Abstract
Background and Objectives:
EUS training is recognized to have a substantial learning curve. To date, few dedicated training programs for EUS have been described. The swine model has been highlighted as a realistic tool to enhance EUS training. Studies extensively describing EUS swine anatomy are lacking in the current literature. The article aims to describe both radial and linear EUS pancreatobiliary swine anatomy.
Materials and Methods:
Four live pigs were endoscoped under general anesthesia using both radial and linear array echoendoscopes. Relevant images and videos were recorded.
Results:
It was possible to effectively image aorta, crus of the diaphragm, celiac trunk, superior mesenteric artery, pancreas, common bile duct, gallbladder, portal vein, kidneys, spleen, and hepatic hilum. Images were comparable to human EUS findings, with some remarkable differences. The pancreas was relatively larger in swine and in contrast to humans has three segments (duodenal, splenic, and connecting lobe).
Conclusions:
The swine model was a highly realistic teaching model for linear and radial pancreatobiliary EUS and a useful tool for training in the setting of in vivo hands-on sessions.
Keywords: Animal laboratory, EUS, EUS simulators, swine model, training
INTRODUCTION
EUS is complimentary to other cross-sectional imaging modalities, in that it allows small lesions to be imaged and it provides an opportunity to obtain tissue for lesion confirmation by EUS-FNA. Furthermore, therapeutic applications of interventional EUS are growing and are increasingly becoming a part of standard clinical practice.
EUS training is recognized to have a substantial learning curve. To date, few dedicated training programs for EUS have been described or implemented,[1,2,3] and there is a growing interest in the most efficient ways to enhance learning and achieve independent practice. To address the current training gap of EUS and the need for structured training, the World Endoscopy Organization (WEO) established the first WEO International School of EUS (WISE) in 2018. WISE is a state-of-the-art EUS training program focused on teaching a specialized group of young doctors with intermediate EUS experience by means of lectures, live case observation, web conferences sharing routine cases and difficulties, and hands-on training sessions using phantoms and live pigs.
The swine model has been highlighted as a realistic tool to enhance EUS training.[4] However, a detailed description of EUS swine anatomy and description of a standard EUS imaging approach are lacking in the current literature.
This article aimed to describe both radial and linear EUS pancreatobiliary swine anatomy and provide a training guide for future endoscopists using the swine model.
MATERIALS AND METHODS
Setting and study design
During the animal laboratory experience of the WISE group, four mini pigs were scoped via an overtube while under general anesthesia by six endosonographers [Table 1] over six sessions lasting 2 h.
Table 1.
Characteristics of the endosonographers of the WISE group
| Endosonographer’s characteristics | n |
|---|---|
| Age (years), mean (range) | 34 (31–37) |
| EUS procedures without supervision, mean (range) | 261 (125–450) |
Animal models
Four mini pigs (mean weight 25 kg) were used. All pigs were fasted for 24 h before examination and 3 h after the procedure. The pigs were sedated with general anesthesia. Induction was achieved using a combination of tiletamine and zolazepam (5 mg/kg, i.m.) (Zoletil 50; Virbac, Korea) and xylazine hydrochloride (1.5–2 mg/kg, i.m.) (Rompun; Bayer, Korea). 0.05 mg/kg of atropine was also given i.m. The animals were then intubated and 2%–2.5% isoflurane (Forane; JW Pharmaceutical, Korea) was administered throughout the procedure, and cardiopulmonary parameters were monitored.
Equipment
Both radial (GF-U260, Olympus, Tokyo, Japan; EG-580UR, Fujifilm, Tokyo, Japan) and linear (GF-UCT260, Olympus, Tokyo, Japan; EG-580UT, Fujifilm, Tokyo, Japan) array echoendoscopes (fully dedicated for exclusive use in swine model) were used for the delineation of pancreatobiliary anatomy. Echoprocessors used were EU-ME2 Premier Plus (Olympus, Tokyo, Japan) and SU-8000 (Fujifilm, Tokyo, Japan), and the videos were recorded by an external capture card (iGrabber nano, MyGica, China).
Ethical approval
Approval of the Institutional Animal Care and Use Committee was obtained before initiation of the study.
RESULTS
It was possible to image aorta, crus of the diaphragm, celiac trunk (CT), superior mesenteric artery (SMA), pancreas, common bile duct (CBD), gallbladder, portal vein (PV), kidneys, spleen, and hepatic hilum effectively by both the radial and linear echoendoscopes.
Swine pancreatobiliary anatomy
The swine pancreas is divided into three lobes [Figure 1]: splenic lobe, duodenal lobe, and connecting lobe. The splenic lobe corresponds to the body and tail of the human pancreas. The duodenal lobe is C-shaped surrounding with respect to the duodenum and corresponds to the head of the pancreas in the human. The connecting lobe is the lower extension of the duodenal lobe near the PV and corresponds to the uncinate process of the human pancreas. There is also a thin anatomical connection (not easily visualized during EUS examination) between the body of the pancreas and the connecting lobe named the “bridge.”[5] For simplification, from now on, we further refer to swine pancreatic lobes similarly to human pancreas using the terms “body-tail,≵ “head,≵ and “uncinate process.”
Figure 1.
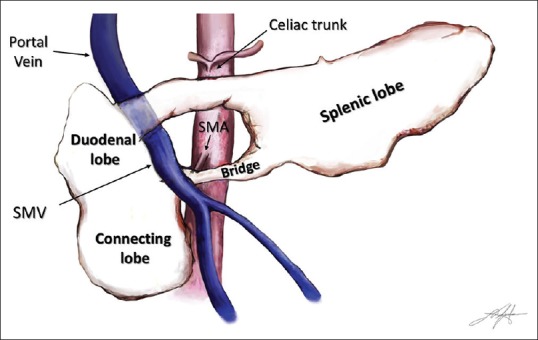
Schematic representation of the swine pancreas and its vascular relations. SMV: Superior mesenteric vein; SMA: Superior mesenteric artery
The PV/SMV passes through a pancreatic ring delineated by the body (superiorly), the head (laterally), the uncinate process (inferiorly), and the “bridge≵ (medially).
Although the pancreatic ductal anatomy is highly variable, it has been classified into four subtypes according to the presence (and its related morphology) of a connection between the splenic and connecting lobe.[2] Actually, in all variants, the main pancreatic duct (PD) drains into the minor papilla, located about 20 cm distally in the descending duodenum [Figure 2].[2]
Figure 2.
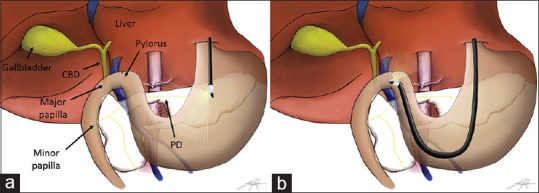
Schematic representation of the swine pancreatobiliary system and EUS scope positions. (a) Examination from the gastroesophageal junction. (b) Examination from the duodenal bulb. Common bile duct drains into the major papilla. Main pancreatic duct (yellow dashed line) drains into the minor papilla located about 20 cm distally in the descending duodenum
EUS examination
EUS examination was performed from two stations: (1) stomach, starting just below the gastroesophageal (GE) junction [Figure 2a], and (2) duodenal bulb [Figure 2b].
Most swine have a Zenker's diverticulum so frequent reintubation, particularly with an oblique viewing EUS endoscope, risks perforation. We then recommend placement of an overtube immediately before each session.
Passing the EUS scope into the second portion of the duodenum was cumbersome in some pigs due to the acute angulation of the superior duodenal angle. Because of this, we considered the descending duodenum unsuitable as a station for EUS examination in the swine model.
Radial examination
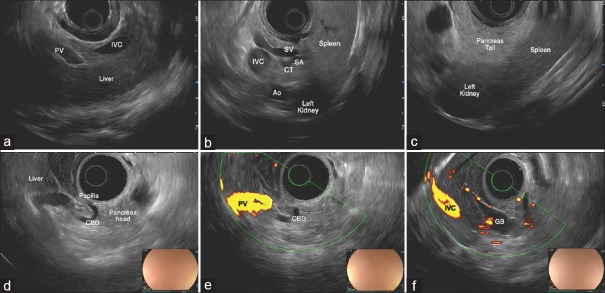
Scanning from the GE junction, a portion of the liver can be visualized along with inferior vena cava (IVC) and PV [Figure 3a]. With clockwise torque, the hilum of the liver comes into view along with the PV, common hepatic artery (CHA), and CBD. If the scope is advanced with gentle clockwise–counterclockwise torque, the PV can be traced to the portal confluence. A portion of the pancreatic body is seen between the transducer and the splenic vein [Figure 3b]. At this level, splenic artery is visualized adjacent to the splenic vein and, by torquing back and forth, can be traced toward the CT and aorta. With the pancreatic body and the splenic vein into view, withdrawing the scope and torquing clockwise will take you toward the tail of the pancreas, which is visualized between the left kidney and the spleen [Figure 3c and Video 1].
Figure 3.
EUS examination of the swine pancreatobiliary system with a radial array echoendoscope. (a) Liver seen just below the gastroesophageal junction. (b) View from the stomach of the pancreatic body (between the transducer and the SV). SA is visible at its origin from the CT. IVC and aorta are also seen. (c) Splenorenal angle delimiting the tail of the pancreas. (d) “U-shaped” CBD wholly seen from the duodenal bulb. (e) Hepatic hilum seen from the bulb. (f) Gallbladder seen from the bulb. PV: Portal vein, IVC: Inferior vena cava, HA: Hepatic artery, CBD: Common bile duct, PVC: Portal vein confluence, SV: Splenic vein, SA: Splenic artery, GB: Gallbladder
Under endoscopic view, the stomach is J shaped. On passing through the pylorus, the major papilla is visualized on the posterosuperior duodenal wall. With the transducer at the level of the papilla, the CBD can be visualized in a “U-shaped” manner, within the head of the pancreas [Figure 3d]. Withdrawing the scope with counterclockwise, torque allows the CBD to be traced toward the hepatic hilum [Figure 3e]. From this position, slightly withdrawing the scope with minute back and forth movements, the gallbladder comes into view [Figure 3f].
Linear Examination
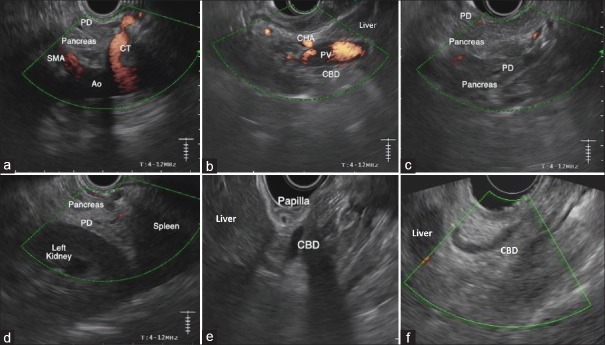
From the GE junction, the aorta is visualized. The swine aorta is smaller than would be expected in humans, but is found by similar maneuvers of almost 180° rotation to visualize the retroperitoneum. The vessels can be seen arising from the aorta: CT is located proximally and the SMA distally. A portion of the pancreatic body is seen between the CT and the SMA [Figure 4a]. With slight withdrawal of the scope and following the CHA from the CT, the hepatic hilum can be seen; CHA is visualized closer to the transducer, PV in the middle, and CBD below [Figure 4b]. From this position with counterclockwise rotation, the CBD can be traced as a thin tubular anechoic structure going down to the duodenal bulb, which is identified as a round structure with air inside located just below the hepatic hilum. Tracing down the PV from the hepatic hilum, the pancreatic body comes into view, closer to the transducer. The PD is also often visualized from this position and can then be traced toward the head of the pancreas by advancing the scope and torquing counterclockwise. At this level, the uncinate process can be seen distally. The connecting lobe in swine has a similar hypoechoic echotexture to the uncinate or dorsal pancreas in humans [Figure 4c]. In some swine, the PV is visible between the body and the uncinate process of the pancreas, but the PV seems to be the easiest structure to locate by tracing it from the hepatic hilum to the confluence. The PD within the uncinate process is visible, but usually very thin. In some cases, it can be traced to its connection with the main PD within the pancreatic body. From the pancreatic body, slight withdrawal of the scope and gentle clockwise torque, keeping the PD of the body into view, will take you over the left kidney and the spleen (splenorenal angle) with the tail of the pancreas in between [Figure 4d]. If the scope is advanced, pushing up on the up-down wheel, the left kidney comes in a more central view on the screen. From this position, torquing counterclockwise the aorta, IVC, and the right kidney are sequentially visualized [Video 2].
Figure 4.
EUS examination of the swine pancreatobiliary system with a linear array echoendoscope. (a) Aorta and take off of the first abdominal branches seen from the stomach, just below the gastroesophageal junction. The PD of the body of the pancreas (splenic lobe) is visible. (b) Hepatic hilum seen from the stomach. (c) Body of the pancreas (splenic lobe) and PD seen from the stomach, closer to the transducer. Distally, the connecting lobe (corresponding to the uncinate process in humans) is visible with its more hypoechoic echotexture. (d) Splenorenal angle delimiting the tail (distal part of the splenic lobe) of the pancreas. (e) CBD and major papilla from the duodenal bulb. Major papilla is seen as a prominent structure arising from the wall of the bulb. (f) “U-shaped” CBD traced from the duodenal bulb. CT: Celiak trunk, SMA: Superior mesenteric artery, PD: Pancreatic duct, CHA: Common hepatic artery, PV: Portal vein, CBD: Common bile duct
On traversing the pylorus, the papilla is immediately visible as a prominent structure arising from the wall of the duodenal bulb. Placing the transducer so it faces the posterosuperior duodenal wall, the hepatic hilum comes into the endosonographic view. From this position, the “U-shaped” CBD can be seen and traced from the hepatic hilum to the papilla with clockwise rotation [Figure 4e and f]. The cystic duct can be followed from the CBD to the gallbladder. From this position, both endoluminal saline injection and/or limited balloon inflation can be useful to aid better coupling and to displace the endoluminal air.
FNA
EUS-guided FNA is also feasible in the pig model. During the study, FNA was attempted from a range of sites in the pancreas and liver. The procedure was performed successfully using 22G FNA needles (EZ Shot 3 Plus, Olympus, Tokyo, Japan). As in humans, the needle could be seen advancing into the organ and moving in real time. Confirmation of a successful FNA was demonstrated by the presence of pancreatic or liver tissue on on-site cytopathological examination.
DISCUSSION
EUS is commonly used in the diagnosis and management of pancreatobiliary conditions, with a growing number of therapeutic applications including drainage of pancreatic fluid collections, tumor diagnosis and therapy, and EUS-guided biliary drainage.[6,7,8]
However, EUS is recognized to be an operator-dependent technique with a substantial learning curve. Defined training methods and programs are lacking, and the number of procedures to reach competency is debated.[9,10] Recognizing that EUS involves both the acquisition of technical and the cognitive skills for image interpretation, some groups have suggested that there is a move away from absolute numbers of procedures to other performance metrics to judge competency for independent practice.[7] The ASGE have defined a number of quality indicators in EUS including being able to document relevant structures, stage cancers, evaluate subepithelial lesions, and perform EUS-FNA.[11] The opportunity to practice each of these metrics is not possible in every case, and exposure can be dependent on the center caseload. Therefore, having animal models, which are highly representative of a human EUS examination, is invaluable for training.
Live pigs for EUS examination should be <30 kg because the larger is the pig, the longer esophagus and stomach are, making very difficult to reach the duodenum for proper examination of the pancreas.
During the training pathway, we did not stipulate any specific sequence of the use of different scopes (e.g., radial scope before linear scope or vice versa). In fact, we believe that interpretation of radial and linear images are largely independent and that endosonographers must develop skills in each technique separately.[12]
In this study, we found that the images obtained in swine were comparable to human EUS findings. There were some remarkable differences: endoscopically, pigs tended to have a J-shaped stomach, with the major papilla arising from the bulb. On imaging the aorta by EUS in swine, it is notably smaller than in humans and best visualized from the stomach just below the GE junction, rather than followed down from the esophagus as would routinely be done in humans. The liver and particularly the spleen are substantially larger in swine, overlapping anteriorly. In pigs, the CBD is very short and “U-shaped” compared to humans and without pathology is similarly thin but as in humans can be successfully traced to the major papilla by both radial and linear echoendoscopes from the stomach and duodenal bulb.
During the linear examination, differently than in humans, from the stomach, CBD goes vertically from the hilum down to the duodenal bulb, which is located very close to the liver. Similarly, from the bulb, the CBD can be traced from the hepatic hilum up to the major papilla in a curvilinear path (U-shape). From this position, the major papilla is seen very close to the liver and close to the transducer.
In linear EUS, the pancreas was easier to locate by tracing the PV from the liver, rather than following the coeliac axis, which is often done in humans.
In swine, the pancreas is relatively larger and in contrast to humans has three segments (duodenal lobe, splenic lobe, and the connecting lobe) [Figure 1]. However, the connecting lobe was approximated well to the uncinate, the duodenal lobe to the head, and the splenic lobe to the body and tail of the pancreas in humans.
In pigs, the anatomical structures are very close one to another compared to humans, so movements of the scope often need to be even more precise, gentle, and slow, which is a good exercise for trainees.
To differentiate the organs is just a part of a standard EUS pancreatobiliary examination and further skills need to be trained. Actually, we believe that learning how to confidently trace structures, independent of their nature (vessel, duct and parenchyma), and to identify their origin is vital training for the intermediate endosonographer. This is the basis of good EUS technique and allows the endosonographer, when faced with altered anatomy (as in the swine model), to confidently identify structures and their relationship to adjacent organs. These skills are immediately applicable to being able to provide a confident screening or staging technique for pancreatobiliary disease. Furthermore, the swine model provides excellent haptic feedback that resembles what is experienced in humans, improving the realism of the examination and training experience which is significantly superior to other phantoms or computer-based simulator models that have been developed to date.[13,14,15]
CONCLUSIONS
The swine model was a very useful aid for teaching intermediate endosonographers. Swine have similar anatomy to humans and can be imaged with standard adult echoendoscopes. The swine model has representative tactile feel and allows repeated opportunities for tracing vessels and structures, which is the backbone of routine EUS practice. This model should be considered for training and up-skilling courses in EUS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.eusjournal.com
Acknowledgment
We thank Federico Amata for graphic assistance.
REFERENCES
- 1.Wang MH, Dy F, Vu VK, et al. Structured endoscopic ultrasonography (EUS) training program improved knowledge and skills of trainees: Results from the Asian EUS group. Dig Endosc. 2015;27:687–91. doi: 10.1111/den.12458. [DOI] [PubMed] [Google Scholar]
- 2.Cho CM. Training in endoscopy: Endoscopic ultrasound. Clin Endosc. 2017;50:340–4. doi: 10.5946/ce.2017.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthet M, Gasmi M, Boustiere C, et al. EUS training in a live pig model: Does it improve echo endoscope hands-on and trainee competence? Endoscopy. 2007;39:535–9. doi: 10.1055/s-2007-966336. [DOI] [PubMed] [Google Scholar]
- 4.Bhutani MS, Hoffman BJ, Hawes RH. A swine model for teaching endoscopic ultrasound (EUS) imaging and intervention under EUS guidance. Endoscopy. 1998;30:605–9. doi: 10.1055/s-2007-1001364. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer J, Scott WE, 3rd, Weegman BP, et al. Pig pancreas anatomy: Implications for pancreas procurement, preservation, and islet isolation. Transplantation. 2008;86:1503–10. doi: 10.1097/TP.0b013e31818bfda1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan SI, Rajan E, et al. ASGE Standards of Practice Committee. Role of EUS. Gastrointest Endosc. 2007;66:425–34. doi: 10.1016/j.gie.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Piraka C, Shah RJ, Fukami N. EUS-guided transesophageal, transgastric, and transcolonic drainage of intra-abdominal fluid collections and abscesses. Gastrointest Endosc. 2009;70:786–92. doi: 10.1016/j.gie.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 8.Ergun M, Aouattah T, Gillain C, et al. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: Long-term outcome. Endoscopy. 2011;43:518–25. doi: 10.1055/s-0030-1256333. [DOI] [PubMed] [Google Scholar]
- 9.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 10.Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: Implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013;77:558–65. doi: 10.1016/j.gie.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Wani S, Wallace MB, Cohen J, et al. Quality indicators for EUS. Gastrointest Endosc. 2015;81:67–80. doi: 10.1016/j.gie.2014.07.054. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Liu Y, Pan P, et al. Prior radial-scanning endoscopic ultrasonography training did not contribute to subsequent linear-array endoscopic ultrasonography study performance in the stomach of a porcine model. Gut Liver. 2015;9:353–7. doi: 10.5009/gnl13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerson LB. Evidence-based assessment of endoscopic simulators for training. Gastrointest Endosc Clin N Am. 2006;16:489–509. doi: 10.1016/j.giec.2006.03.015. vii-viii. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf TE, Matthes K, Lee Y, et al. Evaluation of the EASIE-R simulator for the training of basic and advanced EUS. Gastrointest Endosc. 2009;69:S264. [Google Scholar]
- 15.Kim GH, Bang SJ, Hwang JH. Learning models for endoscopic ultrasonography in gastrointestinal endoscopy. World J Gastroenterol. 2015;21:5176–82. doi: 10.3748/wjg.v21.i17.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.