Abstract
Background and Objective:
EUS-guided fine-needle biopsy has become the standard for tissue sampling. A new 20G ProCore™ (PC) needle has been developed to overcome the limitations of tissue acquisition of the smaller needles (22G, 25G) and the rigidity of the larger one (19G). The aim of this study is to assess the performance of the 20G PC needle.
Materials and Methods:
Patients who underwent EUS-guided tissue acquisition with the 20G PC needle of pancreatic and extra-pancreatic mass lesions were retrospectively identified at three Italian centers (Bologna, Fermo, and Palermo). Diagnostic adequacy, accuracy, and tissue core acquisition were the outcome measures. All the cases were performed without rapid on-site evaluation.
Results:
A total of 384 patients with pancreatic (62.2%) and extra-pancreatic lesions were included in the study. For pancreatic lesions, adequacy, accuracy, sensitivity, and specificity were 92.4%, 91.5%, 90.8%, and 100%, respectively, with a number needed to misdiagnose (NNM) of 11.8. The tissue core was obtained in 72% of cases. Transduodenal approach was performed in 150 pancreatic lesions; adequacy, accuracy, and tissue core acquisition were 88.7%, 90%, and 66%, respectively (NNM 10). For extrapancreatic lesions, adequacy, accuracy, sensitivity, specificity, and tissue core sampling were 95.3%, 95.3%, 92.6%, 100%, and 84.5% (NNM 21.3).
Conclusions:
The 20G PC needle showed high diagnostic adequacy and accuracy, regardless the access route.
Keywords: Cancer, EUS, fine-needle biopsy, pancreas, ProCore needle
INTRODUCTION
Over the past two decades, EUS-FNA has become an indispensable method for obtaining tissue samples from gastrointestinal and peri-intestinal tract lesions, including pancreatic, lymph node, and subepithelial lesions (SELs).[1,2,3,4,5,6] The technique is used to characterize both malignant and nonmalignant lesions,[7] and its diagnostic utility has been largely established. Despite its widespread use, EUS-FNA has several limitations. Its diagnostic sensitivity depends on various factors, such as the availability of a dedicated cytopathologist for rapid on-site evaluation (ROSE), the ability and experience of the endosonographer, the location of the lesions and finally, the needle type used for tissue sampling.[8,9] Moreover, the cytological sample is not always adequate. Lack of histological architecture compromises the analysis aimed at diagnosis, differentiation, invasiveness, and immunohistochemical characterization, particularly important in the case of stromal tumors and lymphomas.[7,10,11] Given the advent of molecular profiling and personalized oncologic therapies, it has become crucial having a whole histologic sample available (fine-needle biopsy [FNB]) for evaluating the molecular markers expression, which ultimately offers the patient the best available treatment.[12,13]
In 2011, in the attempt to overcome the limitations of the 19G Trucut needle,[14] the ProCore™ (PC) needle (Cook Endoscopy Inc., Limerick, Ireland) was introduced, which available in different sizes (i.e., 19G, 22G, and 25G).[15] The main feature of this needle was the presence of a variable length size window, located at different distance from the tip of the needle depending on its size, which had a reverse bevel to engage and cut the tissue in which the needle was put.
Based on the best available evidence, PC needles showed high diagnostic accuracy, regardless the size.[15,16,17,18] However, the 19G PC needle appears to be the best in the histological tissue acquisition, mainly in case of solid pancreatic lesions, with a significantly lower number of passes required to obtain a diagnosis.[9,19] Moreover, in a recent study by Fabbri et al.,[20] it has been demonstrated that the accuracy and adequacy of EUS-guided tissue acquisition with ROSE were comparable to those obtained in the absence of ROSE using the PC needles, as well as obtaining tissue core. This makes the device a valid alternative when a dedicated cytopathologist is not available, leading to a potential reduction of costs and procedure time without affecting the quality of patient care. In addition, the availability of flexible 19G nitinol needle suggested the use of this needle size also for duodenal accessible lesions, the most difficult sampling position. However, the performance of flexible 19G needles seems still suboptimal for obtaining transduodenal tissue suitable for histological examination reporting an overall diagnostic accuracy of only 73.6%.[21]
Recently, Cook Medical has developed a new needle size, the 20G PC needle, which is expected to be a balanced compromise between flexibility and facility of use proper of the smallest needles, and quality of the tissue sampling, typical of the larger needle, providing a new tool to accurately target lesions, regardless size and location.
The primary aim of the current study was to evaluate the diagnostic performance of the EUS-FNB using the 20G PC needle in patients with pancreatic and extra-pancreatic lesions and the secondary aim was to compare it with a historical series of cases performed with PC needles of different sizes.
MATERIALS AND METHODS
We conducted a multicenter retrospective study to assess the performance of the 20G PC FNB needle in three centers in Italy (Maggiore Hospital, Bologna; Murri Hospital, Fermo; ISMETT, Palermo). Patients that underwent EUS-guided tissue acquisition of pancreatic and extra-pancreatic lesions with the 20G PC needle, between May 2016 and 2017, were retrospectively identified within prospectively collected endoscopic databases.
EUS-guided tissue acquisition was performed as previously reported.[20] Briefly, it was performed by convex array echoendoscopes (Pentax Europe, GmbH, Hamburg, Germany; Olympus America, Inc., Melville NY, USA). Tissue acquisition was done with the 20G Echotip® Ultra FNB needle, featuring PC reverse bevel technology and the techniques depended on local preference. All the involved endoscopists involved perform at least 100 EUS-FNA/FNB per year.
All the samplings included in the current study were performed without the presence in the endoscopic suite of a cytopathologist for ROSE. EUS-guided samples were expelled directly into formalin and processed using histologic techniques, for example, cell blocks and/or paraffin-embedded biopsies depending on the presence of macroscopically visible tissue fragments.
At histological analysis, a tissue core was defined as an architecturally intact piece of tissue measuring at least 550 μ in the greatest axis (corresponding approximately to the diameter of a high power microscopic field), as previously reported.[22,23]
As part of an internal quality audit process, all the technical failures (i.e. the need to change the needle because the impossibility to puncture the lesion), as well as both the intraprocedural and within 72 h complications, are routinely recorded in the involved centers.
In 2017, we published a multicenter, retrospective comparative studies evaluating the diagnostic performance of PC needles (i.e., 19G, 22G, and 25G) with and without the presence of ROSE for pancreatic mass lesions.[20] The same study centers were involved. For the purpose of comparing the performance of the 20G PC needle with other PC needles of different sizes, we extracted data from those cases performed without ROSE and compared them with pancreatic cases punctured, in the current study, with the 20G PC.
The protocol was approved by the Institutional Review Board of the coordinating center (Maggiore Hospital, Bologna) in August 07, 2017, and thereafter by the Ethics Committee of each participating center; written informed consent was obtained from all patients.
Definition of standard reference
When available, the histological diagnosis based on surgically resected specimens was considered as the reference standard, while in patients who did not undergo surgery, the final diagnosis was based on the combination of different outcomes (tissue sampling and imaging studies) together with a compatible clinical disease course of at least 3 months.
Outcome measures
The primary outcome measure was the adequacy defined as the rate of cases in which a specific histological diagnosis was rendered. For practical purposes, specific diagnoses were grouped into two categories, namely, benign and malignant, the latter encompassing neoplasms of different malignant potential, for example, lymphoma, adenocarcinoma, and neuroendocrine tumors. The secondary outcome measures were the diagnostic accuracy defined as the proportion of correct diagnoses, and the proportion of cases in which the specimen fulfilled the definition of core at histological evaluation. Intraprocedural and postprocedural (within 72 h) complications were registered.
Statistical analysis
Continuous variables were reported as mean ± standard deviation, whereas categorical variables were reported as proportions. Overall accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) with 95% of confidence interval (CI) were computed. The number needed to diagnose (NND) was computed as 1/(sensitivity + specificity – 1). The number needed to misdiagnose (NNM) was defined as the number of lesions to puncture to obtain a wrong diagnosis (i.e., missing a neoplastic lesion as there were no false-positive cases) and was computed as 1/(1– accuracy). Samples inadequate for cytological/histological evaluation were considered as false-negative cases. Statistical analyses were performed with SPSS version 22 for Mac (IBM Corporation, New York, NY, USA) and with R version 3.1.3 for Mac (R project for statistical computing, Vienna, Austria).
RESULTS
Study population
A total of 384 patients (mean age 67 ± 12.1 years) with pancreatic and extra-pancreatic lesions were enrolled in the study. Pancreatic lesions were in total of 239 patients (62.2%), whereas the extra-pancreatic lesions were in 145 patients (37.8%). The mean lesion size was 33.9 ± 18.9 mm. The final diagnosis was based on surgically resected specimens in 138 of 384 cases (35.9%). Baseline characteristics of the study population are detailed in Tables 1 and 2.
Table 1.
Baseline patients’ characteristics
| Patients’ characteristics | Overall (n=384) |
|---|---|
| Age, years (mean±SD) | 67±12.1 |
| Lesion site, n (%) | |
| Pancreas | 239 (62.2) |
| Other sites | 145 (37.8) |
| Lesion diameter, mm (mean±SD) | 33.9±18.9 |
| Number of passes (mean±SD) | 2.4±1 |
| Center, n (%) | |
| Palermo | 160 (41.7) |
| Bologna | 142 (37) |
| Fermo | 82 (21.4) |
SD: Standard deviation
Table 2.
Locations of the pancreatic and extra-pancreatic solid mass lesions (n=384)
| Lesion site | Frequency (%) |
|---|---|
| Aorto-pulmonary nodes | 3 (0.8) |
| Subcarinal nodes | 21 (5.5) |
| Para-esophageal nodes | 6 (1.6) |
| Perigastric nodes | 4 (1) |
| Periduodenal nodes | 10 (2.6) |
| Hepatic hilum nodes | 13 (3.4) |
| Peri-choledochal nodes | 2 (0.5) |
| Peri-pancreatic nodes | 6 (1.6) |
| Mediastinum | 10 (2.6) |
| Lung | 6 (1.6) |
| Adrenal gland | 2 (0.5) |
| Esophagus | 3 (0.8) |
| Stomach | 19 (4.9) |
| Duodenum | 4 (1) |
| Liver | 9 (2.3) |
| Common bile duct | 17 (4.4) |
| Pancreas (uncinated process) | 19 (4.9) |
| Pancreas (head) | 131 (34.2) |
| Pancreas (body) | 64 (16.7) |
| Pancreas (tail) | 22 (5.7) |
| Jejunum | 1 (0.3) |
| Retroperitoneum | 4 (1) |
| Rectum | 8 (2.1) |
Diagnostic performance
Pancreatic lesions
Sample quality was considered adequate for histological assessment in 218 of 236 pancreatic lesions (diagnostic yield: 92.4%). In 18 cases, the tissue sample was considered inadequate for any sort of evaluation.
Considering the performance of the needle in the detection of malignancies, the overall accuracy was 91.5% (95% CI 87.2%–94.7%); sensitivity 90.8% (95% CI 86.1%–94.3%); specificity 100% (95% CI 82.4%–100%); PPV 100% (95% CI 98.1%–100%); and NPV 48.7% (95% CI 32.4%–65.2%). The NND was 1.1 (95% CI 1.1–1.5), whereas the NNM was 11.8 (95% CI 7.8–18.9).
The analysis of the diagnostic adequacy and accuracy stratified according to the center did not show any significant difference, with all centers reporting results above 90%.
At histological examination, a core was present in 170 cases (72%) with a mean number of 2.8 passes ± 1 [Figure 1]. Overall, two complications were reported in the pancreatic group, one case of intraprocedural self-limiting bleeding (0.3%), and one case of postprocedural pain managed with medical therapy and resolved within 24 h (0.3%). No case of technical failures was recorded [Table 3].
Figure 1.
A whole mount slide of a EUS-guided sample from a 4 cm lesion of the body of the pancreas (H and E, whole mount). The specimen appears highly cellular and admixed with abundant hemorrhagic material
Table 3.
20G ProCore™ performance on pancreatic lesions
| 20G PC performance | Pancreatic lesions (n=236) |
|---|---|
| Age (years), mean±SD | 68.3±11.8 |
| Number of passes, mean±SD | 2.8±1.0 |
| Lesion site, n (%) | |
| Pancreatic head and uncinated process | 150 (63.6) |
| Pancreatic body | 64 (27.1) |
| Pancreatic tail | 22 (9.3) |
| Possibility to obtain histological core, n (%) | 170 (72.0) |
| Possibility to draw histological/cytological diagnosis from tissue sample (diagnostic yield), n (%) | 218 (92.4) |
| Diagnosis at EUS vs. gold standard diagnosis type, n | |
| Malignant lesion | 197/217 |
| Benign lesion | 21/19* |
| No sample or inadequate sample | 18 |
| 20G PC diagnostic performance for malignant lesions, % (95% CI) | |
| Overall accuracy | 91.5 (87.2–94.7) |
| Sensitivity | 90.8 (86.1–94.3) |
| Specificity | 100 (82.4–100) |
| PPV | 100 (98.1–100) |
| NPV | 48.7 (32.4–65.2) |
| NND | 1.1 (1.1–1.5) |
| NNM | 11.8 (7.8–18.9) |
| 20G PC-related complications, n(%) | |
| Bleeding | 1 (0.4) |
| Pain | 1 (0.4) |
*Two lesions were incorrectly diagnosed as benign at EUS-guided sampling, while were found to be malignant. PC: ProCore™, PPV: Positive predictive value, NPV: Negative predictive value, NNM: Number needed to misdiagnose, NND: Number needed to diagnose, CI: Confidence interval, EUS: Endoscopic ultrasound, SD: Standard deviation
Transgastric versus transduodenal approach for pancreatic lesions
A subgroup analysis according to the route of sampling (transgastric vs. transduodenal) was performed.
EUS-guided sampling via transgastric approach was performed in 86 pancreatic cases for lesions located in the body (n = 64) or the tail of the pancreas. Tissue for histological/cytological diagnosis was obtained in 83 cases providing a diagnostic yield of 96.5%. The overall accuracy was 94.2% (95% CI 87.0–98.1), sensitivity 93.5% (95% CI 85.5–97.9), specificity 100% (95% CI 66.4–100), PPV 100% (95% CI 95.0–100), and NPV 64.3% (95% CI 35.1–87.2). The NND was 1.1 (95% CI 1.0–1.9), whereas the NNM was 17.2 (95% CI 7.7–52.6). A core suitable for pathological examination was obtained in 71 cases (82.6%).
EUS-guided sampling via transduodenal approach was performed in 150 cases for lesions located in the pancreatic head and uncinate process. Tissue for histological diagnosis was obtained in 133 cases, providing a diagnostic yield of 88.7%. The overall accuracy was 90% (95% CI 84.0–94.3), sensitivity 89.3% (95% CI 82.9–93.9), specificity 100% (95% CI 69.2–100), PPV 100% (95% CI 97.1–100), and NPV 40.0% (95% CI 21.1–61.3). The NND was 1.1 (95% CI 1.1–1.9), whereas the NNM was 10 (95% CI 6.3–17.5). No EUS-related complications were reported. At histological examination, a tissue core was present in 99 cases (66%).
Diagnostic performance of the 20G PC versus other PC needles for pancreatic lesions
The diagnostic performance of the 20G PC was compared with a historical series previously published.[20] The 20G presented a not significantly higher diagnostic adequacy (92.4%) in comparison to the 19G (90.1%), 22G (88.3%), and 25G (87.1%). Similarly, the accuracy was not significantly higher (20G vs. 19G vs. 22G vs. 25G = 91.5% vs. 90.9% vs. 88.3% vs. 90%) [Figure 2]. Details are reported in Appendix A.
Figure 2.
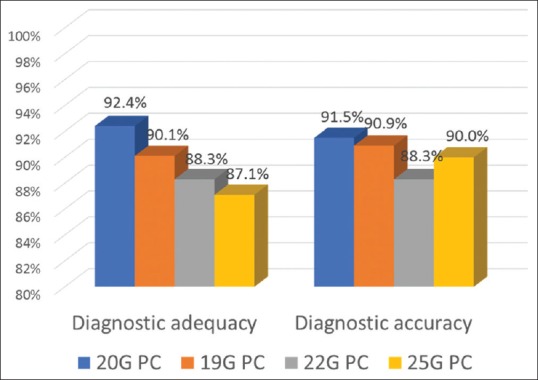
Comparison of the diagnostic performance of the 20G ProCore™ versus ProCore™ needles of different sizes
Extrapancreatic lesions
The diagnostic yield was 95.3% (141/148 cases). When the performance of the needle in the detection of malignant lesions was assessed, an overall accuracy of 95.3% (95% CI 90.5%–98.1%) was found, the sensitivity was 92.6% (95% CI 85.4%–97.0%), specificity 100% (95% CI 93.3%–100%), PPV 100% (95% CI 95.9%–100%), and the NPV was 88.3% (95% CI 77.4%–95.2%). The NND was 1.1 (95% CI 1–1.3), whereas the NNM was 21.3 (95% CI 10.5–52.6). The mean number of passes was 1.9 ± 0.9, and a tissue core was present in 125 cases (84.5%). Neither complications nor technical failures were registered. The results are summarized in Table 4.
Table 4.
20G ProCore™ performance on extra-pancreatic lesions
| 20G PC performance | Extra-pancreatic lesions (n=148) |
|---|---|
| Age (years), mean±SD | 65.0±12.2 |
| Number of passes, mean±SD | 1.9±0.9 |
| Possibility to obtain histological core, n (%) | 125 (84.5) |
| Possibility to draw histological/cytological diagnosis from tissue sample (diagnostic yield), n (%) | 141 (95.3) |
| Diagnosis at EUS vs. gold standard diagnosis type, n | |
| Malignant lesion | 88/95 |
| Benign lesion | 53/53 |
| No sample or inadequate sample | 7 |
| 20G PC diagnostic performance for malignant lesions, % (95% CI) | |
| Overall accuracy | 95.3 (90.5–98.1) |
| Sensitivity | 92.6 (85.4–97.0) |
| Specificity | 100 (93.3–100) |
| PPV | 100 (95.9–100) |
| NPV | 88.3 (77.4–95.2) |
| NND | 1.1 (1–1.3) |
| NNM | 21.3 (10.5–52.6) |
| 20G PC-related complications, n (%) | |
| Bleeding | 0 |
| Pain | 0 |
PC: ProCore™, PPV: Positive predictive value, NPV: Negative predictive value, NNM: Number needed to misdiagnose, NND: Number needed to diagnose, CI: Confidence interval, EUS: Endoscopic ultrasound, SD: Standard deviation
DISCUSSION
To the best of our knowledge, this study represents the largest series reporting the use of the new 20G PC needle in pancreatic and extra-pancreatic solid mass lesions. Main findings are the high diagnostic yield/adequacy and accuracy for pancreatic and extra-pancreatic lesions and the presence of a core at histopathological analyses in >70% of the cases. Furthermore, the diagnostic adequacy was very high (88.7%) even when the transduodenal approach was performed for pancreatic lesions located in the head or the uncinate process. Finally, the results were consistent across study centers. When compared with a historical series, the 20G PC showed a clear trend toward better performance.
These results are important for several reasons. First, the diagnostic adequacy and capability to obtain tissue core sampling were high, despite the absence of ROSE. Nowadays, the need of formalin-fixed and paraffin-embedded tissue has become of crucial importance to improve the possibility of diagnosis, allowing proper histological assessment, immunostaining techniques, and tissue profiling analysis (i.e., molecular markers) for individualized therapy. Diagnostic adequacy and accuracy above 90% have been generally reported in ideal conditions, such as highly experienced endosonographers working in a high volume center with expert pathologists and most importantly with the possibility of ROSE; otherwise fair results should be expected.[24] Our findings confirm a recently published experience with PC needles of different sizes (25G, 22G, and 19G).[20] In that retrospective multicenter study, 333 pancreatic solid mass lesions were sampled with and without ROSE and the adequacy (ROSE vs. no-ROSE: 92.1% vs. 88.1%) as well as the overall accuracy (ROSE vs. no-ROSE: 92.1% vs. 88.1%) were not significantly different. Similarly, the tissue core was available in 61.4% and 53.4% of cases with and without ROSE, respectively, again without any significant difference. The newly developed 20G PC needle seems to further improve these findings since the core was overall reported in 72% of the pancreatic masses sampled. Of note, the same definition of core sampling was adopted in both studies, allowing a direct comparison.
Second, the 20G showed a good performance even when lesions were sampled from the duodenum. It has been suggested that, in case of no availability of ROSE and for lesions accessed from the duodenum, a 19G needle made of nitinol with increased flexibility should be used.[23,25] This suggestion has been questioned by the results of a recently published multicenter international prospective study in which 246 patients with solid lesions (203 cases) or enlarged lymph nodes who needed to undergo EUS sampling only from the duodenum were analyzed.[21] Main outcomes such as diagnostic adequacy and accuracy significantly varied across centers, despite only high volume centers were included, and the procedures were performed by recognized experts in EUS-FNB. In addition, major complications were reported in six cases (two bleeding cases, two acute pancreatitis, one perforation requiring surgery, and one duodenal hematoma). Therefore, the authors concluded that the 19G flexible needle needs a local validation before its routine use from the transduodenal route. The current study showed high adequacy (88.7%) and accuracy (90%) for lesions sampled from the duodenum, without any major complication. In addition, all the samples were processed using histological techniques (cell blocks and formalin-fixed/paraffin-embedded tissue fragments), and the presence of a core was detected in 66% of cases. This result was higher than those reported for PC needles of different sizes.[20] This can be explained by the balance achieved with the 20G PC needle between the inner diameter closer to the 19G and the flexibility similar to that of the 22G. Therefore, more tissue can be captured also in a difficult position. The 19G PC needle appears to be the best in tissue acquisition, with a significantly lower number of passes required,[15] however, it has some limitations, represented by the technical difficulties when transduodenal access is mandatory, due to the rigidity of the device and its gage. On the other hand, smaller size needles, such as 25G PC, although easier to use from a technical point of view, have not proved to be as effective in obtaining a tissue sample suitable for histological evaluation.[17] In this context, the 20G PC needle aims to overcome these limitations, representing a compromise between technical feasibility and diagnostic adequacy.
Third, our study showed very good outcomes for extra-pancreatic mass lesions with diagnostic yield and accuracy >95%. A wide spectrum of lesions was sampled, mainly represented by nodes and a core was obtained in about 85% of the cases. Similarly, Antonini et al.[26] have recently reported the results of a multicenter retrospective study with the 20G PC needle for the diagnosis of SELs. A total of 50 SELs were included and the definitive diagnosis with full histological assessment, including immunohistochemistry was obtained in 88% of the patients, without any major complication.
A systematic review aimed to compare the PC needles with standard needles did not find any significant difference in terms of diagnostic adequacy, accuracy or tissue core acquisition between the two types of needles.[19] However, most of the included studies compared the smaller-caliber needles (i.e., 22G, and 25G), rather than the larger 19G needle which, as shown,[15] has the highest rate of histological tissue acquisition. We performed a retrospective comparison with a historical series of patients with pancreatic mass lesions punctured with PC needles of different sizes and the 20G showed a higher diagnostic performance. Despite all the limitations of this nonrandomized, retrospective comparison, a clear trend toward better performance of the new 20G needle was shown, possibly setting a new referral standard for tissue acquisition. A multicenter international randomized prospective trial aimed to compare the diagnostic accuracy of two EUS-guided tissue acquisition devices, namely, the 25G Echotip® Ultra FNA device and the 20G Echotip® PC FNB device has just completed the enrollment and the results are awaited [ClinicalTrials. gov Identifier: NCT02167074].
The present study had some limitations, mainly due to its retrospective design and the lack of a control group. The external validity of our findings is limited to high-volume centers with endosonographers performing >100 EUS-FNA/FNB per year, however, our data were confirmed in a multicenter setting, with main outcomes consistent across study centers. The definition of tissue core was standardized and already used in the past.[20,22] Despite its arbitrary nature, this definition can be a useful tool to compare different needles and results, becoming a mere histological unite of measure of the sample. From the pathologist's point of view, the presence of a core does not imply that the specimen is diagnostic as it can only be made of fibrous tissue without neoplastic elements. Moreover, free cells and/or small groups of cells are present in the sample, still requiring cytological diagnostic skills. Nevertheless, the higher amount of material obtained with FNB needles rather than with FNA needles offers some advantages: it can be processed only with histological techniques, leading to higher standardization and it can be easily scanned to create digital slides. Differently from smears, which have different thickness throughout the slide, histologic biopsies and cell blocks have one depth of focus only. Digital slides can then be used for audits, teaching, and diagnostic purposes.
CONCLUSIONS
The newly developed 20G PC needle showed high diagnostic adequacy and accuracy for pancreatic and extra-pancreatic lesions, regardless the access route. Whether confirmed by comparative studies, this needle might represent a new standard for EUS tissue sampling.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix
Appendix A.
Different needles performance on pancreatic lesions
| Performance | 20 PC (n=236) | 19 PC (n=11) | 22 PC (n=111) | 25 PC (n=70) |
|---|---|---|---|---|
| Age, years (mean±SD) | 68.3±11.8 | 64.9±8.4 | 64.7±12.6 | 70.2±9.8 |
| Number of passes (mean±SD) | 2.8±1.0 | 1.6±0.8 | 2.8±1.3 | 2.9±0.8 |
| Lesion site, n (%) | ||||
| Pancreatic head and uncinated process | 150 (63.6) | 4 (36.3) | 73 (65.7) | 58 (82.8) |
| Pancreatic body | 64 (27.1) | 7 (63.7) | 34 (30.6) | 11 (15.8) |
| Pancreatic tail | 22 (9.3) | 0 | 4 (3.7) | 1 (1.4) |
| Diagnostic adequacy, n (%) | 218 (92.4) | 10 (90.1) | 98 (88.3) | 61 (87.1) |
| Diagnostic performance for malignant lesions, % (95% CI) | ||||
| Overall accuracy | 91.5 (87.2-94.7) | 90.9 (58.7-99.8) | 88.3 (80.8-93.6) | 90 (80.5-95.9) |
| Sensitivity | 90.8 (86.1-94.3) | 90.9 (58.7-99.8) | 87.5 (79.6-93.2) | 88.5 (77.8-95.3) |
| Specificity | 100 (82.4-100) | - | 100 (59-100) | 100 (66.4-100) |
| Positive predictive value | 100 (98.1-100) | 100 (69.2-100) | 100 (96-100) | 100 (93.4-100) |
| Negative predictive value | 48.7 (32.4-65.2) | 0 (0-97.5) | 35 (15.4-59.2) | 56.2 (29.9-80.2) |
| Number needed to diagnose (NND) | 1.1 (1.1-1.5) | - | 1.1 (1.1-2.6) | 1.1 (1.1-2.3) |
| Number needed to misdiagnose (NNM) | 11.8 (7.8-18.9) | 11 (2.4-500) | 8.5 (5.2-15.6) | 10 (5.1-24.4) |
REFERENCES
- 1.Vander Noot MR, 3rd, Eloubeidi MA, Chen VK, et al. Diagnosis of gastrointestinal tract lesions by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2004;102:157–63. doi: 10.1002/cncr.20360. [DOI] [PubMed] [Google Scholar]
- 2.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: Diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984–9. doi: 10.1055/s-2005-870272. [DOI] [PubMed] [Google Scholar]
- 3.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 4.DeWitt J, Alsatie M, LeBlanc J, et al. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy. 2007;39:65–71. doi: 10.1055/s-2006-945042. [DOI] [PubMed] [Google Scholar]
- 5.DeWitt JM. Endoscopic ultrasound-guided fine-needle aspiration of right adrenal masses: Report of 2 cases. J Ultrasound Med. 2008;27:261–7. doi: 10.7863/jum.2008.27.2.261. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: A prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–7. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European society of gastrointestinal endoscopy (ESGE) clinical guideline – Updated January 2017. Endoscopy. 2017;49:695–714. doi: 10.1055/s-0043-109021. [DOI] [PubMed] [Google Scholar]
- 8.Fuccio L, Larghi A. Endoscopic ultrasound-guided fine needle aspiration: How to obtain a core biopsy? Endosc Ultrasound. 2014;3:71–81. doi: 10.4103/2303-9027.123011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 11.Jhala NC, Jhala DN, Chhieng DC, et al. Endoscopic ultrasound-guided fine-needle aspiration. A cytopathologist's perspective. Am J Clin Pathol. 2003;120:351–67. doi: 10.1309/MFRF-J0XY-JLN8-NVDP. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JH, Aslanian HR, et al. ASGE Technology Committee. Devices for use with EUS. VideoGIE. 2017;2:35–45. doi: 10.1016/j.vgie.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMaio CJ, Kolb JM, Benias PC, et al. Initial experience with a novel EUS-guided core biopsy needle (SharkCore): Results of a large North American multicenter study. Endosc Int Open. 2016;4:E974–9. doi: 10.1055/s-0042-112581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–8. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 15.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 16.Witt BL, Adler DG, Hilden K, et al. A comparative needle study: EUS-FNA procedures using the HD ProCore(™) and EchoTip(®) 22-gauge needle types. Diagn Cytopathol. 2013;41:1069–74. doi: 10.1002/dc.22971. [DOI] [PubMed] [Google Scholar]
- 17.Iwashita T, Nakai Y, Samarasena JB, et al. High single-pass diagnostic yield of a new 25-gauge core biopsy needle for EUS-guided FNA biopsy in solid pancreatic lesions. Gastrointest Endosc. 2013;77:909–15. doi: 10.1016/j.gie.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Antonini F, Fuccio L, Giorgini S, et al. Biliary plastic stent does not influence the accuracy of endoscopic ultrasound-guided sampling of pancreatic head masses performed with core biopsy needles. Dig Liver Dis. 2017;49:898–902. doi: 10.1016/j.dld.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Fuccio L, Fornelli A, et al. The presence of rapid on-site evaluation did not increase the adequacy and diagnostic accuracy of endoscopic ultrasound-guided tissue acquisition of solid pancreatic lesions with core needle. Surg Endosc. 2017;31:225–30. doi: 10.1007/s00464-016-4960-4. [DOI] [PubMed] [Google Scholar]
- 21.Attili F, Fabbri C, Yasuda I, et al. Low diagnostic yield of transduodenal endoscopic ultrasound-guided fine needle biopsy using the 19-gauge flex needle: A large multicenter prospective study. Endosc Ultrasound. 2017;6:402–8. doi: 10.4103/eus.eus_54_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabbri C, Luigiano C, Maimone A, et al. Endoscopic ultrasound-guided fine-needle biopsy of small solid pancreatic lesions using a 22-gauge needle with side fenestration. Surg Endosc. 2015;29:1586–90. doi: 10.1007/s00464-014-3846-6. [DOI] [PubMed] [Google Scholar]
- 23.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–43. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 24.Eisendrath P, Ibrahim M. How good is fine needle aspiration? What results should you expect? Endosc Ultrasound. 2014;3:3–11. doi: 10.4103/2303-9027.127122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varadarajulu S, Hasan MK, Bang JY, et al. Endoscopic ultrasound-guided tissue acquisition. Dig Endosc. 2014;26(Suppl 1):62–9. doi: 10.1111/den.12146. [DOI] [PubMed] [Google Scholar]
- 26.Antonini F, Delconte G, Fuccio L, et al. OC.05.3: Endoscopic ultrasound-guided tissue sampling with a new 20G biopsy needle for the characterization of gastrointestinal subepithelial lesions: Results of a preliminary multicenter study. Dig Liver Dis. 2017;49:e88–9. doi: 10.4103/eus.eus_1_18. [DOI] [PMC free article] [PubMed] [Google Scholar]


