes starch easily digestible.4 Nevertheless, these particular processing conditions, even if characterized by high pressures and temperatures, do not generally guarantee the complete degradation/inactivation of mycotoxins possibly contaminating raw ingredients.5 Consequently, extruded (or commonly named “dry”) pet food results particularly at risk of contamination, as proven by several surveys recently conducted in various world regions6−17 (Table 1).
Table 1. Most Recent Mycotoxin Studies on Commercial Pet Fooda.
| location and year of sampling/publication | type of pet food investigated | mycotoxins detected | most relevant results | ref |
|---|---|---|---|---|
| Poland (2004) | 57 pet foods (41 standard and 16 therapeutic samples) | ZEA (and its derivatives) | ZEA was identified in 84.2% of the samples (mean concentration 36.2 μg/kg). Max values in standard and therapeutic samples were 299.5 and 158 μg/kg, respectively. | (6) |
| Austria (2007) | 29 dry dog foods and 11 wet dog foods | DON and OTA | DON was identified in all the dry samples (range between 22 and 1837 μg/kg); 27% of the samples were positive for DON (range between 95 and 170 μg/kg); OTA contaminated 10% of the dry samples (range between 7 and 40 μg/kg) and 18% of the wet samples (range between 45 and 115 μg/kg). | (7) |
| Austria (2007) | 76 dry dog foods | DON, ZEA, fumonisins, OTA, and aflatoxins | 83% of the samples were positive for DON (mean of 409 μg/kg, max of 1390 μg/kg); 47% of the samples were positive for ZEA (mean of 80 μg/kg, max of 298 μg/kg); 42% of the samples were positive for fumonisins (mean of 178 μg/kg, max of 568 μg/kg); OTA was less frequently found (5% of the positive samples); aflatoxins were not detected. | (8) |
| Brazil (2010/2011) | 100 dry dog foods | ZEA, fumonisins, and aflatoxins | 68% of the samples were positive for fumonisins (max of FB1 + FB2, 380 μg/kg); 95% of the samples were positive for ZEA (max 442.2 μg/kg); 68% of the samples were positive for aflatoxins (max of 3.88 μg/kg). | (9) |
| Italy (2011) | 41 dry dog foods (32 complete and 9 complementary) | FB1 and FB2 | FB1 and FB2 were quantified in 63.4 and 56.1% of the samples, respectively. The range of FB1 + FB2 was between 150 and 8800 μg/kg. Two samples (one complete and one complementary dog food), containing 5190 and 8800 μg/kg of FB1 + FB2, respectively, exceeded the European guidance value (5000 μg/kg). | (10) |
| South Africa (2011) | 60 dog foods | ZEA, fumonisins, aflatoxins, and OTA | 87% of the samples were positive for aflatoxins (mainly AFB1 and AFB2): mean of 248 μg/kg, range between 1.2 and 353 μg/kg; most of the samples (75%) contained levels above the regulatory limits. Fumonisins were detected in 98% of the samples: mean of 1556 μg/kg, range between 5.2 and 4654 μg/kg. OTA was detected in 68% of the samples (mean of 13.7 μg/kg, range between 0.5 and 53.6 μg/kg). ZEA was detected in 96% of the samples (mean value of 354 μg/kg, range between 2.5 and 2351 μg/kg). | (11) |
| Poland (2014) | 25 dry dog foods and 24 dry cat foods | DON, ZEA, fumonisins, aflatoxins, OTA, T-2, and HT-2 | All the samples were positive for DON and ZEA (max of 436 and 123 μg/kg, respectively); T-2 was detected in 88% and HT-2 in 84% of the samples (max of 13.3 and 19.6 μg/kg, respectively); 29% of the samples were positive for fumonisins (max of 108 μg/kg); 45% of the samples were positive for OTA (max of 3 μg/kg); AFB1 was identified at LOD level (0.05 μg/kg) in 8% of the samples | (12) |
| Egypt (2014) | 20 pet food (5 wet dog foods, 5 wet cat foods, 5 dry dog foods, 5 dry cat foods) | ZEA, total aflatoxins, AFB1, and OTA | 15% of the samples were positive for AFB1 (max 18.4 μg/kg); OTA was detected in most of the samples (max 6.65 μg/kg); ZEA was measured in 20% of the samples at levels between 148 and 1170 μg/kg. | (13) |
| Italy (2015) | 48 dry dog foods | DON, ZEA, fumonisins, OTA, and aflatoxins | DON, fumonisins, and OTA were the most common mycotoxins (100, 88, and 81% of the positive samples, respectively); max values were 281, 1746 (FB1 + FB2), and 41.1 μg/kg, respectively. Fumonisin and OTA contamination was higher in standard than in premium dog foods. No sample contained quantifiable amounts of AFB1 and AFG1 (<LOQ, 0.05 μg/kg). AFB2 or AFG2 was measured in 12% of the samples (max of 15.8 μg/kg); 25% of the samples contained ZEA at quantifiable levels (max value of 42.4 μg/kg). | (14) |
| South Africa (2017) | 20 dry dog foods | ZEA, fumonisins, aflatoxins, and OTA | All the samples were positive for fumonisins (levels of FB1 or FB2 above 20 μg/kg; standard dog foods were more contaminated than premium ones); OTA and ZEA were detected in most of the samples at very low concentrations; aflatoxins were identified in all the samples with relatively high concentrations of AFB1 (5 standard and 5 premium dog foods contained AFB1 at levels above 10 μg/kg) | (15) |
| China (2017) | 32 dry pet foods | DON, ZEA, FB1, AFB1, AFG1, OTA and T-2, citrin, and beauvericin | Only one sample was free of contamination. All the other samples (96.9%) contained at least three mycotoxins. DON, ZEA, AFB1, FB1, citrin, and beauvericin displayed a relatively high occurrence (78.1, 62.5, 87.5, 93.8, 68.8, and 96.9%, respectively). AFB1 exceeded the European regulatory limit in all the positive samples (range, 30.3–242.7 μg/kg). T-2 was found in only one sample (15.4 μg/kg) and OTA in two samples (15.1 and 17.3 μg/kg). | (16) |
| Poland (2019) | 42 therapeutic foods (17 samples for cats and 25 samples for dogs) | DON, ZEA, FB1, and nivalenol | ZEA was detected in 69% (range, 1.22–51.7 μg/kg), DON in 52% (24.87–2451 μg/kg), FB1 in 33% (4.89–80.13 μg/kg), and nivalenol in 26% (17.43–200 μg/kg) of the samples. The highest level of mycotoxins was quantified in samples aimed to nutritionally support allergies (ZEA), renal diseases (FB1), and obesity (DON and nivalenol). | (17) |
ZEA, zearalenone; DON, deoxynivalenol; OTA, ochratoxin A; FB1, fumonisin B1; FB2, fumonisin B2; AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; HT-2, HT-2 toxin; T-2, T-2 toxin; LOQ, limit of quantification.
Awareness of the worldwide occurrence of mycotoxins in food and feedstuffs, the consequent risks to human and animal health, and the detrimental effects on global trade have led in recent decades to the adoption of regulatory limits for the most important mycotoxins found in food commodities18 and animal feed:19 aflatoxins, fumonisins, ochratoxins (OTA), zearalenone (ZEA), and trichothecenes (in particular, deoxynivalenol (DON) and T-2 toxin (T-2)). Consequently, these particular mycotoxins are considered the most relevant from both a safety and economic perspective.
In the European Union (EU), legislation concerning mycotoxin contamination in animal feeds (mostly for livestock and only in regard to fumonisins for pets) has been promulgated in the past.20,21
More recently, following some scientific opinions provided by the European Food Safety Authority (EFSA),22−24 specific “guidance values” recommended for DON, ZEA, OTA, T-2, and HT-2 also referred to the compound feed intended for dogs or cats have been introduced.25,26
Beyond the detrimental consequences on economic performance and reputation in the pet food industry, mycotoxin contamination poses severe health risks to pet animals although current knowledge on the toxicological effects in these species is limited.2 Moreover, modern analytical methods based on liquid chromatography tandem mass spectrometry (LC–MS/MS), allowing simultaneous determination of different molecules, have recently highlighted the problem of mycotoxin co-occurrence also in pet food products,9,14,16 as described for other animal feeds.27
The aim of the present study was to investigate the occurrence of the most important mycotoxins that are currently covered by EU legislation in the complete dry pet food intended for cats that is commercially available in Italy.
Results
Chemical analysis of the samples identified similar mean moisture and starch contents in the two cat food categories evaluated. In fact, excluding grain-free products, moisture contents were 46 ± 11 and 64 ± 14 g/kg, while starch contents were 259 ± 54 and 269 ± 35 g/kg (on a dry matter basis) in the premium and standard products, respectively. In cat foods not containing cereals, moisture was 50 ± 13 g/kg, and starch content was 186 ± 45 g/kg.
Positivity for and concentrations of the different mycotoxins in the two cat food categories are reported in Tables 3 and 4, respectively.
Table 3. Positivity for Mycotoxins of Commercial Dry Cat Fooda.
| number
of positive samples |
||||||
|---|---|---|---|---|---|---|
| LODb < mycotoxin < LOQc |
mycotoxin ≥ LOQc |
|||||
| mycotoxin | standard (n = 30) | premium (n = 34) | total (n = 64) | standard (n = 30) | premium (n = 34) | total (n = 64) |
| ZEA | 13 (43%) | 8 (24%) | 21 (33%) | 12 (40%) | 15 (44%) | 27 (42%) |
| DON | 0 | 7 (21%) | 7 (11%) | 30 (100%) | 21 (62%) | 51 (80%) |
| AFB1 | 5 (17%) | 5 (15%) | 10 (16%) | 7 (23%) | 1 (3%) | 8 (13%) |
| AFB2 | 0 | 0 | 0 | 0 | 0 | 0 |
| AFG1 | 0 | 0 | 0 | 0 | 0 | 0 |
| AFG2 | 0 | 0 | 0 | 0 | 0 | 0 |
| FB1 | 1 (3%) | 1 (3%) | 2 (3%) | 29 (97%) | 32 (94%) | 61 (95%) |
| FB2 | 1 (3%) | 2 (6%) | 3 (5%) | 29 (97%) | 31 (91%) | 60 (94%) |
| fumonisinsd | 1 (3%) | 1 (3%) | 2 (3%) | 29 (97%) | 31 (91%) | 60 (94%) |
| OTA | 5 (17%) | 0 | 5 (8%) | 2 (7%) | 0 | 2 (3%) |
| T-2 | 7 (23%) | 6 (18%) | 13 (20%) | 2 (7%) | 3 (9%) | 5 (8%) |
| HT-2 | 3 (10%) | 4 (12%) | 7 (11%) | 0 | 0 | 0 |
| T-2/HT-2e | 1 (3%) | 1 (3%) | 2 (3%) | 0 | 0 | 0 |
ZEA, zearalenone; DON, deoxynivalenol; AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; FB1, fumonisin B1; FB2, fumonisin B2; OTA, ochratoxin A; T-2, T-2 toxin; HT-2, HT-2 toxin.
LOD: limit of detection (FB1, FB2, AFB1, AFB2, AFG1, AFG2 and DON: 1 μg/kg; ZEA and OTA: 2 μg/kg; T-2 and HT-2: 5 μg/kg).
LOQ: limit of quantification (FB1, FB2, AFB1, AFB2, AFG1, AFG2 and DON: 3 μg/kg; ZEA and OTA: 5 μg/kg; T-2: 10 μg/kg; HT-2: 20 μg/kg).
Fumonisins: positivity for both fumonisins (FB1 and FB2).
T-2/HT-2: positivity for both toxins (T-2 and HT-2).
Table 4. Concentrations of Mycotoxins (μg/Kg) in Commercial Dry Cat Fooda.
| standard
cat foods (n = 30) |
premium cat foods (n = 34) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| mycotoxin | mean ± SDb | medianc | mind | maxe | mean ± SDb | medianc | mind | maxe | European guidance values (μg/kg)26 |
| ZEA | 8.89f ± 8.36 | 5.0 | LOD | 34.1 | 20.8f ± 31.3 | 5.0 | LOD | 112 | 100g |
| 200h | |||||||||
| DON | 209f ± 351 | 69.2 | 3.0 | 1588 | 77.7f ± 117 | 28.1 | LOD | 423 | 5000i |
| FB1 | 774 ± 853 | 441 | LOQ | 3277 | 648 ± 929 | 138 | LOD | 3464 | |
| FB2 | 438 ± 558 | 209 | LOQ | 2257 | 724 ± 1180 | 112 | LOD | 4837 | |
| FB1 + FB2 | 1212 ± 1326 | 630 | LOQ + LOQ | 4258 | 1372 ± 2072 | 269 | LOD + LOD | 7933 | 5000j |
ZEA, zearalenone; DON, deoxynivalenol; FB1, fumonisin B1; FB2, fumonisin B2; LOD, limit of detection (DON, FB1, and FB2: 1 μg/kg; ZEA: 2 μg/kg); LOQ, limit of quantification (DON, FB1 and FB2: 3 μg/kg; ZEA: 5 μg/kg). The values for aflatoxins are not reported since AFB2, AFG1 and AFG2 were not detected and AFB1, OTA, T-2 and HT-2 levels were lower than their corresponding limit of quantification in 87%, 97%, 92% and 100% of the samples, respectively.
Arithmetic mean ± standard deviation.
Median of all samples.
Minimum value.
Maximum quantified value.
Means within a row differ (P < 0.05).
Guidance value relative to a compound feed for puppies, kittens and dogs and cats for reproduction.
Guidance value relative to a compound feed for adult dogs and cats other than for reproduction.
Guidance value relative to a compound feed for cats.
Guidance value relative to a compound feed for pets.
ZEA was identified in 75% of the samples. Higher concentrations of this mycotoxin than those of the corresponding LOQ (5 μg/kg) were found in 42% of the samples. Standard cat foods were less contaminated than premium ones (8.89 vs 20.8 μg/kg, respectively; P < 0.05). Furthermore, in this last category, three dietetic samples (one for obesity management and two for renal diseases), containing 112, 101, and 104 μg of ZEA/kg, exceeded the reference guidance value recently established for cats used for reproduction and for kittens (100 μg/kg) (Figure 1). On the other hand, these three last samples respected the limit referred to compound feed intended for adult cats other than for reproduction (200 μg/kg).26
Figure 1.
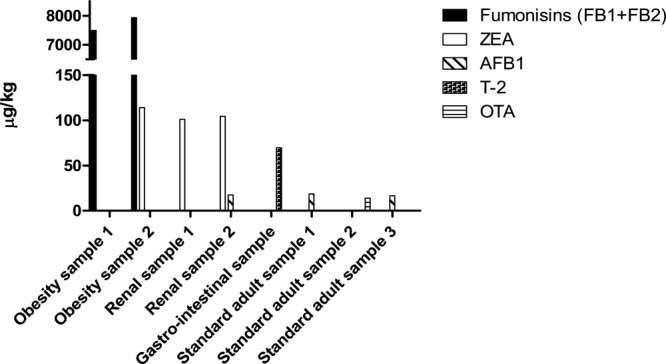
Samples of commercial dry cat food that did not comply with the current EU legislation concerning mycotoxin contamination.
DON was quantified in 62% of the premium and 100% of the standard cat foods (at concentrations ≥ the corresponding LOQ, 3 μg/kg), with higher values in this last price category (77.7 vs 209 μg/kg in premium and standard samples, respectively; P < 0.05). All of the positive samples resulted in compliance with the corresponding European guidance value recommended for cat food (5000 μg/kg).26
Concerning aflatoxins, analyses revealed a general low occurrence among the cat foods evaluated. In particular, no sample contained AFB2, AFG1, and AFG2 at levels above the corresponding LOD (1 μg/kg) (Table 3). AFB1 was identified in trace amounts in 16% of the samples (values > the corresponding LOD, 1 μg/kg, but < the corresponding LOQ, 3 μg/kg) and quantified in eight samples (one premium and seven standard cat foods) at concentrations between 3 and 18.4 μg/kg. In particular, three of these last positive samples (one premium sample for renal diseases and two standard samples for adult cats), containing values of 17.4, 18.4, and 16.5 μg of AFB1/kg, exceeded the regulatory maximum content established for compound feeds for animals other than for livestock (10 μg/kg)20 (Figure 1).
Fumonisins were the most common mycotoxins. With only one exception (represented by a grain-free product), all of the samples presented at least one of the two types of fumonisins evaluated (FB1 or FB2) at concentrations above the corresponding LOD (1 μg/kg). In particular, FB1 and/or FB2 were quantified in 95% of the samples (their concentration was ≥ the LOQ, 3 μg/kg), with no differences between standard and premium samples. The average levels of total fumonisins (FB1 + FB2) were relatively high although a wide range of contaminations was found (overall mean concentration of 1297 μg/kg and median concentration of 376 μg/kg). Furthermore, two dietetic products formulated for obesity management (containing 7494 and 7933 μg of total fumonisins/kg, respectively) exceeded the corresponding guidance value for pets (5000 μg/kg)26 (Figure 1).
OTA was identified in seven standard samples among which only two (both intended for adult cats) contained quantifiable amounts of this mycotoxin (at concentrations of 5.1 and 14 μg/kg, respectively). According to the current European guidance value for compound feeds for dogs and cats (10 μg/kg),26 one sample was illegal (Figure 1).
Finally, T-2 and HT-2 were detected in trace amounts (when the concentration was between the corresponding LOD (5 μg/kg for both toxins) and LOQ (10 μg/kg for T-2 and 20 μg/kg for HT-2)) in 28 and 11% of the total samples, respectively. Only T-2 was quantified in five samples (two standard and three premium), with mean and maximum concentrations of 35.6 and 69.6 μg/kg, respectively. This last value, found in a dietetic product intended for cats affected by gastrointestinal diseases, was higher than the current guidance value established for T-2 + HT-2 regarding compound feeds for cats (corresponding to 50 μg/kg)26 (Figure 1).
In regard to grain-free products, one sample did not contain detectable levels of any mycotoxin, while in the other four samples, only fumonisins were quantified (at concentrations ≥ the corresponding LOQ, 3 μg/kg) in a range between 11.1 and 125 μg of FB1 + FB2/kg.
The present study showed that 80% of the cat foods evaluated (51 of 64 samples) contained quantifiable concentrations of at least two types of mycotoxins. In particular, 28% of the cat foods (11 standard and 7 premium samples) were contaminated by two different mycotoxins (among which 89% contained DON + fumonisins), 42% (14 standard and 13 premium samples) by three (among which 82% contained ZEA + DON + fumonisins), 8% (3 standard and 2 premium samples) by four, and 2% (1 standard sample) by five mycotoxins (ZEA + DON + fumonisins + OTA + AFB1) (Table 5). The simultaneous quantification of at least DON and fumonisins (FB1 and/or FB2) was the most frequent: 77% of the samples evaluated (29 standard and 20 premium) revealed measurable concentrations of these two Fusarium mycotoxins.
Table 5. Mycotoxins Co-occurrence in Commercial Dry Cat Food.
| number of mycotoxins | standard cat foods (n = 30) | premium cat foods (n = 34) |
|---|---|---|
| 1 | 1 | 11 |
| 2 | 11 | 7 |
| 3 | 14 | 13 |
| 4 | 3 | 2 |
| 5 | 1 | 0 |
In regard to the correlation study between starch content (on a dry matter basis) and mycotoxin concentration, only total fumonisins, ZEA, and DON were considered because of their widespread occurrence in over half of the samples. Nevertheless, the Pearson coefficient test failed to show a linear relationship for all three mycotoxins evaluated (r = 0.058, r = 0.002, and r = 0.195 in regard to the correlation between starch content and total fumonisins, ZEA, and DON, respectively).
Discussion
The present monitoring compared, for the first time, the occurrence of the most important mycotoxins in cat food with the guidance values for pets that were recently introduced by EU legislation.26 Based on our results, a relatively high number of samples (five premium dietetic samples and three standard samples for adult cats) exceeded the European regulatory limits established for some mycotoxins (Figure 1). Astonishingly, two dietetic samples even exceeded the guidance values for two mycotoxins simultaneously. Nevertheless, it must be emphasized that cat food sampling preceded the adoption of the current European Recommendation concerning DON, ZEA, and OTA contamination in pet food.26
The lack of compliance with European rules appears particularly critical since this outcome mainly concerned dietary products aimed at nutritional support for cats affected by common pathological conditions such as obesity and renal and gastrointestinal disorders. In fact, such animals might present a suboptimal immunological status, and prolonged consumption of mycotoxin-contaminated pet food could be detrimental to their health.2 Dietetic pet food is poorly investigated in this regard as most of the published studies on mycotoxin contamination have been carried out on pet food formulated for healthy dogs and cats (Table 1).
The results from the present monitoring partially disagreed with those highlighted by our previous similar study on Italian dry dog food.14 In the previous work, interesting evidence such as a widespread multicontamination in most of the samples and differences in the concentration of some mycotoxins (fumonisins and OTA) based on the price category was obtained.14 Conversely, the present investigation on cat food showed that a high price does not necessarily guarantee a mycotoxin-free product.
The mean starch content in standard and premium categories was similar given the high variability of this complex carbohydrate among the cat foods evaluated. Furthermore, the correlation study between the starch content and the concentration of the most prevalent mycotoxins (total fumonisins, ZEA, and DON) failed to demonstrate a linear relationship. On the other hand, the five grain-free samples evaluated, containing starch from legumes and potatoes, showed an overall low contamination. In most of the other samples, cereals and cereal byproducts represented the first or second ingredient (or ingredient category) listed on the label (corn in primis when the type of cereals was specified). Anyway, information on the exact amount of the different ingredients was not provided. Consequently, it is only possible to speculate on the relationship between mycotoxin contamination and the relatively large quantity of cereals used to produce dry pet food.
Mold growth and mycotoxin production strongly depend on several factors along the cereal supply chain, such as weather and storage conditions (i.e., temperature and humidity), microbial and insect damages, and mechanical injuries.28,29 This situation partially explains the difficulties in the prediction and control of the problem as well as the heterogeneous levels of contamination emerging from the comparison of the results described by the different studies conducted on pet food published in recent years (Table 1).
In the present study, in accordance with our previous investigation on dry dog food14 and other similar investigations,8,12,16,17 a widespread occurrence of the most important Fusarium mycotoxins (DON, fumonisins, and, to a lesser extent, ZEA) was observed.
These toxic molecules are commonly found in cereals (corn, in primis) and in compound feeds (typically containing different grains).30 Furthermore, DON, ZEA, and in particular fumonisins are particularly heat-stable, and only temperatures above 150 °C have demonstrated a significant reduction of their toxicity.31 Consequently, these toxins are a matter of great concern for the pet food industry (particularly fumonisins) since extrusion typically provides lower temperatures.
According to previous investigations, the situation concerning aflatoxin occurrence in pet food is widely heterogeneous (Table 1). Surprisingly, our study revealed a non-negligible presence of AFB1 in the cat foods evaluated, with three samples exceeding the European regulatory limit established for animal feeds including pet food.20
Several outbreaks of canine aflatoxicosis are reported in the literature.32−34 Conversely, to our knowledge, no case involving cats has been described. Anyway, aflatoxins represent a common cause of pet food recalls.35 Given the absence of critical control points for aflatoxins along the manufacturing process (due to their stability to the extrusion process), routine screening for these contaminants in cereal ingredients (especially corn and corn byproducts) is necessary.36 Furthermore, even if lower levels of AFB1 than 20 μg/kg have previously shown to be insufficient for causing noticeable clinical signs in companion animals, the chronic exposure should not be ignored.2 Surely, the situation concerning AFB1 as indicated in the present study represents an important warning sign and suggests for rigorous screening tests on incoming raw ingredients.
OTA contamination was very limited even if one of the two positive standard samples contained a level above the current European guidance value recently established for companion animals.26 OTA represents a nephrotoxic compound usually detected not only in several agricultural commodities such as corn, wheat, and barley but also in animal-derived products (in particular, meat and milk) given its high fat solubility and the consequent accumulation in animal tissues, particularly in swines.18 For this reason, OTA could be detected both in dry and wet pet food since the latter product typically contains large amounts of animal-derived ingredients such as muscles and offal.37 Different levels of contamination have been reported by several studies carried out both in Europe and in African countries (Table 1). Interestingly, a recent retrospective study by Meucci et al.38 evaluating the blood concentration of OTA in healthy and nephropathic dogs showed a higher incidence of OTA positivity in this last group. In this regard, other authors had previously pointed out this mycotoxin etiology in canine renal failure syndrome.39 Certainly, these results contribute to supporting the need for rigorous monitoring by the pet food industry regarding OTA.
According to toxicological studies, T-2 represents the most toxic compound among trichothecenes. Given the rapid in vivo conversion of T-2 to HT-2, in vivo toxicity of T-2 is recognized to include that of HT-2.31 According to a recent EFSA opinion, cats are extremely sensitive to this mycotoxin category,40 and probably for this reason, some years ago, the European legislation was implemented with a guidance value for T-2 + HT-2 only referred to this last species.25 Recently, some studies have reported a wide occurrence of T-2 and HT-2 in cereals and animal feed.28,29 In regard to pet food, only a few studies have evaluated the occurrence of these mycotoxins, and they reported conflicting results.12,16 In the present monitoring, probably because of the corresponding relatively high LODs and LOQs, few positive samples were identified among which a dietetic product did not comply with the current legislation.26
The present study confirmed the noteworthy problem of the co-occurrence of different mycotoxins, previously reported by other surveys on pet food (Table 1) as well as on feed raw materials and feedingstuffs.28,29 Certainly, the detection of one single mycotoxin in a feed sample represents the exception, and it is well-known that both humans and animals are usually exposed to several mycotoxins (mainly at low levels) at the same time.27 Nevertheless, worldwide regulations and past toxicological studies usually refer to individual mycotoxins. Recently, an increasing number of investigations evaluating the toxicological effects of different mycotoxin combinations have been published, both in vivo41 and in vitro conditions,27 even if the experimental doses were usually higher than those found in “natural” contaminations and often exceeding the international regulatory limits.42 In the present study, one of the most common co-occurrence was ZEA + DON + fumonisins, with these mycotoxins being the most frequent in the samples evaluated. In this regard, several Fusarium strains producing ZEA also produce trichothecenes such as DON, and in general, a frequent co-occurrence of ZEA with other Fusarium toxins has been described in cereals, especially in corn.43 Furthermore, a synergy among these Fusarium toxins has been recognized42 even though additive, synergistic, and antagonistic effects have been described under in vitro conditions, often depending on the dose of the toxins.27
Pets are traditionally fed with the same type of diet for long periods of their life. Therefore, the scientific community should be aware of the potential chronic exposure of dogs and cats to relatively low levels of different mycotoxins and the consequential detrimental risks to their health.
The results from the present study showed that mycotoxin contamination represents a critical point for pet food safety. Certainly, given the high stability of mycotoxins through the cooking process used to produce dry pet food,5 scrupulous monitoring of incoming ingredients undoubtedly represents the most effective strategy to prevent mycotoxin contamination.
Materials and Methods
Sampling
In order to evaluate a representative selection of the different types of cat food available on the Italian market, 64 complete commercial dry products of different brands were collected from stores in the province of Bologna (Italy) from June to September 2015. Specifically, they included 30 standard cat foods (5 for kittens, 5 for senior, and 20 for adult cats) and 34 premium cat foods (5 for kittens, 5 for senior cats, 5 grain-free, and 19 dietetic products including 6 for obesity management, 7 for renal diseases, and 6 for gastrointestinal disorders). Standard samples consisted of products ranging in price from 0.80 to 4.00 €/kg that were sold by discount and mass-market retailers. Conversely, premium samples consisted of more costly diets ranging from 4.00 to 15.00 €/kg that were purchased in specialized pet stores. The size of the packages ranged from 250 g to 2 kg. In grain-free samples, the main sources of starch declared on the label were legumes (lentils, peas, chickpeas, and beans) and potatoes. The main cereals and cereal by-products listed on the package of the other cat foods were corn, wheat, rice, corn gluten, wheat gluten, corn starch, barley, spelt, and yellow millet. In particular, corn and corn byproducts (corn starch and corn gluten) represented the most common vegetable ingredients placed in the first positions of the ingredient list declared on cat food labels.
All of the cat foods were stored in a cool, dry place inside their original hermetically sealed package until chemical analysis. With the aim of obtaining a representative sample of each cat food, approximately half of the content of each product was randomly taken from the original package (from four different sites), finely ground, stored at −20 °C, and analyzed within its shelf life.
Nutrient Analyses
The cat food samples were chemically analyzed to determine the moisture and starch content according to the official methods of the Association of Official Analytical Chemists (method 950.46 for moisture and method 996.11 for starch).44
Determination of Mycotoxin Concentration
The most important mycotoxins currently regulated in the EU regarding pet food have been evaluated in cat food samples.
Chemicals and Reagents
Aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), fumonisin B1 (FB1), fumonisin B2 (FB2), deoxynivalenol (DON), zearalenone (ZEA), ochratoxin A (OTA), T-2 toxin (T-2), and HT-2 toxin (HT-2) standards were purchased from Sigma-Aldrich (Steinheim, Germany).
U-[13C17]-aflatoxin B1 (13C AFB1), U-[13C34]-fumonisin B1 (13C FB1), U-[13C15]-deoxynivalenol (13C DON), U-[13C18]-zearalenone (13C ZEA), U-[13C20]-ochratoxin A (13C OTA), and U-[13C24]-T-2 toxin (13C T-2) were obtained from Romer Lab Inc. – Biopure (Tulln, Austria).
Methanol, formic acid, and ammonium acetate, used as mobile phases, were of LC–MS grade and were purchased from Riedel-de Haën (Seelze, Germany). Acetonitrile and acetic acid, used in the extraction procedures, were purchased from Merck (Darmstadt, Germany).
Reverse osmosis and ultrapure water, respectively used as an extraction solvent and the mobile phase, were produced using a human-powered apparatus from Human Co. (Seoul, South Korea).
Sample Preparation
The method set up by Zhang et al.45 was slightly modified and used in this work. A ground sample (500 mg) was weighed in a beaker, fortified with labeled standards, and extracted with 5 mL of acetonitrile/water (50:50). The sample was shaken for 15 min, and 500 μL of the supernatant was collected and transferred in an Amicon ultra centrifugal filter (0.5 mL, 3 K; Millipore, Carrigtwohill, Ireland). After a centrifugation step, 14,000 rpm for 30 min at 20 °C, the sample, once filtered, was analyzed using ultra-performance liquid chromatography in combination with tandem mass spectrometry (UPLC–MS/MS). The samples with a mycotoxin concentration greater than 1,000 μg/kg were diluted to obtain a correct analyte concentration in the curve range.
UPLC–MS/MS Equipment and Conditions
Analyses were conducted with a UPLC–MS/MS system, composed of a Waters Acquity UPLC binary pump, equipped with a Waters Acquity BEH C18 reversed-phase column (50 × 2.1 mm, 1.7 μm) coupled to a VanGuard guard column with identical packaging (Waters, Milford, MA, USA).
Two different mobile phases were optimized, one for DON and the other for all of the other analytes.
For all the analytes, water containing 0.1% formic acid and methanol containing 0.1% formic acid were employed as mobile phases under programmed conditions at a flow rate of 0.42 mL/min. Analyses were carried out over 16 min using a previous method developed by Jackson et al.46
Some changes about LC and MS conditions for the DON analysis were necessary to improve the sensitivity of the determination. For DON, the mobile phase consisted of 5 mM ammonium acetate (A) and methanol (B), and the flow rate was 0.3 mL/min. The following gradient program, time (%A–%B), was applied: 0 min (99–1), 1 min (95–5), 2 min (25–75), 2.1 min (1–99), 4 min (99–1), 6 min (99–1). For both methods, the column heater temperature was set at 40 °C, and the volume injection was 10 μL.
The mass spectrometer was a Quattro Premier XE, a triple quadrupole instrument equipped with an ESCI multimode ionization source (Waters, Milford, MA, USA).
The mass spectrometer was operated in the positive electrospray ionization mode (ESI+) using multiple reaction monitoring (MRM). The capillary voltage was set at 3.5 kV for all analytes and 3.0 kV for DON; the MRM transitions, cone voltages, and collision energies are shown in Table 2.
Table 2. Mass Spectrometry Parameters for the Selected Mycotoxinsa.
| compound | precursor ion (m/z) | product ionsb (m/z) | cone voltage (kV) | collision energy (eV) |
|---|---|---|---|---|
| DON | 297.1 | 249.20 | 18 | 10 |
| 231.2 | 18 | 13 | ||
| 13C DON | 312.2 | 263.4 | 18 | 12 |
| 216.4 | 18 | 16 | ||
| AFB1 | 312.20 | 285.30 | 45 | 22 |
| 241.30 | 45 | 36 | ||
| AFB2 | 315.05 | 287.10 | 45 | 33 |
| 259.10 | 45 | 38 | ||
| AFG1 | 329.10 | 243.30 | 45 | 26 |
| 283.30 | 45 | 24 | ||
| AFG2 | 331.10 | 313.25 | 46 | 33 |
| 313.25 | 46 | 39 | ||
| 13C AFB1 | 330.3 | 301.2 | 45 | 22 |
| 255.4 | 45 | 38 | ||
| FB1 | 722.20 | 334.50 | 52 | 45 |
| 352.50 | 52 | 43 | ||
| FB2 | 706.30 | 336.50 | 50 | 38 |
| 318.50 | 50 | 40 | ||
| 13C FB1 | 756.3 | 356.5 | 52 | 45 |
| 374.6 | 52 | 40 | ||
| OTA | 404.15 | 239.20 | 25 | 25 |
| 221.20 | 25 | 37 | ||
| 13C OTA | 424.1 | 232.4 | 52 | 40 |
| 356.6 | 52 | 45 | ||
| HT-2 | 447.25 | 345.3 | 36 | 20 |
| 345.3 | 36 | 22 | ||
| T-2 | 489.2 | 245.1 | 36 | 27 |
| 387.0 | 36 | 22 | ||
| 13C T-2 | 513.3 | 406.2 | 40 | 23 |
| 260.3 | 40 | 28 | ||
| ZEA | 319.3 | 283.20 | 20 | 12 |
| 185.20 | 20 | 30 | ||
| 13C ZEA | 377.3 | 301.3 | 17 | 12 |
| 199.4 | 17 | 18 |
DON, deoxynivalenol; AFB1, aflatoxin B1; AFB2, aflatoxin B2; AFG1, aflatoxin G1; AFG2, aflatoxin G2; FB1, fumonisin B1; FB2, fumonisin B2; HT-2, HT-2 toxin; OTA, ochratoxin A; T-2, T-2 toxin; ZEA, zearalenone.
Quantification ions are reported in bold.
Data acquisition and processing was performed using Mass Lynx 4.1 Software (Waters Corporation, Milford, USA).
Method Validation
The proposed method was validated in-house according to the European Commission Decision 2002/657/EC47 and Commission Regulation 2006/401/EC.48 The following parameters were evaluated: specificity, linearity, trueness, precision, limits of quantification (LOQs), and limits of detection (LODs).
One grain-free sample exhibiting no measurable concentrations of the analytes of interest was used for the validation of the analytical method.
Fortified samples were obtained adding different volumes of mycotoxin standard solutions to blank samples before the extraction steps. Spiked samples were left at least for 2 h at room temperature to allow for the evaporation of the solvents and the equilibration between the analytes and matrix.
The matrix-matched calibration curve (R2 > 0.98) occurred over the range of 0 to 1000 μg/kg using seven calibration standards (0, 5, 20, 50, 100, 200, and 1000 μg/kg).
The recovery and precision of the methods were evaluated at three quality control levels (20, 50, and 100 μg/kg).
The injection of four replicates of three quality control levels demonstrated satisfying precision, with maximum relative standard deviations to the mean of 12.2% as well as good trueness values expressed as the relative bias between the mean value measured and the spiked concentration, ranging from −12.1 to 4.5%. The trueness and precision (in terms of repeatability) values obtained fulfill the performance criteria established by the abovementioned regulations.47,48 Specificity was assessed excluding the presence of potential interferences around mycotoxin retention times in the chromatograms of non-contaminated samples. Moreover, the blank sample used for the method validation was used to evaluate the matrix effects, as ion suppression or enhancement; in particular, it was injected while standard solutions (1 μg/mL) were directly infused with a flow of 10 μg/min through a T-connection device into the LC eluate.49 The ion currents were stable, and no interference was recorded at the specific retention times of the considered mycotoxins.
Limits of quantification of the method, defined as the concentrations providing a chromatographic signal with a signal-to-noise ratio equal to 10, were 3 μg/kg for FB1, FB2, AFB1, AFB2, AFG1, AFG2, and DON, 5 μg/kg for ZEA and OTA, 10 μg/kg for T-2, and 20 μg/kg for HT-2. Limits of detection of the method, defined as the concentrations providing a chromatographic signal with a signal-to-noise ratio equal to 3 for the qualification transition, were 1 μg/kg for FB1, FB2, AFB1, AFB2, AFG1, AFG2, and DON, 2 μg/kg for ZEA and OTA, and 5 μg/kg for T-2 and HT-2.
Standard curves and quality controls were run at the beginning and end of each analytical run day.
Statistical Analyses
The concentrations of the different mycotoxins in the two price categories of cat foods were statistically analyzed by using Student’s t test. For samples in which they were not detected or quantified, a specific mycotoxin was assigned according to the corresponding LOD or LOQ. The level of significance was set at P < 0.05. Furthermore, the correlations between the starch content (expressed on a dry matter basis) and the concentration of the different mycotoxins were analyzed using the Pearson correlation test. All of the statistical computations were performed with Statistica 10.0 (Stat Soft Italia, Italy).
Glossary
Abbreviations used
- AFB1
aflatoxin B1
- AFB2
aflatoxin B2
- AFG1
aflatoxin G1
- AFG2
aflatoxin G2
- DON
deoxynivalenol
- EFSA
European Food Safety Authority
- EU
European Union
- FB1
fumonisin B1
- FB2
fumonisin B2
- HT-2
HT-2 toxin
- LC–MS/MS
liquid chromatography coupled to mass spectrometry
- LOD
limit of detection
- LOQ
limit of quantification
- MRM
multiple reaction monitoring
- OTA
ochratoxin A
- T-2
T-2 toxin
- UPLC-MS/MS
ultra-performance liquid chromatography coupled to tandem mass spectrometry
- ZEA
zearalenone
Appendix
Guidelines of the European Commission
Guidance values recommended by the European Commission:26
-
1.
For total fumonisins (FB1 + FB2): 5000 μg/kg relative to compound feeds for pets.
-
2.
For ZEA: 100 μg/kg relative to compound feeds for puppies, kittens, and dogs and cats for reproduction.
-
3.
For T2 + HT2: 50 μg/kg relative to compound feeds for cats.
-
4.
For OTA: 10 μg/kg relative to compound feeds for dogs and cats.
Maximum content established by the European Commission for AFB1:20 10 μg/kg for complete feeds for animal species other than for livestock.
The authors declare no competing financial interest.
References
- White G. A.; Ward L.; Pink C.; Craigon J.; Millar K. M. ″Who’s been a good dog?″ - Owner perceptions and motivations for treat giving. Prev. Vet. Med. 2016, 132, 14–19. 10.1016/j.prevetmed.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Boermans H. J.; Leung M. C. Mycotoxins and the pet food industry: toxicological evidence and risk assessment. Int. J. Food Microbiol. 2007, 119, 95–102. 10.1016/j.ijfoodmicro.2007.07.063. [DOI] [PubMed] [Google Scholar]
- Sweeney M. J.; Dobson A. D. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–58. 10.1016/S0168-1605(98)00112-3. [DOI] [PubMed] [Google Scholar]
- Twomey L. N.; Pethick D. W.; Rowe J. B.; Choct M.; Pluske J. R.; Brown W.; Laviste M. C. The use of sorghum and corn as alternatives to rice in dog foods. J. Nutr. 2002, 132, 1704S–5S. 10.1093/jn/132.6.1704S. [DOI] [PubMed] [Google Scholar]
- Kaushik G. Effect of processing on mycotoxin content in grains. Crit. Rev. Food Sci. Nutr. 2013, 55, 1672–1683. 10.1080/10408398.2012.701254. [DOI] [PubMed] [Google Scholar]
- Zwierzchowski W.; Gajecki M.; Obremski K.; Zielonka L.; Baranowski M. The occurrence of zearalenone and its derivatives in standard and therapeutic feeds for companion animals. Pol. J. Vet. Sci. 2004, 7, 289–293. [PubMed] [Google Scholar]
- Songsermsakul P.; Razzazi-Fazeli E.; Böhm J.; Zentek J. Occurrence of deoxynivalenol (DON) and ochratoxin A (OTA) in dog foods. Mycotoxin Res. 2007, 23, 65–67. 10.1007/BF02946027. [DOI] [PubMed] [Google Scholar]
- Böhm J.; Koinig L.; Razzazi-Fazeli E.; Blajet-Kosicka A.; Twaruzek M.; Grajewski J.; Lang C. Survey and risk assessment of the mycotoxins deoxynivalenol, zearalenone, fumonisins, ochratoxin A and aflatoxins in commercial dry dog food. Mycotoxin Res. 2010, 26, 147–153. 10.1007/s12550-010-0049-4. [DOI] [PubMed] [Google Scholar]
- Bissoqui L. Y.; Frehse M. S.; Freire R. L.; Ono M. A.; Bordini J. G.; Hirozawa M. T.; de Oliveira A. J.; Ono E. Y. Exposure assessment of dogs to mycotoxins through consumption of dry feed. J. Sci. Food Agric. 2016, 96, 4135–4142. 10.1002/jsfa.7615. [DOI] [PubMed] [Google Scholar]
- Pagliuca G.; Lugoboni B.; Gazzotti T.; Cipollini I.; Zaghini G. Fumonisin B1 and B2 in dry dog food: preliminary study on commercial samples. World Mycotoxin J. 2011, 4, 439–446. 10.3920/WMJ2011.1309. [DOI] [Google Scholar]
- Mulunda M.; Ndou R. V.; Dzoma B.; Nyirenda M.; Bakunzi F. Canine aflatoxicosis outbreak in South Africa (2011): A possible multi-mycotoxins aetiology. J. S. Afr. Vet. Assoc. 2013, E1–E5. 10.4102/jsava.v84i1.133. [DOI] [PubMed] [Google Scholar]
- Błajet-Kosicka A.; Kosicki R.; Twarużek M.; Grajewski J. Determination of moulds and mycotoxins in dry dog and cat food using liquid chromatography with mass spectrometry and fluorescence detection. Food Addit. Contam., Part B 2014, 7, 302–308. 10.1080/19393210.2014.933269. [DOI] [PubMed] [Google Scholar]
- Abd-Elhakim Y. M.; El Sharkawy N. I.; Moustafa G. G. An investigation of selected chemical contaminants in commercial pet foods in Egypt. J. Vet. Diagn. Invest. 2016, 28, 70–75. 10.1177/1040638715624733. [DOI] [PubMed] [Google Scholar]
- Gazzotti T.; Biagi G.; Pagliuca G.; Pinna C.; Scardilli M.; Grandi M.; Zaghini G. Occurrence of mycotoxins in extruded commercial dog food. Anim. Feed Sci. Technol. 2015, 202, 81–89. 10.1016/j.anifeedsci.2015.02.004. [DOI] [Google Scholar]
- Singh S. D.; Chuturgoon A. A. A comparative analysis of mycotoxin contamination of supermarket and premium brand pelleted dog food in Durban, South Africa. J. S. Afr. Vet. Assoc. 2017, 88, e1–e6. 10.4102/jsava.v88i0.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M.; Li L.; Gu Z.; Yao M.; Xu D.; Fan W.; Yan L.; Song S. Mycotoxins in commercial dry pet food in China. Food Addit. Contam., Part B 2018, 11, 237–245. 10.1080/19393210.2018.1475425. [DOI] [PubMed] [Google Scholar]
- Witaszak N.; Stępień Ł.; Bocianowski J.; Waśkiewicz A. Fusarium Species and Mycotoxins Contaminating Veterinary Diets for Dogs and Cats. Microorganisms 2019, 7, 26. 10.3390/microorganisms7010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshannaq A.; Yu J.-H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. 10.3390/ijerph14060632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinotti L.; Ottoboni M.; Giromini C.; Dell’Orto V.; Cheli F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. 10.3390/toxins8020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commission Directive 2003/100/EC of 31 October 2003 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council on undesirable substances in animal feed. Off. J. Eur. Union 2003, L285, 33–37. [Google Scholar]
- Commission Recommendation 2006/576/EC of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to ochratoxin A (OTA) as undesirable substance in animal feed. EFSA J. 2004, 2, 1–36. 10.2903/j.efsa.2004.101. [DOI] [Google Scholar]
- Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 2, 1–42. 10.2903/j.efsa.2004.73. [DOI] [Google Scholar]
- Scientific Opinion on risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. 10.2903/j.efsa.2011.2481. [DOI] [Google Scholar]
- Commission Recommendation (EC) No 637/2013 of 4 November 2013 amending Recommendation (EC) No 576/2006 as regards T-2 and HT-2 toxin in compound feed for cats. Off. J. Eur. Union 2013, L294, 44. [Google Scholar]
- Commission Recommendation 2016/1319/EC of 29 July 2016 amending Commission Recommendation 2006/576/EC on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2016, L208, 58–60. [Google Scholar]
- Smith M.-C.; Madec S.; Coton E.; Hymery N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. 10.3390/toxins8040094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosicki R.; Błajet-Kosicka A.; Grajewski J.; Twarużek M. Multiannual mycotoxin survey in feed materials and feedingstuffs. Anim. Feed Sci. Technol. 2016, 215, 165–180. 10.1016/j.anifeedsci.2016.03.012. [DOI] [Google Scholar]
- Abdallah M. F.; Girgin G.; Baydar T.; Krska R.; Sulyok M. Occurrence of multiple mycotoxins and other fungal metabolites in animal feed and maize samples from Egypt using LC-MS/MS. J. Sci. Food Agric. 2017, 97, 4419–4428. 10.1002/jsfa.8293. [DOI] [PubMed] [Google Scholar]
- Placinta C. M.; D’Mello J. P. F.; MacDonald A. M. C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. 10.1016/S0377-8401(98)00278-8. [DOI] [Google Scholar]
- Marin S.; Ramos A. J.; Cano-Sancho G.; Sanchis V. Mycotoxins: occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. 10.1016/j.fct.2013.07.047. [DOI] [PubMed] [Google Scholar]
- Dereszynski D. M.; Center S. A.; Randolph J. F.; Brooks M. B.; Hadden A. G.; Palyada K. S.; McDonough S. P.; Messick J.; Stokol T.; Bischoff K. L.; Gluckman S.; Sanders S. Y. Clinical and clinicopathologic features of dogs that consumed foodborne hepatotoxic aflatoxins: 72 cases (2005-2006). J. Am. Vet. Med. Assoc. 2008, 232, 1329–1337. 10.2460/javma.232.9.1329. [DOI] [PubMed] [Google Scholar]
- Arnot L. F.; Duncan N. M.; Coetzer H.; Botha C. J. An outbreak of canine aflatoxicosis in Gauteng Province, South Africa. J. S. Afr. Vet. Assoc. 2012, 83, 01. [DOI] [PubMed] [Google Scholar]
- Wouters A. T. B.; Casagrande R. A.; Wouters F.; Watanabe T. T. N.; Boabaid F. M.; Cruz C. E. F.; Driemeier D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J. Vet. Diagn. Invest. 2013, 25, 282–287. 10.1177/1040638713477409. [DOI] [PubMed] [Google Scholar]
- Bischoff K.; Rumbeiha W. K. Pet food recalls and pet food contaminants in small animals. Vet. Clin. Small Anim. Pract. 2012, 42, 237–250. 10.1016/j.cvsm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- Carrión P. A.; Thompson L. J.. Pet Food. In Food Safety Management. A Practical Guide for the Food Industry; Motarjemi J.; Lelieveld H., Eds.; Academic Press: Whaltam, MA, 2014, pp. 379–396. [Google Scholar]
- Razzazi E.; Böhm J.; Grajewski J.; Szczepaniak K.; Kübber-Heiss A. J.; Iben C. H. Residues of ochratoxin A in pet foods, canine and feline kidneys. J. Anim. Physiol. Anim. Nutr. 2001, 85, 212–216. 10.1046/j.1439-0396.2001.00331.x. [DOI] [PubMed] [Google Scholar]
- Meucci V.; Luci G.; Vanni M.; Guidi G.; Perondi F.; Intorre L. Serum levels of ochratoxin A in dogs with chronic kidney disease (CKD): a retrospective study. J. Vet. Med. Sci. 2017, 79, 440–447. 10.1292/jvms.16-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W. I.; Do S. H.; Jeong D. H.; Chung J. Y.; Yang H. J.; Yuan D. W.; Hong I. H.; Park J. K.; Goo M. J.; Jeong K. S. Canine renal failure syndrome in three dogs. J. Vet. Sci. 2006, 7, 299–301. 10.4142/jvs.2006.7.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcella D.; Gergelova P.; Innocenti M. L.; Steinkellner H. Human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. 10.2903/j.efsa.2017.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier B.; Oswald I. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. 10.3920/WMJ2011.1281. [DOI] [Google Scholar]
- Alassane-Kpembi I.; Schatzmayr G.; Taranu I.; Marin D.; Puel O.; Oswald I. P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2016, 57, 3489–3507. 10.1080/10408398.2016.1140632. [DOI] [PubMed] [Google Scholar]
- Escrivá L.; Font G.; Manyes L. In vivo toxicity studies of fusarium mycotoxins in the last decade: a review. Food Chem. Toxicol. 2015, 78, 185–206. 10.1016/j.fct.2015.02.005. [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) . Official methods of analysis of the Association of Official Analytical Chemists. 17th rev. ed. AOAC: Washington, DC, USA, 2000. [Google Scholar]
- Zhang K.; Wong J. W.; Krynitsky A. J.; Trucksess M. W. Determining mycotoxins in baby foods and animal feeds using stable isotope dilution and liquid chromatography tandem mass spectrometry. J. Agric. Food Chem. 2014, 62, 8935–43. 10.1021/jf503943r. [DOI] [PubMed] [Google Scholar]
- Jackson L. C.; Kudupoje M. B.; Yiannikouris A. Simultaneous multiple mycotoxin quantification in feed samples using three isotopically labeled internal standards applied for isotopic dilution and data normalization through ultra-performance liquid chromatography/electrospray ionization tandem mass spe. Rapid Commun. Mass Spectrom. 2012, 26, 2697–713. 10.1002/rcm.6405. [DOI] [PubMed] [Google Scholar]
- Commission Decision 657/2002/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of the analytical methods and the interpretation of results. Off. J. Eur. Union 2002, L221, 8–34. [Google Scholar]
- Commission Regulation 401/2006/EC of 23 February 2006 laying down the methods oh sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, L70, 12–33. [Google Scholar]
- Antignac J.-P.; de Wasch K.; Monteau F.; de Brabander H.; Andre F.; le Bizec B. The ion suppression phenomenon in liquid chromatography-mass spectrometry and its consequences in the field of residue analysis. Anal. Chim. Acta 2005, 529, 129–136. 10.1016/j.aca.2004.08.055. [DOI] [Google Scholar]


