Key Points
Question
Does low-dose arginine vasopressin supplementation decrease the need for blood product transfusions in patients with trauma and hemorrhagic shock?
Findings
In this randomized clinical trial of 100 adult trauma patients in hemorrhagic shock, arginine vasopressin supplementation significantly decreased the volume of blood products given in the first 48 hours by a median of 1.4 L without increasing complications.
Meaning
Including low-dose arginine vasopressin supplementation when resuscitating trauma patients in hemorrhagic shock may safely decrease the need for blood products.
This randomized clinical trial assesses whether low-dose supplementation of arginine vasopressin during the resuscitation of hemorrhagic shock decreases the need for transfusions in patients with trauma.
Abstract
Importance
Current therapies for traumatic blood loss focus on hemorrhage control and blood volume replacement. Severe hemorrhagic shock, however, is associated with a state of arginine vasopressin (AVP) deficiency, and supplementation of this hormone may decrease the need for blood products in resuscitation.
Objective
To determine whether low-dose supplementation of AVP in patients with trauma (hereinafter referred to as trauma patients) and with hemorrhagic shock decreases their need for transfused blood products during resuscitation.
Design, Setting, and Participants
This randomized, double-blind placebo-controlled clinical trial included adult trauma patients (aged 18-65 years) who received at least 6 U of any blood product within 12 hours of injury at a single urban level 1 trauma center from May 1, 2013, through May 31, 2017. Exclusion criteria consisted of prehospital cardiopulmonary resuscitation, emergency department thoracotomy, corticosteroid use, chronic renal insufficiency, coronary artery disease, traumatic brain injury requiring any neurosurgical intervention, pregnancy, prisoner status, or AVP administration before enrollment. Data were analyzed from May 1, 2013, through May 31, 2017, using intention to treat and per protocol.
Interventions
After administration of an AVP bolus (4 U) or placebo, participants received AVP (≤0.04 U/min) or placebo for 48 hours to maintain a mean arterial blood pressure of at least 65 mm Hg.
Main Outcomes
The primary outcome was total volume of blood product transfused. Secondary end points included total volume of crystalloid transfused, vasopressor requirements, secondary complications, and 30-day mortality.
Results
One hundred patients underwent randomization (49 to the AVP group and 51 to the placebo group). Patients were primarily young (median age, 27 years [interquartile range {IQR}, 22-25 years]) and male (n = 93) with penetrating trauma (n = 79). Cohort characteristics before randomization were well balanced. At 48 hours, patients who received AVP required significantly less blood products (median, 1.4 [IQR, 0.5-2.6] vs 2.9 [IQR, 1.1-4.8] L; P = .01) but did not differ in requirements for crystalloids (median, 9.9 [IQR, 7.9-13.0] vs 11.0 [8.9-15.0] L; P = .22) or vasopressors (median, 400 [IQR, 0-5900] vs 1400 [IQR, 200-7600] equivalent units; P = .22). Although the groups had similar rates of mortality (6 of 49 [12%] vs 6 of 51 [12%]; P = .94) and total complications (24 of 44 [55%] vs 30 of 47 [64%]; P = .37), the AVP group had less deep venous thrombosis (5 of 44 [11%] vs 16 of 47 [34%]; P = .02).
Conclusions and Relevance
Low-dose AVP during the resuscitation of trauma patients in hemorrhagic shock decreases blood product requirements. Additional research is necessary to determine whether including AVP improves morbidity or mortality.
Trial Registration
ClinicalTrials.gov identifier: NCT01611935
Introduction
Trauma remains the leading cause of death for individuals 45 years and younger in the United States, with hemorrhage accounting for approximately 72% of mortality within 24 hours of injury.1,2 Although resuscitation with fluids and blood products remains the cornerstone of care, vigorous volume replacement can lead to serious complications, including coagulopathy, acute lung injury, and abdominal compartment syndrome.3,4,5 Massive hemorrhage also profoundly alters the neuroendocrine milieu needed to maintain vasomotor tone, and prolonged shock may progress to a state of refractory hypotension.6 The inclusion of vasoactive hormones during resuscitation, therefore, may limit the need for aggressive blood product transfusion and decrease resuscitation-associated complications.
Arginine vasopressin (AVP), a hormone secreted by the posterior pituitary in response to increased osmolarity or hypotension, has been used widely as a vasopressor in critically ill patients.7,8 Arginine vasopressin is also essential during hemorrhagic shock.9 Although 10% to 20% of the total pituitary AVP stores can be released rapidly during the onset of acute blood loss, secretion diminishes over time, despite persistent stimulation; and low levels are associated with catecholamine-resistant hypotension and increased venous capacitance.10,11,12 Patients with trauma (hereinafter referred to as trauma patients) and hemorrhage who receive large-volume transfusion during resuscitation may be at particular risk of developing AVP deficiency during the first 48 hours of resuscitation.13,14 In addition to AVP’s fixed secretion rate and relatively short half-life (10-35 minutes), trauma patients lose AVP in shed blood and are resuscitated with AVP-poor crystalloids and blood products. As such, AVP levels decrease dramatically in severely injured patients who require at least 5 U of blood product.13
Supplementing AVP restores serum levels and may improve hemorrhage control. In addition to potentially vasoconstricting injured vessels, AVP preferentially shunts blood away from nonessential vascular beds to more vital structures such as the heart and brain.15 When given in a physiologic dosage (≤0.04 U/min), however, AVP does not augment blood pressure in healthy volunteers and only acts as a vasopressor in deficient states.6,16 Arginine vasopressin supplementation may also improve hemostasis by enhancing platelet function and thus augmenting clot formation.17
Although exogenous AVP has been demonstrated to improve survival in animal models of lethal hemorrhage, its use in trauma patients is limited to a number of case reports, a retrospective study suggesting increased mortality, and 1 prospective but underpowered trial.15,18,19,20 We conducted a randomized, double-blind clinical trial to determine whether low-dose AVP supplementation decreases the need for blood product transfusion in trauma patients with hemorrhage. Our secondary hypotheses were that AVP supplementation decreases the need for resuscitative support (eg, crystalloids or vasopressors) and would result in fewer complications.
Methods
This single-center randomized clinical trial was conducted from May 1, 2013, through May 31, 2017, at an urban level 1 trauma center with approval from the institutional review board of the University of Pennsylvania. Because this study was conducted using the US Food and Drug Administration (FDA) exception from informed consent for emergency research policy (FDA 21 CFR 50.24), an extensive community consultation process was performed,21 and an Investigational New Drug application was filed with the FDA. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. The trial protocol is available in Supplement 1.
Study Patients
Trauma patients (aged 18-65 years) who received 6 U of any blood product within 12 hours of injury were eligible to participate. Enrollment occurred as soon as the sixth unit of any blood product (eg, packed red blood cells [PRBC], fresh frozen plasma [FFP], or platelets) had been infused. Cryoprecipitate was not included in the cumulative product volume but was analyzed separately. Exclusion criteria consisted of interhospital transfer, prehospital cardiopulmonary resuscitation, emergency department thoracotomy, recent corticosteroid use, chronic renal insufficiency, significant coronary artery disease, traumatic brain injury requiring neurosurgical intervention, pregnancy, being younger than 18 years or older than 65 years, and/or prisoner status. Patients who opted out were also excluded.
Demographic characteristics, physiologic data, laboratory values, resuscitation requirements, and hemostatic agents were recorded. Injury characteristics were extracted from the record after enrollment (Table 1).
Table 1. Baseline Demographics and Clinical Characteristics by Treatment Group.
| Factor | Study Group | ASDa | |||
|---|---|---|---|---|---|
| AVP Supplementation (n = 49) | Placebo (n = 51) | ||||
| No. Missing | Data | No. Missing | Data | ||
| Patient demographics | |||||
| Age, median (IQR), y | 0 | 26 (21-31) | 0 | 27 (24-36) | 0.35 |
| Male, No. (%) | 0 | 46 (94) | 0 | 47 (92) | 0.07 |
| Race/ethnicity, No. (%) | |||||
| Black | 0 | 42 (86) | 0 | 40 (78) | 0.36 |
| White | 0 | 7 (14) | 0 | 8 (16) | |
| Hispanic | 0 | 0 | 0 | 2 (4) | |
| Asian | 0 | 0 | 0 | 1 (2) | |
| Injury characteristics | |||||
| Penetrating mechanism, No. (%) | 0 | 39 (80) | 0 | 40 (78) | 0.03 |
| Injury Severity Score, median (IQR)b | 0 | 19 (14-26) | 0 | 26 (17-34) | 0.29 |
| PATI, median (IQR)c | 0 | 32 (27-44) | 0 | 26 (16-40) | 0.38 |
| Primary source of hemorrhage, No. (%) | |||||
| Head | 0 | 0 | 0 | 1 (2) | 0.31 |
| Neck | 0 | 2 (4) | 0 | 3 (6) | |
| Thoracic | 0 | 14 (29) | 0 | 12 (24) | |
| Abdominal | 0 | 21 (43) | 0 | 26 (51) | |
| Extremity | 0 | 12 (24) | 0 | 9 (18) | |
| Hemorrhage control, No. (%) | |||||
| Operating room | 0 | 48 (98) | 0 | 50 (98) | 0.29 |
| Interventional radiology | 0 | 0 | 0 | 1 (2) | |
| Both | 0 | 1 (2) | 0 | 0 | |
| AIS ≥4, No. (%) | |||||
| Neck | 0 | 5 (10) | 0 | 1 (2) | 0.35 |
| Chest | 0 | 17 (35) | 0 | 16 (31) | 0.07 |
| Abdomen | 0 | 20 (41) | 0 | 16 (31) | 0.19 |
| Extremity | 0 | 5 (10) | 0 | 5 (10) | 0.01 |
| Trauma bay admission vital signs, mean (SD) | |||||
| SBP, mm Hg | 0 | 113 (29) | 0 | 114 (35) | 0.02 |
| MAP, mm Hg | 1 | 76 (24) | 0 | 82 (33) | 0.20 |
| HR, bpm | 0 | 105 (27) | 0 | 109 (26) | 0.15 |
| Glasgow Coma Score, median (IQR)d | 0 | 14 (10-15) | 0 | 14 (8-15) | 0.17 |
| Time to enrollment, median (IQR), min | 0 | 97 (69-122) | 0 | 80 (56-102) | 0.29 |
| Enrollment vital signs | |||||
| Lowest SBP, mean (SD), mm Hg | 0 | 77 (21) | 0 | 69 (20) | 0.38 |
| SBP, mean (SD), mm Hg | 0 | 99 (31) | 0 | 90 (29) | 0.30 |
| MAP, mean (SD), mm Hg | 0 | 70 (19) | 0 | 66 (21) | 0.18 |
| HR, mean (SD), bpm | 0 | 103 (17) | 0 | 106 (25) | 0.14 |
| Temperature, median (IQR), °C | 5 | 36 (35-37) | 5 | 36 (35-36) | 0.33 |
| Trauma evaluation laboratory values | |||||
| Lactate level, median (IQR), mg/dL | 9 | 6 (4-9) | 11 | 7 (5-11) | 0.35 |
| Sodium level, mean (SD), mEq/L | 4 | 139 (3) | 8 | 140 (4) | 0.26 |
| Creatinine level, mean (SD), mg/dL | 5 | 1.35 (0.25) | 8 | 1.33 (0.25) | 0.03 |
| Hemoglobin level, mean (SD), g/dL | 4 | 12.6 (1.7) | 8 | 12.0 (1.9) | 0.33 |
| Platelet count, mean (SD), ×103/μL | 5 | 226 (74) | 8 | 222 (77) | 0.06 |
| Prothrombin time, INR, median (IQR) | 5 | 1.2 (1.1-1.4) | 9 | 1.2 (1.2-1.4) | 0.22 |
| Partial thromboplastin time, median (IQR), s | 5 | 27 (25-32) | 9 | 29 (26-35) | 0.11 |
| Preenrollment resuscitation requirements | |||||
| Crystalloids, median (IQR), L | 0 | 2.1 (1.5-3.6) | 0 | 2.0 (1.1-2.8) | 0.32 |
| Blood products, median (IQR), L | 0 | 1.8 (1.8-2.3) | 0 | 2.0 (1.8-2.2) | 0.07 |
| PRBC, median (IQR), L | 0 | 1.5 (1.2-1.8) | 0 | 1.5 (1.5-1.8) | 0.08 |
| FFP, median (IQR), L | 0 | 0.5 (0.3-0.5) | 0 | 0.5 (0.1-0.5) | 0.18 |
| Platelets, median (IQR), mL | 0 | 0 (0-0) | 0 | 0 (0-0) | 0.14 |
| Cryoprecipitate, median (IQR), mL | 0 | 0 (0-0) | 0 | 0 (0-0) | <0.001 |
| Estimated blood loss, median (IQR), mL | 0 | 250 (0-800) | 1 | 25 (0-675) | 0.15 |
| Factor VII, No. (%) | 0 | 0 | 0 | 0 | <0.001 |
| Tranexamic acid, No. (%) | 0 | 18 (37) | 0 | 23 (45) | 0.17 |
Abbreviations: AIS, Abbreviated Injury Score; ASD, absolute standardized difference; bpm, beats per minute; AVP, arginine vasopressin; FFP, fresh frozen plasma; HR, heart rate; INR, international normalized ratio; IQR, interquartile range; MAP, mean arterial pressure; PATI, Penetrating Abdominal Trauma Index; PRBC, packed red blood cells; SBP, systolic blood pressure.
SI conversion factors: To convert creatinine to micromoles per liter, multiply by 88.4; hemoglobin to grams per liter, multiply by 10.0; lactate to millimoles per liter, multiply by 0.111; platelets to 109 per liter, multiply by 1.0; sodium to millimoles per liter, multiply by 1.0.
Defined as the absolute difference in means, mean ranks, or proportions between groups divided by the pooled SD. Variables with ASD of greater than 0.392 are defined as imbalanced between groups. An ASD of no greater than 0.392 will capture 95% of the participants if the true ASD is zero for that variable.
Scores range from 0 to 75, with higher scores indicating more severe injury.
Applicable only for patients with an abdominal injury; summary includes 25 in the AVP group and 23 in the placebo group. Scores range from 0 to 200, with higher scores indicating greater severity.
Scores range from 3 to 15, with higher scores indicating greater level of consciousness.
Treatment Assignments and Infusions
Group assignment was performed by an independent investigational drug service using a computer-generated randomization scheme in blocks of 6. Study kits containing AVP or placebo were prepared off-site. The clinical team, research personnel, patients’ families, and patients were blinded to group assignment for the duration of the trial.
Arginine vasopressin was mixed in saline with a final concentration of 0.4 U/mL. A 4-U bolus of AVP or an equivalent volume of saline placebo was given before starting the study infusion (either AVP or placebo) at 0.04 U/min. After the operating surgeon declared definitive hemorrhage control, the study infusion could be titrated (0 to 0.04 U/min) to maintain a mean arterial blood pressure (MAP) of at least 65 mm Hg for a total of 48 hours. The study infusion, therefore, could change depending on the patient’s hemodynamic stability. In the operating room or interventional suite, patients ideally were resuscitated with blood products in a 1:1:1 fashion. After the procedure, patients received crystalloids and blood products at the discretion of the treating physician to address lactic acidosis, urine output, anemia (hemoglobin level <10 g/dL [to convert to grams per liter, multiply by 10.0]), and/or coagulopathy (international normalized ratio, ≥1.4). All health care professionals were blinded to treatment assignment. On enrollment, a strict MAP goal of at least 65 mm Hg was maintained for the next 48 hours. If vasopressors were needed, neosynephrine, norepinephrine bitartrate, and/or epinephrine were used. All vasopressor treatments were titrated and stopped before tapering the study infusion. If vasopressor support was again required, the study infusion was restarted before adding vasopressors. Open-label AVP use was not permitted. The MAP goal was determined by the consensus of a multidisciplinary panel of trauma surgeons and anesthesiologists (including C.A.S., D.H., P.K., J.P., B.S., N.M., and P.R.). A study coordinator monitored all resuscitations in real time to facilitate enrollment and ensure protocol compliance.
End Points
The primary outcome was the cumulative volume of blood product transfused within 48 hours and included PRBC, FFP, and platelets after enrollment. Secondary outcomes included total volume of crystalloids transfused, estimated blood loss, overall fluid balance, and total vasopressor requirement within the first 48 hours. All doses of vasopressors were normalized to norepinephrine equivalents: norepinephrine (in micrograms) + epinephrine (in micrograms) + 2.2 × phenylephrine (in micrograms) + (ephedrine/80.9) × 0.4545.22 Outcomes at 30 days included mortality, length of stay (LOS), and complications as defined by the Pennsylvania Trauma Outcomes Study.23 We also investigated outcomes potentially affected by resuscitation, including acute kidney injury, acute respiratory distress syndrome, mechanical ventilator–free days, and open abdomen–free days (eMethods in Supplement 2). Patients underwent weekly screening for deep venous thrombosis (DVT) using lower-extremity ultrasonography for the first 3 weeks of admission and then as clinically needed. Predefined adverse events were monitored in real time and evaluated by an independent medical monitor, the data safety monitoring board, and the institutional review board.
Statistical Analysis
Data were analyzed from May 1, 2013, through May 31, 2017. Based on a previous study,19 a power analysis was performed assuming a baseline mean (SD) use of 5.4 (6.6) L of blood products. Assuming 80% power and a 2-sided α error of .05, 50 patients per group would be required to detect more than a 50% reduction in the total volume of blood product (ie, mean [SD], 2.7 [4.8]). Additional power analyses were not performed for secondary outcomes. An independent statistician blinded to group assignment performed planned interim analyses after the 25th, 50th, and 75th patient enrollments. Safety boundaries were established a priori.24 Primary and secondary outcomes, as well as complications, were compared between groups at each interim without adjusting P values for multiple analyses. The external data and safety monitoring board blindly reviewed interim results and recommended the study continue without modification.
Demographics, clinical characteristics, and preenrollment resuscitation variables were summarized. Balance between groups was assessed using absolute standardized difference (ASD), defined as the absolute difference in means, mean ranks, or proportions divided by the pooled SD. The ASD assesses the degree to which groups differ from each other, with larger ASDs indicating larger differences between groups. Any characteristic with ASD of greater than 1.96 × √(1/ηvasopressin + 1/ηplacebo) was considered imbalanced, where η corresponded to the number of patients randomized to AVP and placebo, respectively. Thus, an ASD of less than 0.392 would capture 95% of participants if the true ASD was zero for that variable.25 The ASD was used because relying on multiple significance tests to evaluate baseline variables can be misleading and exaggerates the rate of type I errors. In contrast, the use of ASD mitigates the risk of type I error amplification.
We performed an intention-to-treat and a per-protocol analysis. For the intention-to-treat analysis, patients who died within 48 hours were assigned the worst observed outcome among survivors in the combined groups to control for potential bias of the estimated treatment effect associated with early mortality. The per-protocol analysis excluded patients who died in the operating room, because these patients were deemed to have nonsurvivable injuries by a blinded panel of trauma surgeons and could not reach the end points of interest. Total complications were compared using a 2-tailed χ2 test. The relative risk of any adverse event was estimated using a log-linked logistic regression model with any adverse event as a function of AVP vs placebo.26 Similarly, crystalloid volume transfused, estimated blood loss, fluid balance at 48 hours, days in the intensive care unit, hospital LOS, days of mechanical ventilation, and days left with an open abdomen were assessed using Wilcoxon rank sum tests with a Hodges-Lehmann estimation of location shift between groups.
We used a 2-sided significance criterion of .05, and no adjustment was made for assessing multiple secondary outcomes. Analyses were performed using R, version 3.3.2 (R Project for Statistical Computing).
Results
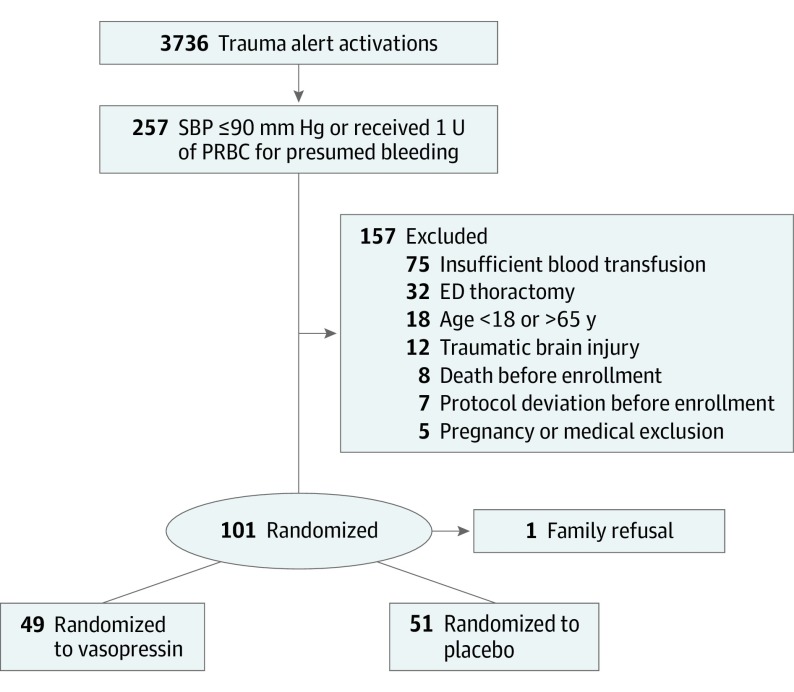
A total of 3736 trauma activations occurred during the study period; 257 patients were hypotensive (SBP ≤ 90 mm Hg) or received at least 1 U of blood product during their initial evaluation. A total of 157 patients were excluded, mostly secondary to insufficient blood product transfusion within the 12-hour enrollment period (Figure). Seven patients who received more than 15 U of blood product or were treated with AVP before randomization were excluded. One family declined enrollment. Of the 100 patients enrolled (93 men and 7 women; median age, 27 years [IQR, 22-25 years]), 49 were randomized to the AVP group and 51 to the placebo group. Seventy-nine patients had penetrating trauma. Time to enrollment did not differ between groups. Most patients were young black men with penetrating trauma. Preenrollment demographics, vital signs, laboratory values, resuscitation volume, and vasopressor requirements were well balanced between the groups (Table 1).
Figure. Flowchart for Screening and Enrollment.
ED indicates emergency department; SBP, systolic blood pressure.
Intention-to-Treat Analysis
Blood products and volume of crystalloid transfused, total dose of vasopressor, and fluid balance at 48 hours were compared. When analyzed individually, AVP was associated with significantly less volume of FFP (median, 0.9 [IQR, 0.8-1.3] vs 1.0 [IQR, 0.5-1.8] L), platelets (median, 200 [IQR, 0-300] vs 300 [IQR, 0-600] mL), and cryoprecipitate (mean [SD], 12.6 [75.4] vs 34.7 [84.8] mL) (Table 2) and significantly less cumulative volume of all blood products with an estimated median difference of −1.00 L (95% CI, −2.03 to 0.00 L; P = .03) (Table 3). Although AVP did not affect the overall complication rate (29 of 49 [59%] vs 34 of 51 [67%]; P = .44), it was associated with a decreased rate of DVTs (10 of 49 [20%] vs 20 of 51 [39%]; P = .05). Arginine vasopressin did not significantly influence resuscitation-related complications such as acute respiratory distress syndrome (34 of 49 [69%] vs 40 of 51 [78%]; P = .31), length of mechanical ventilation (median, 3 [IQR, 1-6] vs 3 [IQR, 2-18] days; P = .43), acute kidney injury (8 of 49 [16%] vs 14 of 51 [27%]; P = .19), or time needed for damage control of open abdomen (median, 29 [IQR, 28-29] vs 28 [IQR, 27-29] days; P = .36). Arginine vasopressin also did not significantly affect median LOS in the intensive care unit (5 [IQR, 3-15] vs 9 [IQR, 3-27] days; P = .28) or hospital (16 [IQR, 10-32] vs 22 [IQR, 14-44] days; P = .46), risk of operative death (5 of 49 [10%] vs 4 of 51 [8%]; P = .68), or overall mortality (6 of 49 [12%] vs 6 of 51 [12%]; P = .94).
Table 2. Cumulative Blood Products, Fluids, and Vasopressors Infused Within the First 48 Hours After Enrollment.
| Factor | Analysis by Study Group | |||||
|---|---|---|---|---|---|---|
| Intention to Treat | Per Protocol | |||||
| AVP Supplementation (n = 49) | Placebo (n = 51) | P Valuea | AVP Supplementation (n = 44) | Placebo (n = 47) | P Valuea | |
| Total blood products, median (IQR), L | 1.7 (0.7-3.1) | 3.0 (1.4-5.2) | .03 | 1.4 (0.5-2.6) | 2.9 (1.1-4.8) | .01 |
| PRBC, median (IQR), L | 0.9 (0.1-1.8) | 1.5 (0.6-3.0) | .08 | 0.6 (0.0-1.5) | 1.2 (0.6-2.6) | .02 |
| FFP, median (IQR), L | 0.9 (0.8-1.3) | 1.0 (0.5-1.8) | .03 | 0.5 (0.3-1.0) | 1.0 (0.5-1.5) | .01 |
| Platelets, median (IQR), mL | 200 (0-300) | 300 (0-600) | .02 | 98 (0-300) | 300 (0-600) | .01 |
| Cryoprecipitate, mean (SD), mL | 12.6 (75.4) | 34.7 (84.8) | .04 | 2.3 (15.1) | 37.2 (87.9) | .02 |
| Crystalloids, median (IQR), L | 9.7 (7.2-13.0) | 10.7 (8.7-14.4) | .24 | 9.9 (7.9-13.0) | 11.0 (8.9-15.0) | .22 |
| Estimated blood loss, median (IQR), L | 0.8 (0.1-19.0) | 1.0 (300-14.6) | .41 | 0.8 (0.3-1.6) | 1.0 (0.3-2.3) | .35 |
| Urine output, median (IQR), L | 5.0 (3.8-6.2) | 4.1 (3.3-5.1) | .03 | 5.1 (4.1-6.4) | 4.2 (3.7-5.1) | .01 |
| Ratio of fluid total input to total output, median (IQR) | 5.0 (2.1-7.8) | 6.7 (4.1-11.8) | .03 | 5.0 (2.5-7.0) | 6.7 (4.0-11.4) | .03 |
| AVP or placebo infused, median (IQR), U | 32.8 (18.5-83.8) | 50.8 (20.0-91.0) | .44 | 39 (22-87) | 56 (23-94) | .58 |
| NE infused, median (IQR), μgb | 581 (1.2-11 255) | 1536 (227-8491) | .40 | 400 (0-5900) | 1400 (200-7600) | .22 |
Abbreviations: AVP, arginine vasopressin; FFP, fresh frozen plasma; IQR, interquartile range; NE, norepinephrine equivalent; PRBC, packed red blood cells.
Values for 48-hour fluids determined from separate Wilcoxon rank sum tests.
All vasopressor doses converted to the NE dose.
Table 3. Primary and Secondary Outcomes.
| Outcome | Study Group, Intention-to-Treat Populationa | Study Group, Per-Protocol Populationb | ||||||
|---|---|---|---|---|---|---|---|---|
| AVP Supplementation (n = 49) | Placebo (n = 51) | Analysisc | P Valued | AVP Supplementation (n = 44) | Placebo (n = 47) | Analysisc | P Valued | |
| Primary outcome | ||||||||
| 48-h Cumulative blood products, median (95% CI), L | 1.7 (0.7 to 3.1) | 3.0 (1.4 to 5.2) | Difference, −1.00 (−2.03 to 0.00) | .03 | 1.4 (0.5 to 2.6) | 2.9 (1.1 to 4.8) | Difference, −1.10 (−2.04 to 0.00) | .01 |
| Secondary outcomes | ||||||||
| 48-h Total vasopressor equivalents, median (95% CI), g | 0.6 (0.0 to 14) | 1.5 (0.2 to 14) | Difference, −0.11 (−1.35 to 0.19) | .38 | 0.4 (0.0 to 5.9) | 1.4 (0.2 to 7,6) | Difference, −0.23 (−1.37 to 0.53) | .22 |
| 48-h Crystalloid, median (95% CI), L | 9.6 (6.3 to 13) | 10 (8.6 to 15) | Difference, −1.31 (−3.43 to 0.80) | .31 | 9.9 (7.9 to 13) | 11 (8.9 to 15) | Difference, −1.07 (−3.04 to 0.62) | .22 |
| Fluid balance at 48 h, median (95% CI), L | 6.0 (3.0 to 9.2) | 7.0 (4.5 to 12) | Difference, −1.89 (−4.40 to 0.28) | .10 | 5.0 (2.5 to 7.0) | 6.7 (4.0 to 11.0) | Difference, −2.22 (−4.40 to −0.13) | .03 |
| ARDS, No. (%) | 34 (69) | 40 (78) | RR (95% CI), 0.88 (0.70 to 1.12) | .31 | 29 (66) | 36 (77) | RR (95% CI), 0.86 (0.66 to 1.12) | .27 |
| Acute kidney injury, No. (%) | 8 (16) | 14 (27) | RR (95% CI), 0.59 (0.27 to 1.29) | .19 | 2 (5) | 8 (17) | 0.27 (0.06 to 1.19) | .08 |
| Death, No. (%) | 6 (12) | 6 (12) | RR (95% CI), 1.04 (0.36 to 3.01) | .94 | NA | NA | NA | NA |
| Death in OR, No. (%) | 5 (10) | 4 (8) | RR (95% CI), 1.30 (0.37 to 4.56) | .68 | NA | NA | NA | NA |
| Open abdomen–free days, median (95% CI)e | 29 (28 to 29) | 28 (27 to 29) | HR (95% CI), 0.78 (0.46 to 1.33) | .36 | 28 (27 to 29) | 28 (26 to 29) | Difference, 0.00 (−1.00 to 1.00) | .87 |
| Time to ventilator removal, median (95% CI), d | 3 (1 to 6) | 3 (2 to 18) | HR (95% CI), 1.17 (0.79 to 1.75) | .43 | 2 (1 to 4) | 3 (1 to 12) | Difference, −1.00 (−2.00 to 0.00) | .11 |
| ICU LOS, median (95% CI), d | 5 (3 to 15) | 9 (3 to 27) | HR (95% CI), 1.26 (0.83 to 1.92) | .28 | 4 (2 to 11) | 9 (3 to 19) | Difference, −2.00 (−6.00 to 0.00) | .06 |
| Hospital LOS, median (95% CI), d | 16 (10 to 32) | 22 (14 to 44) | HR (95% CI), 1.17 (0.77 to 1.78) | .46 | 14 (10 to 25) | 20 (14 to 31) | Difference, −4.00 (−10.0 to 1.00) | .12 |
| Post hoc outcome | ||||||||
| Any complication, No (%) | 29 (59) | 34 (67) | RR (95% CI), 0.89 (0.66 to 1.20) | .44 | 24 (55) | 30 (64) | RR (95% CI), 0.85 (0.61 to 1.21) | .37 |
| DVT, No. (%) | 10 (20) | 20 (39) | RR (95% CI), 0.52 (0.27 to 1.00) | .05 | 5 (11) | 16 (34) | RR (95% CI), 0.33 (0.13 to 0.83) | .02 |
| No. of complications, median (95% CI) | NA | NA | NA | NA | 1 (1 to 2) | 2 (1 to 3) | Difference, 0 (−1 to 0) | .12 |
Abbreviations: ARDS, acute respiratory distress syndrome; HR, hazard ratio; ICU, intensive care unit; LOS, length of stay; NA, not applicable; OR, operating room; RR, relative risk.
Includes all randomized patients. Patients who died before outcome occurred were assumed to have the worst observed outcome.
Includes patients who experienced the end point. Patients who died before the end point were excluded.
Median difference in AVP vs saline groups was estimated using the Wilcoxon rank sum test and Hodges-Lehmann estimation of location shift between groups; RR, from a logistic regression model with a log link; and HR, from a Cox proportional hazards regression model. Patients who died before event were assigned a censoring time equal to the longest observed time to outcome.
Primary and secondary analyses are each assessed at P < .05 significance criterion.
Analysis only includes patients who had an open abdomen.
Per-Protocol Analysis
The per-protocol analysis excluded 9 patients who died in the OR of nonsurvivable injuries. Blood products and volume of crystalloid transfused, total dose of vasopressor required, clinical variables, and laboratory values at 48 hours were compared. Patients receiving AVP received significantly less blood products (median, 1.4 [IQR, 0.5-2.6] vs 2.9 [IQR, 1.1-4.8] L; P = .01), including fewer PRBC (median, 0.6 [IQR, 0.0-1.5] vs 1.2 [IQR, 0.6-2.6] L; P = .02), FFP (median, 0.5 [IQR, 0.3-1.0] vs 1.0 [IQR, 0.5-1.5] L; P = .01), platelets (median, 98 [IQR, 0-300] vs 300 [IQR, 0-600] mL; P = .01), and cryoprecipitate (mean [SD], 2.3 [15.1] vs 37.2 [87.9] mL; P = .02), and had improved fluid balance (median, 5.0 [IQR, 2.5-7.0] vs 6.7 [IQR, 4.0-11.4] L; P = .03) (Table 2). Although the AVP group received a lower volume of vasopressors, these differences did not reach statistical significance. Clinical and laboratory variables were similar between groups at 48 hours (eFigures 1 and 2 and eTable in Supplement 2)
Complications occurring within 30 days were common, but the incidence of developing 1 or more complications was not significantly different between treatment groups (Table 4). Although patients in the AVP group had a lower positive fluid balance at 48 hours, this did not significantly alter the incidence of resuscitation-related complications (Table 3). Arginine vasopressin also did not affect the overall complication rate (24 of 44 [55%] vs 30 of 47 [64%]; P = .37), but was associated with decreased DVTs (5 of 44 [11%] vs 16 of 47 [34%]; P = .02). Notably, the median time to starting DVT prophylaxis was not statistically different between groups (2 vs 2 days; P = .72). Median intensive care unit LOS (4 [IQR, 2-11] vs 9 [IQR, 3-19] days; P = .06) was not significant, and AVP did not significantly affect hospital LOS (14 [IQR, 10-25]vs 20 [IQR, 14-31] days; P = .12).
Table 4. Adverse Events by Treatment Group.
| Adverse Event | Study Group | |
|---|---|---|
| AVP Supplementation (n = 44) | Placebo (n = 47) | |
| Any adverse event, No. (%) of patientsa | 35 (80) | 39 (83) |
| No. of adverse events | 69 | 98 |
| Adverse event, No. (%) of patients | ||
| Deep venous thrombosis | 5 (11) | 16 (34) |
| Pulmonary embolus | 2 (5) | 3 (6) |
| Urinary tract infection | 1 (2) | 1 (2) |
| Ventilator-associated pneumonia | 7 (16) | 7 (15) |
| Acute renal failure | 2 (5) | 8 (17) |
| Acute respiratory distress syndrome | 29 (66) | 36 (77) |
| Gastrointestinal bleeding | 2 (5) | 1 (2) |
| Major dysrhythmia | 0 | 1 (2) |
| Wound infection | 4 (9) | 5 (11) |
| Sepsis | 2 (5) | 6 (13) |
| Extremity compartment syndrome | 0 | 3 (6) |
| Coagulopathy | 2 (5) | 3 (6) |
| Soft tissue infection | 4 (9) | 2 (4) |
| Ischemia | 1 (2) | 2 (4) |
| Hyponatremia | 5 (11) | 3 (6) |
| Urticaria | 1 (2) | 0 |
| Arterial thrombosis | 1 (2) | 0 |
| Rhabdomyolysis | 1 (2) | 1 (2) |
Abbreviation: AVP, arginine vasopressin.
P = .67 based on χ2 test.
Discussion
Hemorrhage is a leading cause of death in patients with trauma. Although blood products remain the criterion standard for treating hemorrhagic shock, they are a limited and perishable resource. Moreover, concerns are increasing that blood products are immunomodulatory and may negatively affect clinical outcomes.27,28,29 Resuscitation strategies that decrease the need for transfusions without increasing complications, therefore, would represent a clinically important innovation. In this single-center, randomized, double-blind clinical trial, the early administration of AVP during the resuscitation of patients with hemorrhagic shock significantly decreased the use of all blood products and improved fluid balance at 48 hours without increasing overall complications.
Arginine vasopressin can affect the pathophysiology of shock in several ways. First, AVP counteracts hypotension by activating vascular smooth muscle V1 receptors independent of α-adrenergic stimulation.30 Arginine vasopressin also mitigates the vasoplegia and increased venous capacitance observed in late-stage shock by inhibiting vascular adenosine triphosphate–sensitive potassium channels and by blunting nitric oxide–induced vasodilation.6 Although exogenous low-dose AVP (<0.04 U/min) has minimal vasopressor effects in normotensive individuals, it dramatically improves vascular tone in shock states associated with AVP deficiency.8,31 Unlike catecholamines, AVP enhances renal perfusion at low doses by preferentially causing efferent arteriolar vasoconstriction with relatively little effect on the afferent circulation.32,33 Arginine vasopressin may also promote hemostasis by inducing the exocytosis of von Willebrand factor from endothelial cells,34 by enhancing the procoagulant capacity of platelets, and by significantly increasing platelet-dependent thrombin generation.17,35 Finally, AVP can conserve intravascular volume by activating renal V2 receptors.36
Although case reports have suggested that AVP is beneficial in life-threatening hemorrhage,37 it is not recommended by Advanced Trauma Life Support guidelines.38 Indeed, vasopressors have been traditionally eschewed in trauma given concerns about exacerbating tissue ischemia and worsening outcomes. In a retrospective evaluation of trauma patients who required vasopressors within the first 72 hours, Collier et al20 reported that AVP use increased mortality by 20%. Similarly, in a retrospective analysis of 921 injured patients, mortality increased 2-fold if vasopressors were given within the first 24 hours.39 Arginine vasopressin, however, was the only vasopressor in that study not associated with increased mortality on logistic regression. Given the retrospective nature of these studies, AVP may represent a marker of increased severity of illness rather than a direct contributor to adverse outcomes. Interestingly, in our study, vasopressors were frequently required to maintain an MAP of at least 65 mm Hg, suggesting that severely injured patients frequently require vasoactive support. Indeed, the median requirement for patients receiving placebo was 1400 μg of norepinephrine equivalents at 48 hours.
Our study demonstrates that using low-dose AVP supplementation in hemorrhagic shock significantly decreases the need for blood products without increasing morbidity. Patients treated with AVP received significantly less PRBC, fewer FFP units, and decreased platelet transfusions. Overall, this process led to a median transfusion reduction of 1.0 L, which translates to a decrease by roughly 3 U of PRBC or 4 U of FFP. In addition to being a limited and expensive resource, blood products may independently increase the risk of complications, including venous thromboembolism, multiple-organ failure, and death.28,40,41,42,43 Moreover, given that blood products are proinflammatory, transfusions may actually promote a hypercoagulable state and possibly increase the risk of DVT.44,45,46 Thus, strategies that result in decreased transfusion requirements could potentially have significant clinical effects.
We were surprised to discover that AVP supplementation was associated with fewer DVTs. Because AVP can activate platelets and thus possibly promote a procoagulable state, we tracked DVTs as a secondary safety outcome. Arginine vasopressin may have indirectly affected the risk of DVT because AVP decreased the amount of blood product transfused. Several retrospective studies28,29,47,48 have raised the concern that blood transfusions increase the risk of venous thromboembolism in a dose-dependent fashion. In a propensity-matched study of more than 750 000 patients undergoing surgery,44 the risk of venous thromboembolism was 2-fold higher in patients who received 1 U of PRBC and 4.5 times higher in those who received at least 3 U. Although a similar dose-dependent risk has been reported in trauma patients, further research is needed to validate this association.45 Alternatively, AVP may modulate the inflammatory response to trauma, thereby quelling the hypercoagulable state and decreasing the risk of DVT. This hypothesis has not been previously explored, but AVP receptors have been identified on human macrophages and lymphocytes. Moreover, in murine sepsis models, AVP has been shown to downregulate nuclear factor κβ activity, decrease serum interleukin 6 levels, and mitigate pulmonary inflammation.49 As such, the potential for AVP to modulate the inflammatory response after hemorrhagic shock is intriguing and warrants further exploration.
Limitations
Several limitations of our trial deserve mention. First, the total dose of AVP infused varied depending on the patient’s hemodynamic stability, and, given the technical challenges of measuring serum AVP levels, we did not use them to guide dosing or duration. In addition, the ideal posthemorrhage blood pressure control remains controversial. That being said, AVP infusion at the physiologic dose of 0.04 U/min did not influence the blood pressure in non–AVP-deficient participants, and only those who are AVP-deficient would be expected to have a hemodynamic response to AVP. Moreover, both groups were required to maintain an MAP of at least 65 mm Hg and received vasopressors if needed. Therefore, patients treated with placebo would have the same likelihood of being resuscitated with blood products and/or vasopressors as the AVP group to achieve the goal MAP. Second, although our institution maintains resuscitation guidelines, the clinical team directed the treatment plan of each patient. Although variations in care may have occurred, the clinical team was blinded to group assignment. Third, our study was conducted at a single institution that cares for a large percentage of patients with penetrating trauma. A larger multiple-institution trial with a more diverse population of trauma patients would be needed to assess the generalizability of our findings. Finally, with a cohort size of only 100 patients, we were underpowered to detect significant differences in many clinically relevant outcomes. Similarly, given the small sample size, we did not adjust for multiple comparisons. As such, a larger study will be needed to determine the effect of AVP on acute kidney injury, acute respiratory distress syndrome, mechanical ventilation, and LOS.
Conclusions
In this randomized clinical trial, low-dose AVP supplementation decreased blood product requirements and DVTs in trauma patients who presented in hemorrhagic shock. A larger study is needed to determine the effect of AVP on morbidity and mortality.
Trial Protocol
eFigure 1. 48-Hour Cumulative Blood Products for Per-Protocol Analysis
eFigure 2. Vital Signs and Urine Output for Each 8-Hour Period
eTable. Laboratory Values on Postoperative Days 1 and 2
eMethods. Pennsylvania Trauma Outcomes Study Definitions of Complications
Data Sharing Statement
References
- 1.Heron M. Deaths: Leading Causes for 2016. Natl Vital Stat Rep. 2018;67(6):1-77. [PubMed] [Google Scholar]
- 2.Tisherman SA, Schmicker RH, Brasel KJ, et al. . Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann Surg. 2015;261(3):586-590. doi: 10.1097/SLA.0000000000000837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotton BA, Guy JS, Morris JA Jr, Abumrad NN. The cellular, metabolic, and systemic consequences of aggressive fluid resuscitation strategies. Shock. 2006;26(2):115-121. doi: 10.1097/01.shk.0000209564.84822.f2 [DOI] [PubMed] [Google Scholar]
- 4.Sihler KC, Napolitano LM. Complications of massive transfusion. Chest. 2010;137(1):209-220. doi: 10.1378/chest.09-0252 [DOI] [PubMed] [Google Scholar]
- 5.Gearhart MM, Luchette FA, Proctor MC, et al. . The risk assessment profile score identifies trauma patients at risk for deep vein thrombosis. Surgery. 2000;128(4):631-640. doi: 10.1067/msy.2000.108224 [DOI] [PubMed] [Google Scholar]
- 6.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345(8):588-595. doi: 10.1056/NEJMra002709 [DOI] [PubMed] [Google Scholar]
- 7.Landry DW, Levin HR, Gallant EM, et al. . Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95(5):1122-1125. doi: 10.1161/01.CIR.95.5.1122 [DOI] [PubMed] [Google Scholar]
- 8.Landry DW, Levin HR, Gallant EM, et al. . Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25(8):1279-1282. doi: 10.1097/00003246-199708000-00012 [DOI] [PubMed] [Google Scholar]
- 9.Altura BM. Evidence that endogenous vasopressin plays a protective role in circulatory shock: role for reticuloendothelial system using Brattleboro rats. Experientia. 1980;36(9):1080-1082. doi: 10.1007/BF01965981 [DOI] [PubMed] [Google Scholar]
- 10.Errington ML, Rocha e Silva M Jr. Vasopressin clearance and secretion during haemorrhage in normal dogs and in dogs with experimental diabetes insipidus. J Physiol. 1972;227(2):395-418. doi: 10.1113/jphysiol.1972.sp010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver JA, Landry DW. Endogenous and exogenous vasopressin in shock. Curr Opin Crit Care. 2007;13(4):376-382. doi: 10.1097/MCC.0b013e3282435e16 [DOI] [PubMed] [Google Scholar]
- 12.Morales D, Madigan J, Cullinane S, et al. . Reversal by vasopressin of intractable hypotension in the late phase of hemorrhagic shock. Circulation. 1999;100(3):226-229. doi: 10.1161/01.CIR.100.3.226 [DOI] [PubMed] [Google Scholar]
- 13.Sims CA, Guan Y, Bergey M, et al. . Arginine vasopressin, copeptin, and the development of relative AVP deficiency in hemorrhagic shock. Am J Surg. 2017;214(4):589-595. doi: 10.1016/j.amjsurg.2017.06.015 [DOI] [PubMed] [Google Scholar]
- 14.Cohn SM, DeRosa M, McCarthy J, et al. . Characterizing vasopressin and other vasoactive mediators released during resuscitation of trauma patients. J Trauma Acute Care Surg. 2013;75(4):620-628. doi: 10.1097/TA.0b013e31829eff31 [DOI] [PubMed] [Google Scholar]
- 15.Voelckel WG, Raedler C, Wenzel V, et al. . Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit Care Med. 2003;31(4):1160-1165. doi: 10.1097/01.CCM.0000060014.75282.69 [DOI] [PubMed] [Google Scholar]
- 16.Möhring J, Glänzer K, Maciel JA Jr, et al. . Greatly enhanced pressor response to antidiuretic hormone in patients with impaired cardiovascular reflexes due to idiopathic orthostatic hypotension. J Cardiovasc Pharmacol. 1980;2(4):367-376. doi: 10.1097/00005344-198007000-00004 [DOI] [PubMed] [Google Scholar]
- 17.Colucci G, Stutz M, Rochat S, et al. . The effect of desmopressin on platelet function: a selective enhancement of procoagulant COAT platelets in patients with primary platelet function defects. Blood. 2014;123(12):1905-1916. doi: 10.1182/blood-2013-04-497123 [DOI] [PubMed] [Google Scholar]
- 18.Stadlbauer KH, Wagner-Berger HG, Raedler C, et al. . Vasopressin, but not fluid resuscitation, enhances survival in a liver trauma model with uncontrolled and otherwise lethal hemorrhagic shock in pigs. Anesthesiology. 2003;98(3):699-704. doi: 10.1097/00000542-200303000-00018 [DOI] [PubMed] [Google Scholar]
- 19.Cohn SM, McCarthy J, Stewart RM, Jonas RB, Dent DL, Michalek JE. Impact of low-dose vasopressin on trauma outcome: prospective randomized study. World J Surg. 2011;35(2):430-439. doi: 10.1007/s00268-010-0875-8 [DOI] [PubMed] [Google Scholar]
- 20.Collier B, Dossett L, Mann M, et al. . Vasopressin use is associated with death in acute trauma patients with shock. J Crit Care. 2010;25(1):173.e9-173.e14. doi: 10.1016/j.jcrc.2009.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Sims CA, Isserman JA, Holena D, et al. . Exception from informed consent for emergency research: consulting the trauma community. J Trauma Acute Care Surg. 2013;74(1):157-165. doi: 10.1097/TA.0b013e318278908a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown SM, Lanspa MJ, Jones JP, et al. . Survival after shock requiring high-dose vasopressor therapy. Chest. 2013;143(3):664-671. doi: 10.1378/chest.12-1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennsylvania Outcomes and Performance Improvement Measurement System (POPIMS) Operations Manual. Pennsylvania Trauma Systems Foundation. 2015. http://ptsf.org/upload/POPIMS_Manual_2015_Final.pdf. Accessed May 1, 2017.
- 24.Bolland K, Whitehead J. Formal approaches to safety monitoring of clinical trials in life-threatening conditions. Stat Med. 2000;19(21):2899-2917. doi: [DOI] [PubMed] [Google Scholar]
- 25.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083-3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940-943. doi: 10.1093/aje/kwg074 [DOI] [PubMed] [Google Scholar]
- 27.Al-Refaie WB, Parsons HM, Markin A, Abrams J, Habermann EB. Blood transfusion and cancer surgery outcomes: a continued reason for concern. Surgery. 2012;152(3):344-354. doi: 10.1016/j.surg.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 28.Glance LG, Dick AW, Mukamel DB, et al. . Association between intraoperative blood transfusion and mortality and morbidity in patients undergoing noncardiac surgery. Anesthesiology. 2011;114(2):283-292. doi: 10.1097/ALN.0b013e3182054d06 [DOI] [PubMed] [Google Scholar]
- 29.Turan A, Yang D, Bonilla A, et al. . Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anaesth. 2013;60(8):761-770. doi: 10.1007/s12630-013-9937-3 [DOI] [PubMed] [Google Scholar]
- 30.Holmes CL, Landry DW, Granton JT. Science review: vasopressin and the cardiovascular system part 2: clinical physiology. Crit Care. 2004;8(1):15-23. doi: 10.1186/cc2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams TD, Da Costa D, Mathias CJ, Bannister R, Lightman SL. Pressor effect of arginine vasopressin in progressive autonomic failure. Clin Sci (Lond). 1986;71(2):173-178. doi: 10.1042/cs0710173 [DOI] [PubMed] [Google Scholar]
- 32.Rudichenko VM, Beierwaltes WH. Arginine vasopressin–induced renal vasodilation mediated by nitric oxide. J Vasc Res. 1995;32(2):100-105. doi: 10.1159/000159082 [DOI] [PubMed] [Google Scholar]
- 33.Edwards RM, Trizna W, Kinter LB. Renal microvascular effects of vasopressin and vasopressin antagonists. Am J Physiol. 1989;256(2, pt 2):F274-F278. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J Thromb Haemost. 2003;1(4):682-689. doi: 10.1046/j.1538-7836.2003.00190.x [DOI] [PubMed] [Google Scholar]
- 35.Pecci A, Balduini CL. Desmopressin and super platelets. Blood. 2014;123(12):1779-1780. doi: 10.1182/blood-2014-01-551242 [DOI] [PubMed] [Google Scholar]
- 36.Barrett LK, Singer M, Clapp LH. Vasopressin: mechanisms of action on the vasculature in health and in septic shock. Crit Care Med. 2007;35(1):33-40. doi: 10.1097/01.CCM.0000251127.45385.CD [DOI] [PubMed] [Google Scholar]
- 37.Haas T, Voelckel WG, Wiedermann F, Wenzel V, Lindner KH. Successful resuscitation of a traumatic cardiac arrest victim in hemorrhagic shock with vasopressin: a case report and brief review of the literature. J Trauma. 2004;57(1):177-179. doi: 10.1097/01.TA.0000044357.25191.1B [DOI] [PubMed] [Google Scholar]
- 38.American College of Surgeons Advanced Trauma Life Support 10th Edition Student Course Manual. Chicago, IL: American College of Surgeons; 2018. [Google Scholar]
- 39.Sperry JL, Minei JP, Frankel HL, et al. . Early use of vasopressors after injury: caution before constriction. J Trauma. 2008;64(1):9-14. doi: 10.1097/TA.0b013e31815dd029 [DOI] [PubMed] [Google Scholar]
- 40.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667-2674. doi: 10.1097/CCM.0b013e3181844677 [DOI] [PubMed] [Google Scholar]
- 41.Boutin A, Moore L, Lauzier F, et al. . Transfusion of red blood cells in patients with traumatic brain injuries admitted to Canadian trauma health centres: a multicentre cohort study. BMJ Open. 2017;7(3):e014472. doi: 10.1136/bmjopen-2016-014472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spinella PC, Perkins JG, Grathwohl KW, et al. . Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2)(suppl):S69-S77. doi: 10.1097/TA.0b013e318160ba2f [DOI] [PubMed] [Google Scholar]
- 43.Watson GA, Sperry JL, Rosengart MR, et al. ; Inflammation and Host Response to Injury Investigators . Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J Trauma. 2009;67(2):221-227. doi: 10.1097/TA.0b013e3181ad5957 [DOI] [PubMed] [Google Scholar]
- 44.Goel R, Patel EU, Cushing MM, et al. . Association of perioperative red blood cell transfusions with venous thromboembolism in a North American registry. JAMA Surg. 2018;153(9):826-833. doi: 10.1001/jamasurg.2018.1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhillon NK, Smith EJT, Ko A, et al. . The risk factors of venous thromboembolism in massively transfused patients. J Surg Res. 2018;222:115-121. doi: 10.1016/j.jss.2017.09.038 [DOI] [PubMed] [Google Scholar]
- 46.Karcutskie CA, Meizoso JP, Ray JJ, et al. . Association of mechanism of injury with risk for venous thromboembolism after trauma. JAMA Surg. 2017;152(1):35-40. doi: 10.1001/jamasurg.2016.3116 [DOI] [PubMed] [Google Scholar]
- 47.Douros A, Jobski K, Kollhorst B, Schink T, Garbe E. Risk of venous thromboembolism in cancer patients treated with epoetins or blood transfusions. Br J Clin Pharmacol. 2016;82(3):839-848. doi: 10.1111/bcp.13019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xenos ES, Vargas HD, Davenport DL. Association of blood transfusion and venous thromboembolism after colorectal cancer resection. Thromb Res. 2012;129(5):568-572. doi: 10.1016/j.thromres.2011.07.047 [DOI] [PubMed] [Google Scholar]
- 49.Russell JA, Walley KR. Vasopressin and its immune effects in septic shock. J Innate Immun. 2010;2(5):446-460. doi: 10.1159/000318531 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure 1. 48-Hour Cumulative Blood Products for Per-Protocol Analysis
eFigure 2. Vital Signs and Urine Output for Each 8-Hour Period
eTable. Laboratory Values on Postoperative Days 1 and 2
eMethods. Pennsylvania Trauma Outcomes Study Definitions of Complications
Data Sharing Statement