Abstract
Patients with unresectable pancreatic cancer have a poor prognosis. The analysis of prognostic factors before treatment may be helpful in determining the best therapeutic strategies. The aim of the PEACE study is to assess the vascularity of pancreatic malignant tumors using contrast-enhanced harmonic EUS (CEH-EUS) and to clarify the prognostic value of tumor vascularity in patients with locally advanced and metastatic pancreatic cancer. Hereby, we present the protocol of a prospective, nonrandomized, single-arm, multicenter study aiming to assess changes in tumor vascularity using CEH-EUS before and 2 months after treatment initiation in patients with unresectable, locally advanced/metastatic pancreatic cancer and to examine the correlation between vascular changes and treatment response, progression-free survival, and overall survival.
Keywords: Contrast-enhanced endoscopic, endoscopic ultrasound, pancreatic cancer, prognosis, ultrasound
INTRODUCTION
Pancreatic cancer is one of the most lethal and therapeutically resistant malignancies, with a grim prognosis that is attributed to the late clinical presentation and the relative chemoresistance of the disease.[1] At the time of diagnosis, over 80% of patients present with locally advanced or metastatic disease and are, therefore, not suitable for curative resection.[2] There is currently no uniform consensus regarding standard of care for the treatment of unresectable, locally advanced/metastatic pancreatic cancer. Treatment options include chemotherapy alone or induction chemotherapy followed by chemoradiotherapy or stereotactic body radiation therapy (SBRT).[3] However, even with identical therapy regimens, some patients experience improvements in survival and tumor response, whereas other patients only experience inconvenience and increased toxicity. It has been suggested that the burden of treatment should not be added to the suffering of those with advanced pancreatic cancer. Therefore, understanding prognostic factors before treating patients may be helpful in selecting those predicted to have an improved survival and tumor response after treatment.
Studies have shown that angiogenesis is an important factor that influences the prognostic of solid tumors.[4,5,6] Contrast-enhanced (CE) imaging methods can offer detailed information on tumor vascularity. Changes in tumor vascularity under CE ultrasonography (CE-US) were employed for evaluating the effectiveness of chemotherapy. Sofuni et al.[7] used CE-US in patients with unresectable pancreatic cancer treated by chemotherapy. They found that patients with abundant intratumoral blood flow had a significantly better response to treatment, and changes in intratumoral blood flow after treatment were related to prognosis (P = 0.006). On the other hand, Masaki et al.[8] assessed tumor vascularity of pancreatic cancer using CE-US before systemic chemotherapy. They revealed that the median survival was longer in patients who had avascular tumors compared with patients who had vascular tumors.
Contrast-enhanced EUS (CE-EUS) is a new method which allows detailed characterization of focal pancreatic masses.[9] CE-EUS offers high-resolution images of the pancreas that far surpass those achieved by computed tomography (CT), US, or magnetic resonance imaging. CE-EUS can detect intratumoral vessels in the pancreatic lesions.[10] Dedicated contrast-enhanced harmonic EUS (CEH-EUS) technique, based on a low mechanical index, is available in new EUS systems. It allows high-resolution continuous real-time assessment of the microvascularization during the contrast uptake period (real-time perfusion imaging).[11,12] Several research groups already reported the feasibility of CEH-EUS with low mechanical index.[13,14] Quantitative analysis of tumor vascularity can be performed using time-intensity curve (TIC) analysis-derived parameters, obtained from processing CEH-EUS recordings with a commercially available software. TIC analysis is increasingly being recognized as a standardized quantification tool for perfusion characteristics of intra-abdominal tumors.[15] Rapid processing of CEH-EUS recordings allows trained physicians to objectively analyze otherwise qualitative data provided by contrast enhancement techniques.[16,17] Yamashita et al.[10] performed CE-EUS on 39 patients with unresectable pancreatic cancer who were scheduled to undergo chemotherapy. They showed that both progression-free survival and overall survival were significantly longer in patients with intratumoral large vessels (P = 0.037 and P = 0.027, respectively) and that a positive vessel sign was an independent factor associated with longer survival. However, whether low vascular tumors correlate with the chemoresistance and poor prognosis is still unclear.
RATIONALE AND OBJECTIVES
Patients with unresectable pancreatic cancer have an especially poor prognosis and many severe symptoms. The analysis of prognostic factors before treatment may be helpful in selecting appropriate candidates for chemotherapy and in determining treatment strategies. For example, patients who have a poor prognosis may be treated best with only supportive care because of their short survival. Consequently, the main aim of the PEACE study is to assess the vascularity of pancreatic malignant tumors with CEH-EUS and to clarify the prognostic value of tumor vascularity in patients with advanced pancreatic cancer.
Our hypothesis is that tumors with intratumoral vessels have a better prognosis and are chemosensitive. In an orthotopic model of pancreatic cancer, AsPC-1 cells were less sensitive to gemcitabine when cultured under hypoxic conditions compared with cells treated under normoxic conditions.[18] Therefore, it is possible that hypoxic condition in tumor tissue leads to chemoresistance and poor prognosis in patients with pancreatic carcinoma who received systemic chemotherapy.[19]
Moreover, studies have shown that angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumor blood vessels. Chauhan et al.[15] demonstrated that the angiotensin inhibitor losartan reduces stromal collagen and hyaluronan production. Consequently, losartan reduces solid stress in tumors resulting in increased vascular perfusion. Through this physical mechanism, it can improve drug and oxygen delivery to tumors, thereby potentiating chemotherapy and reducing hypoxia in breast and pancreatic cancer models. Accordingly, another aim of our study is to examine the correlation between tumor vascularity and angiotensin inhibitors use in patients using these drugs to control arterial hypertension.
The objectives of the PEACE trial are summarized in Table 1.
Table 1.
PEACE trial objectives
| Primary objective | Secondary objectives |
|---|---|
| To determine the correlation between CEH-EUS parameters before and after treatment and tumor response. Tumor response will be assessed by contrast-enhanced computed tomography, according to the RECIST |
To register CEH-EUS parameters before and after chemotherapy and to describe tumor changes in vascularity after treatment To determine the correlation between CEH-EUS parameters before treatment and overall survival and progression-free survival To determine the correlation between changes in tumor vascularity and PFS and OS To assess quantitative elastography parameters during EUS, before, and after systemic treatment and determine their correlation with overall survival and progression-free survival To examine the correlation between tumor vascularity and angiotensin inhibitors use To compare genomic changes based on whole-exome sequencing and transcriptome sequencing from pre- and post-treatment FNA samples |
TIC: Time-intensity curve, CEH-EUS: Contrast-enhanced harmonic EUS, PFS: Progression-free survival, OS: Overall survival, RECIST: Response evaluation criteria in solid tumors, FNA: Fine needle aspiration
STUDY DESIGN
This is a prospective, nonrandomized, single-arm, interventional, multicenter study aiming to assess the changes in tumor vascularity using CEH-EUS before and 2 months after treatment initiation in patients with unresectable, locally advanced/metastatic pancreatic cancer and to examine the correlation between vascular changes and treatment response, progression-free survival, and overall survival [Figure 1].
Figure 1.
Study design
All patients with a suspicion of pancreatic masses will undergo EUS, including EUS-FNA for confirmation of diagnosis, with sequential elastography (EG)-EUS and CEH-EUS. Inclusion and exclusion criteria are listed in [Table 2]. A positive cytological diagnosis will be taken as a final proof of malignancy of the pancreas mass. The diagnoses obtained by EUS-FNA will be further verified during a clinical follow-up of at least 6 months. Contrast enhanced CT will be performed as pretreatment staging study to assess the diagnosis of pancreatic cancer, local extension of the tumor, and presence of distant and lymph node metastasis. Patients who have received previous chemotherapy or radiotherapy will be excluded from the analysis.
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age 18–90-year-old men or women Signed informed consent for CH-EUS, EH-EUS, and FNA The diagnosis of pancreatic cancer histologically confirmed by FNA/clinical follow-up Unresectable, locally advanced, and/or metastatic disease Both pancreatic adenocarcinomas and pancreatic neuroendocrine tumors were included |
Previous chemotherapy or radiotherapy Resectable pancreatic tumors |
CH-EUS: Contrast-enhanced EUS
Patients with a confirmed diagnosis of pancreatic cancer (both adenocarcinomas and neuroendocrine tumors were included) will undergo systemic treatment. Selection of the specific treatment regimen will be according to the individual physicians’ choice.
Two months after the first course of treatment, CT and EUS (with sequential EG-EUS, CEH-EUS, and EUS-FNA) will be repeated. CT will be performed to evaluate the tumor response. Tumor response will be assessed according to the response evaluation criteria in solid tumors (RECIST).
The patients will be followed up for at least 6 months through clinical examination, biological examinations, and transabdominal ultrasound, eventually with a repeat spiral CT/EUS after 6 months.
METHODS
All patients with a suspicion of pancreatic masses will undergo EUS and CT before and 2 months after the first course of chemotherapy.
EUS and EUS-FNA
Protocol of EUS with EUS-FNA will include linear EUS instruments with complete examinations of the pancreas. Tumor characteristics (echogenicity, echostructure, and size) will be described as well as the presence/absence of power Doppler signals. EUS-FNA will be performed in all pancreatic masses with at least four passes in the absence of an on-site cytopathologist.
Contrast-enhanced-harmonic EUS procedure
A two-panel image with the usual conventional gray scale B-mode EUS image on the right side and with the contrast harmonic image on the left side will be used, according to the preestablished presets. The starting point of the timer will be considered the moment of intravenous contrast injection (Sonovue 4.8 mL).
CEH-EUS will be performed during usual EUS examinations, with the whole movie (T0-T120s) recorded in a DICOM format on the embedded HDD of the ultrasound system, for later analysis.
A low mechanical index procedure (dynamic wideband contrast harmonic imaging mode) will be used, with a mechanical index of 0.2 and corresponding powers. The following presettings will be used in all centers: contrast mode dCHI-W, WPI-R/P (resolution/penetration for superficial vs. deep structures), mechanical index (preferred MI of 0.2 for Pentax-Hitachi and 0.3 for Olympus-Aloka), MI gray scale (0.03), gray map 4, AGC 0, R-filter C, persistence 2, dynamic range 50, B-color 21, smoothing 3, and gamma curve linear.
The contrast agents used for CEH-EUS is Sonovue®, which consists of phospholipids stabilized bubbles of sulfur hexafluoride (SF6).[16] Sonovue® is isotonic, stable, and resistant to pressure, with a viscosity similar to blood. It does not diffuse into the extravascular compartment remaining within the blood vessels until the gas dissolves and is eliminated in the expired air (blood pool contrast agent).[17] The safety profile of Sonovue® showed a very low incidence of side effects; it is not nephrotoxic and the incidence of severe hypersensitivity is similar to other magnetic resonance imaging contrast agents. Sonovue® is approved for clinical use in the European Union countries. The blood supply of the pancreas is entirely arterial, making CH examinations feasible and readily available. Based on the European Federation Societies of Ultrasound in Medicine and Biology Guidelines and Recommendations, updated in 2008, two phases were defined for CE-US and CE-EUS of the pancreas: an early/arterial phase (starting from 10 to 30 s) and a venous/late phase (from 30 to 120 s).[20]
To minimize human bias, the processing and computer analysis of the digital movies will be performed within the coordinating IT center, with all programmers and statisticians being blinded to the clinical, imaging, and pathological data. Offline analysis of TICs will be performed using Vue-Box, which yields the following quantitative parameters: peak enhancement, wash-in area under the curve (Wi-AUC), rise time, mean transit time (mTT), time to peak (TTP), wash-in rate (WiR), and wash-in perfusion index (WiPI). The software also provides referenced values (expressed in percentages), aligning the set of values for the tumors’ regions of interest (ROI) to the parenchymal ones.
EG-EUS procedure
EG-EUS will be performed during usual EUS examinations, before, and 2 months after the first course of treatment, with two movies of 10 s recorded on the embedded HDD to minimize variability and to increase repeatability of acquisition.
A two-panel image with the usual conventional gray scale B-mode EUS image on the right side and with the EG image on the left side will be used. The ROI for EUS-EG is preferably larger than the focal mass (approximately 50%–50%), to include the surrounding structures. If the focal mass is larger than 3 cm, part of the mass will be included in the ROI, as well as the surrounding structures (preferably avoiding large vessels). Very large ROI for the EG calculations will be avoided due to the appearance of side artifacts.
The following presettings will be used in all centers: EG color map 1, frame rejection 2, noise rejection 2, persistence 3, dynamic rage 4, smoothing 2, and blend 50%.
Strain ratio (SR) and strain histogram will be measured, with three measurements made and recorded on the embedded HDD. For SR, the reference area should be placed at the same level with the lesion, if possible.
Contrast-enhanced CT
Contrast-enhanced CT will be obtained before treatment to assess the local extension of the tumor, the presence of lymph nodes, and distant metastases.
A template will be used to report the imaging results [Appendix 1]. It includes morphologic, arterial, venous, and extrapancreatic evaluations. The morphologic evaluation includes the documentation of tumor appearance, size, and location, as well as the presence of narrowing or abrupt cutoff of pancreatic duct or biliary tree. The arterial evaluation includes the assessment of the celiac axis, the superior mesenteric artery, and the common hepatic artery. Arterial variations should be noted, such as vessel contact, solid tissue contact, hazy attenuation or stranding contact, and focal vessel narrowing or contour irregularity. Venous evaluation includes the assessment of the portal vein and the superior mesenteric vein. Documentation of thrombus within the vein and venous collaterals should also be done. The extrapancreatic evaluation includes the documentation of liver lesions, peritoneal or omental nodules, ascites, suspicious lymph nodes, and other present extrapancreatic disease sites.
Contrast enhanced CT will be performed 2 months after the first course of treatment, using the same template, to evaluate the tumor response. Tumor response will be assessed according to the RECISTs. Based on RECIST guidelines, complete response (CR) is defined as the complete disappearance of the tumor, partial response (PR) is defined as ≥30% decrease in the longest diameter (LD), progressive disease (PD) is defined as ≥20% increase in LD, and stable disease (SD) is defined as a decrease or increase less than PR or PD based on the anatomic assessment. Patients with CR or PR are defined as responders, whereas those with PD or SD are defined as nonresponders.[21]
Treatment
Depending on the performance status, monosystemic or combination systemic chemotherapy may be considered as initial therapy for patients with unresectable, locally advanced/metastatic pancreatic adenocarcinoma. If patients present with poorly controlled pain or local invasion with bleeding, starting with upfront chemoradiation therapy or SBRT can be an option. Selection of treatment will be according to the individual physicians’ choice. It will be continued until disease progression or unacceptable toxicity.
Moreover, the selection of the systemic treatment for unresectable and/or metastatic neuroendocrine tumors of the pancreas will also be according to the individual physicians’ choice.
Data collection
For each patient, the following information will be recorded and uploaded to http://oncobase.umfcv.ro/: age, gender, primary tumor location, primary tumor size, tumor status (metastatic or locally advanced), site of metastasis, serum carcinoembryonic antigen level, serum carbohydrate antigen 19-9 level, prior biliary drainage (presence or absence), antitumoral agent (chemotherapy regimen), angiotensin inhibitors use (drug, dose), parameters of the pancreatic cancer CT reporting template, EUS, CH-EUS, and EG-EUS parameters (echogenicity, echostructure, size, presence/absence of power Doppler signals, SR, SH, PE, Wi-AUC, RT, mTT, TTP, WiR, and WiPI).
Statistical analysis
The progression-free survival and overall survival will be measured from the 1st day of chemotherapy to the date of PD and death, respectively. The statistical significance of the correlation between CEH-EUS and EG-EUS parameters and clinicopathologic parameters will be assessed with the Mann–Whitney U-test, the Kruskal–Wallis test, or the Spearman rank correlation test. PFS and OS will be estimated using the Kaplan–Meier method, and statistical comparisons will be made with the log-rank test. Univariate and multivariate analyses will be performed to determine significant variables related to prognosis with a Cox proportional hazards model. All P values will be obtained with a two-tailed statistical analysis, and P < 0.05 will be considered statistically significant.
Supplementary materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
This work was financially supported by a grant of the Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
Conflicts of interest
There are no conflicts of interest.
APPENDIX 1
CT Pancreatic Cancer

Omnipaque-350 IV bolus contrast administration: mL.
Axial 2.5 mm images and sagittal, coronal, and oblique sagittal/coronal images were obtained in the late arterial and portal-venous phases.

Clinical information
Pancreatic mass.
Comparison

Findings PANCREAS
Primary tumor: cm
cm mass in the
mass in the  (series,image).
(series,image).
Pancreatic duct:  mm.
mm.

MESENTERIC ARTERIES
Arterial anatomy 
Arterial tumor abutment or encasement: 
MESENTERIC VEINS
Superior mesenteric vein (SMV) first jejunal branch  to SMA.
to SMA.
SMV terminates as 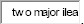
Inferior mesenteric vein (IMV) drains into the 
Venous tumor abutment or encasement:

Portal venous system: 
Inferior vena cava (IVC): 
HEPATOBILIARY SYSTEM
Focal liver lesions: 
Biliary tree  CBD mm.
CBD mm.
Gallbladder: 
LOCOREGIONAL SPREAD
Lymph nodes: 
Peritoneum: 
Omentum: 
Ascites: 
OTHER FINDINGS
Stomach, small bowel, and large bowel: 
Genitourinary system: 
Adrenal glands: 
Spleen: 
Lower chest: 
Bones: 
SUMMARY - RECIST 1.1
Primary Tumor:  cm (largest dimension), series , image .
cm (largest dimension), series , image .
Lymph Node #1: mm (largest short axis), series , image .
Lymph Node #2: mm (largest short axis), series , image .
Liver Lesion #1:  cm (largest dimension), segment
cm (largest dimension), segment  , image .
, image .
Liver Lesion #2:  cm (largest dimension), segment
cm (largest dimension), segment  , image .
, image .
Conclusion
1. Pancreatic mass.
Location: 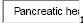 mass
mass
size:  cm,
cm,  .
.
2. Metastatic disease: 
3. Adenopathy: 
4. Vascular involvement: 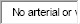
5. 
REFERENCES
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Ralph H. Hruban. Pancreatic Cancer. In World Cancer Report 2014. In: Stewart B, Wild CP, editors. IARC, Lyon, France: 2014. pp. 413–6. [Google Scholar]
- 3.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–8. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda N, Adachi M, Taki T, et al. Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer. 1999;79:1553–63. doi: 10.1038/sj.bjc.6690248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sofuni A, Itoi T, Itokawa F, et al. Usefulness of contrast-enhanced ultrasonography in determining treatment efficacy and outcome after pancreatic cancer chemotherapy. World J Gastroenterol. 2008;14:7183–91. doi: 10.3748/wjg.14.7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masaki T, Ohkawa S, Amano A, et al. Noninvasive assessment of tumor vascularity by contrast-enhanced ultrasonography and the prognosis of patients with nonresectable pancreatic carcinoma. Cancer. 2005;103:1026–35. doi: 10.1002/cncr.20875. [DOI] [PubMed] [Google Scholar]
- 9.Saftoiu A, Vilmann P, Bhutani MS. The role of contrast-enhanced endoscopic ultrasound in pancreatic adenocarcinoma. Endosc Ultrasound. 2016;5:368–72. doi: 10.4103/2303-9027.190932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamashita Y, Ueda K, Itonaga M, et al. Tumor vessel depiction with contrast-enhanced endoscopic ultrasonography predicts efficacy of chemotherapy in pancreatic cancer. Pancreas. 2013;42:990–5. doi: 10.1097/MPA.0b013e31827fe94c. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich CF, Ignee A, Frey H. Contrast-enhanced endoscopic ultrasound with low mechanical index: A new technique. Z Gastroenterol. 2005;43:1219–23. doi: 10.1055/s-2005-858662. [DOI] [PubMed] [Google Scholar]
- 12.Napoleon B, Alvarez-Sanchez MV, Gincoul R, et al. Contrast-enhanced harmonic endoscopic ultrasound in solid lesions of the pancreas: Results of a pilot study. Endoscopy. 2010;42:564–70. doi: 10.1055/s-0030-1255537. [DOI] [PubMed] [Google Scholar]
- 13.Fusaroli P, Spada A, Mancino MG, et al. Contrast harmonic echo-endoscopic ultrasound improves accuracy in diagnosis of solid pancreatic masses. Clin Gastroenterol Hepatol. 2010;8:629–34. e2. doi: 10.1016/j.cgh.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Seicean A, Badea R, Stan-Iuga R, et al. Quantitative contrast-enhanced harmonic endoscopic ultrasonography for the discrimination of solid pancreatic masses. Ultraschall Med. 2010;31:571–6. doi: 10.1055/s-0029-1245833. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan VP, Martin JD, Liu H, et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seicean A, Badea R, Stan-Iuga R, et al. The added value of real-time harmonics contrast-enhanced endoscopic ultrasonography for the characterisation of pancreatic diseases in routine practice. J Gastrointestin Liver Dis. 2010;19:99–104. [PubMed] [Google Scholar]
- 17.Sanchez MV, Varadarajulu S, Napoleon B. EUS contrast agents: What is available, how do they work, and are they effective? Gastrointest Endosc. 2009;69:S71–7. doi: 10.1016/j.gie.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Galardi E, Duquette M, et al. Antiangiogenic treatment with three thrombospondin-1 type 1 repeats versus gemcitabine in an orthotopic human pancreatic cancer model. Clin Cancer Res. 2005;11:5622–30. doi: 10.1158/1078-0432.CCR-05-0459. [DOI] [PubMed] [Google Scholar]
- 19.Bao B, Ali S, Ahmad A, et al. Hypoxia-induced aggressiveness of pancreatic cancer cells is due to increased expression of VEGF, IL-6 and miR-21, which can be attenuated by CDF treatment. PLoS One. 2012;7:e50165. doi: 10.1371/journal.pone.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) – Update 2008. Ultraschall Med. 2008;29:28–44. doi: 10.1055/s-2007-963785. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]


