Abstract

Redox flow batteries have received wide attention because of their unique advantages such as high efficiency, long cycle life, low operating cost, and independent adjustment of energy power. In this study, five types of anthraquinone derivative organic redox couple were selected, and the surfaces of graphite felt were modified. When the number of functional groups is increased or the substitution position is closer to the carbonyl (C=O) groups, a more pronounced hindrance for the C=O reaction on the benzene ring is observed; thus, the electrochemical performance and reversibility decreases. Sodium 9,10-anthraquinone-2-sulfonate solution is the best organic redox couple in terms of both reversibility and electrochemical performance. It was also found that all the surface treatment methods of graphite felt are beneficial for improving their electrochemical performances. All these superior results demonstrate that the graphite felt treated under air exposure at 550 °C for 3 h exhibited the best electrochemical performance, which might be attributed to the increase in the content of C–OH functional groups.
1. Introduction
Redox flow batteries (RFBs) are not good for mobility applications but they are important for stationary grid storage. Their poor volumetric power density and need for subsystem components for enabling continuous flow do not make them useful for mobile electric vehicles.1,2 Grid storage for peak shaving, coupling for solar and wind storage, and so forth are the domains of application and for 1 MW or more storage, their expected project cost comes less than that of lead acid, such as vanadium and Zn–Br RFBs. Currently, RFBs are divided into two categories: inorganic metal-ion flow batteries and organic flow batteries. In the field of inorganic metal-ion flow batteries, vanadium flow batteries are the most developed devices. The high and variable price of vanadium, however, renders these batteries too expensive and risky for wide-scale commercialization.3 In recent years, researchers have found that the development of RFBs based on inexpensive and sustainable redox-active organic materials can overcome these drawbacks.4 According to the characteristics of the supporting electrolyte, the organic flow battery system can be divided into water (aqueous organic RFBs) and nonwater (non-aqueous RFBs) systems.5,6 Water-based flow batteries use aqueous solutions of acids, alkaline, or salts as the supporting electrolyte. The main research on these batteries focuses on increasing their energy density, reducing the cost of active materials, and reducing the overall costs of the batteries. Nonaqueous flow batteries use organic substances as the supporting electrolyte and organic active materials dissolved in organic solvent as reactants and the main research direction for these batteries is toward obtaining a relatively high potential window;7 moreover, it was found that nonaqueous RFBs have higher energy densities than those of aqueous RFBs; however, because of the low conductivity of the nonaqueous electrolyte, their capacities are limited. In aqueous organic redox-flow batteries (AORFBs), organic charge-storage materials offer structural diversity, tenable redox potential, and optimizable solubility.8 High aqueous solubility, well-separated reduction potentials barely avoiding water splitting, stability, safety, and low costs at mass-production scales constitute the most critical attributes of novel aqueous organic electrolytes.8 Therefore, AORFBs have been the subject of recent research.9−14 At present, most of the studies on AORFBs have focused on the family of organic molecules called quinones.9,11,14
Quinones are a special class of cyclic unsaturated diketones, and their conjugated structure affords excellent electrochemical activity. Yang et al. suggested that the proximity of the transfer coefficients to 0.5 and the high rate constants suggest an “outer-sphere” process.14 Depending on the skeletal structure, these compounds can be divided into benzoquinones, naphthoquinones, anthraquinones, phenanthrenequinones, and four other quinone types. Among these, anthraquinones are the most common, and most of their species are found in plants; thus, they are relatively easy to obtain.
In 2009, Xu et al.
pioneered the use of organic quinones as active materials in RFBs.15 Subsequently, Huskinson et al.9 used a solution of 9,10-anthraquinone-2,7-disulfonic acid
(AQDS) as the catholyte in a similar battery. The reaction shows the
balanced electrochemical reaction in a reversible manner with equilibrium
potential (standard potential).
Anthraquinone derivatives undergo a kinetically fast two-electron transfer reaction that is accompanied by the transfer of two protons, provided the protons are necessary for the reaction.14 Gerhardt et al. applied AQDS and other quinone derivatives as the catholyte in an aqueous quinone-bromide redox flow battery.12 Chen et al. reported a cycling analysis of the quinone/bromide RFB.16 Hofmann et al. suggested that the incorporation of electron-withdrawing substituents, such as −NO2, −COOCH3, or −CN, leads to stronger oxidants, which increases the resultant redox potential, thereby making these species well-suited as active catholyte materials.13 In these studies, however, there is no complete study on the effects of the position and number of functional groups on the electrocatalytic performance of modified graphite felt (GF) electrodes. Additionally, in the above studies, researchers mostly used carbon paper as the electrode material. However, in practical applications of AORFBs, GF was used as the electrode materials. The thickness and small surface area per unit volume of carbon paper compared to that of traditional GF electrodes could result in a relatively large concentration polarization, particularly at high current densities, in a single cell. In addition, many studies have shown that the type and number of functional groups on the surface of carbon materials have a significant effect on the catalytic activity of the redox couple,17−20 which, in turn, has a significant effect on the performance of the flow batteries. The electrocatalytic mechanism of AORFBs working with GF electrodes is different from those of traditional vanadium RFBs and other organic liquid flow batteries. However, the effect of the surface modification of GF electrodes on the electrochemical reactions of the quinone-based redox couple has not been reported thus far, and there are few detailed conclusions on the electrochemical reaction mechanism of anthraquinone derivatives on the surface of modified GF.
In this study, five types of anthraquinone derivative electrolytes were selected, and the surfaces of GF electrodes were modified by heat treatment, acid treatment, mixed treatment, and an elemental nitrogen-doping method. The surface morphology, crystal structure, and surface chemical states of the modified GF electrodes were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD) measurement, and X-ray photoelectron spectroscopy (XPS). Furthermore, we used cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) to investigate the electrocatalytic performance of the modified GF electrodes. The electrocatalytic reaction mechanism of the anthraquinone derivative on the GF surface is also discussed.
2. Experimental Section
2.1. Materials
The analytical-grade polyacrylonitrile-based GF was purchased from Beijing Sanye Carbon (China) Co., Ltd. 9,10-Anthraquinone-1-sulfonate sodium (1-AQS, 98%) and 9,10-anthraquinone-2-sulfonate sodium (2-AQS, 97%) were supplied by Shanghai Macklin Biochemical Technology Co., Ltd. 9,10-Anthraquinone-2,7-disulfonate (2,7-AQDS, > 95%) and 9,10-anthraquinone-1,5-disulfonate (1,5-AQDS, > 98%) were purchased from Tstachi (Shanghai) Industrial Development Co., Ltd. Dopamine hydrochloride (98%, Aladdin) and tris(hydroxymethyl)aminomethane hydrochloride (Tris HCl, 99%, Aladdin) were of analytical grade and used as provided without further purification. All the water used in the whole experiment was deionized. Before each pretreatment, the GF was cut to a size of 20 × 20 mm2.
2.2. Modification of GF
2.2.1. Method 1
The GF was put in concentrated sulfuric acid (98.3 wt %) and soaked for 6, 12, 24, 36, or 48 h in room temperature. Then, the felt was washed repeatedly with distilled water, soaked in distilled water for 24 h, and dried in a vacuum oven for 24 h. The samples were named S-M h (where M is 6, 12, 24, 36, or 48)
2.2.2. Method 2
The GF was soaked in concentrated sulfuric acid (98.3 wt %) and concentrated nitric acid (68 wt %) solution that was mixed in a respective volume ratio of 1:3 (S1N3), 1:1(S1N1), or 3:1(S3N1). After immersing in solution for 24 h at room temperature, the felt was washed repeatedly with distilled water, then soaked in distilled water for 24 h, and dried in a vacuum oven (60 °C) for 24 h. The samples were named SaNb-24 (where a is 1 or 3, and b is 1 or 3).
2.2.3. Method 3
The GF was calcined in a muffle furnace at 450, 500, 550, or 600 °C and held for a time (2 or 3 h). Then, the materials were naturally cooled to room temperature. (Hereafter, A-M °C-N h is used to represent the GF sample under an air atmosphere at a temperature of M °C for N h, where M is 450, 500, 550, and 600 and N is 2 or 3.)
2.2.4. Method 4
The GF was calcined in a vacuum furnace at a heating rate of 5 °C min–1, and the temperature was maintained at 450, 500, 550, or 600 °C and held for a time (2 or 3 h). Finally, the GF was cooled to room temperature at the rate of 5 °C per min–1. (Hereafter, V-M °C-N h is used to represent the GF in a vacuum at a temperature of M °C for N h, where M is 450, 500, 550, and 600 and N is 2 or 3.)
2.2.5. Method 5
The GF was placed in a muffle furnace, and the temperature is fixed at 500, 550, or 600 °C and held for some time (2 or 3 h). Then, it was naturally cooled to room temperature to complete the heat treatment in air. Subsequently, the heat-treated GF was placed in a vacuum furnace, and then, the temperature was raised at the rate of 5 °C per min–1 and maintained at 500, 550, or 600 °C for 2 or 3 h. The sample was finally cooled to room temperature at the rate of 5 °C per min–1. The temperature and time of the sample for heat treatment in the vacuum are the same as those used for the heat treatment in air. (Hereafter, M–M °C-N h is used to indicate that the GF samples are heated at a temperature of M °C for N h, where the second M is 500, 550, and 600 and N is 2 or 3.)
2.2.6. Method 6
The experiment is handled in two sequences. For the method with acid soaking followed by heat treatment, the GF was first soaked in concentrated sulfuric acid (98.3 wt %) for 24 h, washed repeatedly with distilled water, placed in distilled water for 24 h, and finally dried in a vacuum oven (60 °C) for 24 h. Then, the GF was heated in a muffle furnace to 550 °C for 2 or 3 h and naturally cooled to room temperature. (Hereafter, 24 h–550 °C-N h represents the GF sample first soaked in concentrated sulfuric acid (98.3 wt %) for 24 h and then held at a temperature of 550 °C for N h, in which N is 2 and 3). In the method with heat treatment followed by the acid soaking process, GF was first placed in a muffle furnace in which the temperature was held constant at 550 °C for 2 or 3 h and then was naturally cooled to room temperature. The heat-treated GF was placed in concentrated sulfuric acid (98.3 wt %), soaked for 24 h, washed repeatedly with distilled water, placed in distilled water for 24 h, and finally dried in a vacuum oven for 24 h. (Hereafter, 550 °C-N h-24 h represents the GF first heated at a temperature of 550 °C for N h and then soaked in concentrated sulfuric acid for 24 h, in which N is 2 or 3.)
2.2.7. Method 7
First, the two combustion boats were placed in the tubular furnace. One of the combustion boats was filled with 3 g of urea and 1 g of water. The two GFs were placed in the other combustion boat. Then, the interior of the tube was evacuated. After that, the tubular furnace was heated from 25 to 160 °C at the rate of 5 °C per min–1 and held for 30 min at 160 °C. Subsequently, at the same rate, the temperature was raised from 160 to 700 °C and held for 5, 10, 15, or 20 h. In the operation, if the pressure was higher than one atmosphere, the outlet shutoff valve would be operated so that the part of the gas in the tubular furnace enters the bottle that is filled with the acid solution. The rate of temperature decrease was 5 °C min–1. (Hereafter, 700 °C-N h was used to indicate that the GF samples were held at a constant temperature of 700 °C for N h, in which N is 5, 10, and 15.)
2.3. Electrochemical Measurements
CV and EIS were carried out using a PAR2273 potentiostat/galvanostat (Princeton Applied Research, USA) at room temperature. In the three-electrode cell, a saturated calomel electrode (SCE) acts as the reference electrode. A GF (10 mm × 10 mm × 10 mm) is used as the working electrode. The platinum plate (6.0 cm2 geometry surface area) served as the counter electrode. The CVs were carried out at the potential versus SCE. The CV curves were recorded in 1.00 mol L–1 H2SO4 + 1.00 mmol L–1 anthraquinone derivative. The potential sweep rate is 25 mV s–1. EIS was performed by applying an alternated voltage of 10 mV over the frequency ranging from 0.01 to 105 Hz at the open-circuit potential.
Rotating-disc electrode (RDE) experiments were conducted using an MSR RDE (Pine Research Instrumentation, Inc.) instrument equipped with a 3 mm diameter glassy carbon disk working electrode, an SCE reference electrode, and a platinum counter electrode. The electrolyte was 1 M of H2SO4 and 1 mM anthraquinone derivative.
2.4. Characterization of PAN-Based GF before and after Treatment
The XRD of the sample was recorded in an X-ray diffractometer (D/MAX-2500/PC, Rigaku Co., Japan) equipped with a Cu Kα radiation source operated at 45 KV and 200 mA. The tests were carried out in the angle (2θ) range from 5° to 80° at the scanning rate of 4° min–1. The morphology of the samples was observed with a scanning electron microscope (FEI-Nanosem 430, USA). In this paper, XPS (GENESIS 60S XP spectrometer,USA) was used to characterize the chemical composition of the surfaces. The XP spectra were recorded with Al Kα radiation as an excitation source. The survey spectra were collected with pass energy of 187.85 eV at a step of 1.0 eV, and the high-resolution spectra were collected with pass energy of 29.35 eV at a step of 0.25 eV. The binding energy scales for the samples were corrected by referencing the C 1s binding energy to graphite carbon at 284.5 eV. To determine the quantification of the elemental concentrations and the binding states, a Shirley background was subtracted. The high-resolution spectra were fitted with Gaussian/Lorentzian functions to determine the concentrations of the atoms in the various binding states. Viscosity measurement of the anthraquinone derivative was conducted by a MCR 301 Viscometer (Anton Paar).
3. Results and Discussion
3.1. Materials’ Characterization
The XRD of the GF before and after treatment (Figure S1 and Table S1) show that the seven pretreatment methods modified the structure of the GF. The SEM images of the samples (Figure S2) show that in addition to the heat treatment, the other three treatments have minimal effect on the apparent morphology of the GF fibers. To further delve into the surface chemical composition of GF, the XPS analyses of four samples were conducted, as shown in Figure 1. As observed, the obvious characteristic peaks located at 285 and 531 eV correspond to the C 1s and O 1s energy levels, respectively.21 The analysis of the XP Spectra revealed that the O/C ratio of the pristine GF had a minimum value of 9.4%, and the O/C ratio of the GF after acid treatment had a maximum value of 14.9%, as shown in Table 1. To further analyze the changes in the content of oxygen-containing functional groups on the surface of GF fibers, the peak fitting of C 1s for four samples, as shown in Figure 1, was performed to compare the valence bonds of carbon, thus indicating the presence of sp2 C=C (284.6 eV), C–OH (286.1–286.3 eV), and C=O (287.3–287.6 eV) groups.22−24 According to the XPS analysis, the hydroxyl content (C–OH) in the GF increases from 6.8 to 7.4% after air-heat treatment, and the carbonyl (C=O) content increases from 2 to 3.6%. The C–OH content in the GF samples after the concentrated sulfuric acid treatment increases from 6.8 to 13.5%, whereas the C=O content decreases from 2 to 1.85%, probably because contamination could affect the results.
Figure 1.
XP Spectra of (a) full survey scan (b–e) C 1s. (b) pristine GF, (c) V-550 °C-3 h, (d) A-550 °C-3 h, (e) S-24 h; fits are shown in color as labeled.
Table 1. Experimental Data from XP Spectra Fitting Based on the Shirley Background.
| C 1s fitting
results |
||||||
|---|---|---|---|---|---|---|
| methods | C 1s/% | O 1s/% | O/C | C–C/% | C–OH/% | C=O/% |
| pristine GF | 90.0 | 8.5 | 0.094 | 81 | 6.8 | 2.0 |
| V-550 °C-3 h | 90.3 | 7.2 | 0.080 | |||
| A-550 °C-3 h | 87.4 | 12.87 | 0.146 | 76 | 7.4 | 3.6 |
| S-24 h | 84.2 | 12.53 | 0.149 | 68.9 | 13.5 | 1.85 |
3.2. Electrochemistry
3.2.1. CV and RDE
There are many types of organic quinone compounds. The five main compounds used in this study are as follows: three anthraquinone derivatives containing one sulfonate group, and two anthraquinone derivatives containing two sulfonate groups. According to the previous studies,8−14 the structures of the five anthraquinone derivatives and the proposed electrochemical reactions processes are shown in Figure S3. The specific cyclic unsaturated diketone structure shown in the figure is the basic structure of the anthraquinone derivatives; however, the types of substituents and the positions of the substitutions for the different anthraquinone derivatives are not the same. In general, we expect the molecules with conjugated carbon–carbon bonds and keto- and enol-groups that allow the delocalization and rearrangement of π electrons to undergo these redox transformations with extraordinary ease.14 However, the results of most studies show that the dissociation and rearrangement of C–C and C–H bonds do not occur in these electrochemical reactions.
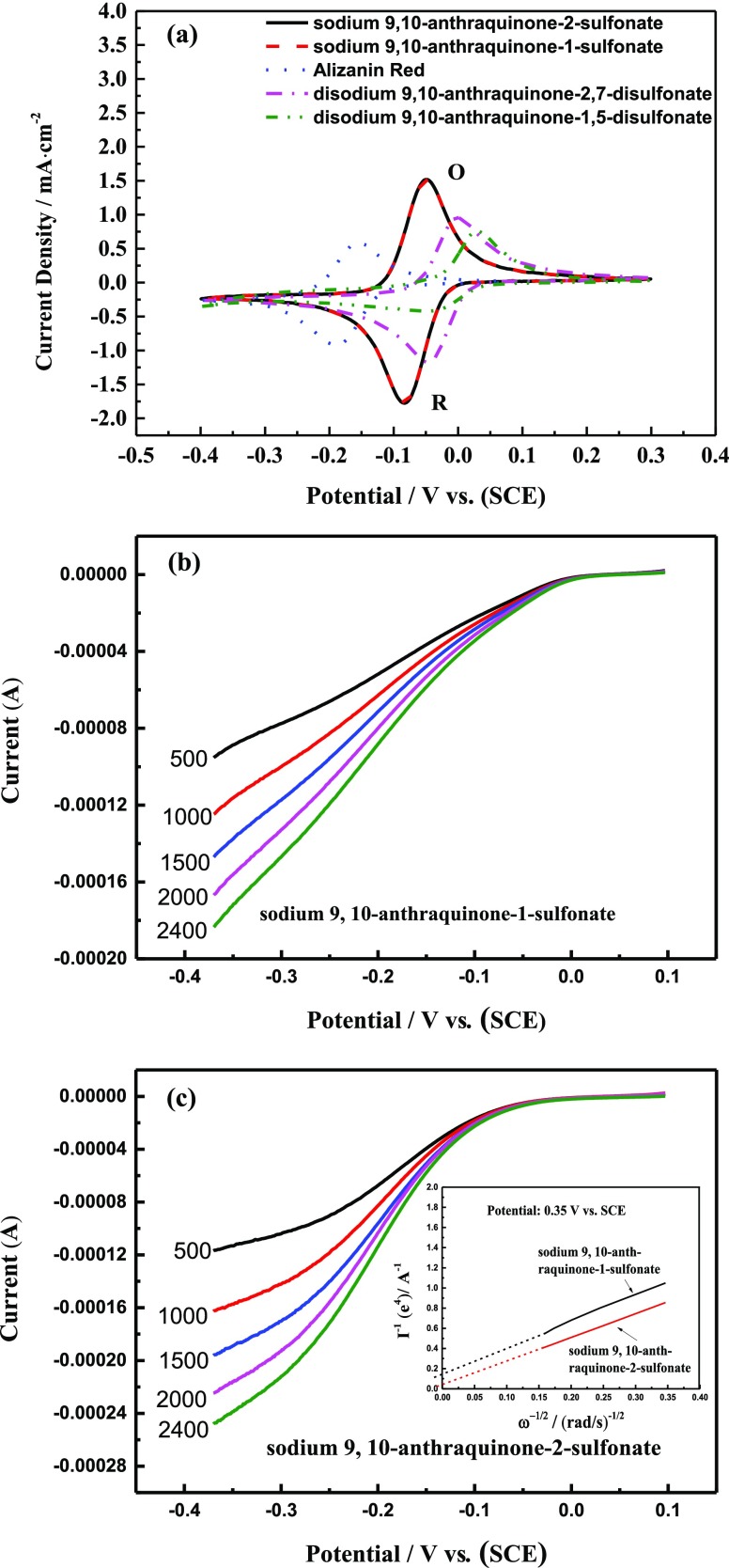
According to the reports of González et al.25 and Jeong et al.,26 this study uses the peak current density as a measure of the electrode reactivity, in which a relatively high peak current density corresponds to excellent catalytic activity of the reaction. Figure 2a shows that the CV of the pristine GF in 2,7-AQDS solution is in accordance with that reported by Huskinson et al.9Table S2 shows the electrochemical data from the cyclic voltammograms of the pristine GF. The redox potential (E), defined as the average value of anodic peak potential (Epa) and cathodic peak potential (Epc), as measured by CV, is found to be −0.024 V for 2,7-AQDS (with 1 α-SO3H and 1 β-SO3H) and −0.066 V for 2-AQS (with 1 β-SO3H), respectively. This shows that substituting sulfonyl groups onto the α and β positions and number can possibly change significantly the redox potential of anthraquinone derivatives, which will be further discussed in the following content. We also employed the peak potential separation (ΔEp) values to evaluate the reversibility of the electrochemical reaction, where a relatively small ΔEp corresponds to excellent reversibility of the reaction.25,26 It can be seen from Figure 4a that the potential and the current density of the oxidation–reduction peaks for the five curves are all different, which indicates that the type of substituent and the substitution position significantly influence the electrochemical activity of the anthraquinone compounds. The results are presented in Table S2. According to that, the current densities of the oxidation and reduction peaks for the pristine GF in the 2-AQS solution is 1.521 and 1.793 mA cm–2, respectively, and the ΔEp is 33.26 mV. The maximum is 74.1 mV. Therefore, both the catalytic activity and the reversibility of the pristine GF in the 2-AQS solution are better than those in the other four anthraquinone derivative solutions. To eliminate and estimate the influence of the liquid phase mass transfer, linear sweep voltammetry measurements of two anthraquinone derivatives (2-AQS and 1-AQS) at a rotating disk electrode at various rotation rates were conducted. Figure 2b,c show the results of these measurements.
Figure 2.
(a) Cyclic voltammograms of pristine GF, (b) and (c) Linear sweep voltammetric data (scan rate of 5 mV s–1) at a glassy carbon rotating disk electrode for 1 mM concentration of 2-AQS and 1-AQS at the rotation rates indicated.
Figure 4.
EIS plots of three samples (a–f) and equivalent circuits (g) used to simulate the EIS plots(f), points: tested data, line: simulated results. The electrolyte solution contains the 2-AQS. (h) Schematic diagram of electronic exchange for 2-AQS on the graphite fiber.
The electrochemical activities of two anthraquinone derivatives are evaluated using the Koutecky–Levich equation (eq 1) to calculate the kinetic current densities (Ik); the diffusion current (Id) can be expressed by the Levich equation (eq 2)
| 1 |
| 2 |
| 3 |
where I is the measured current density; ω, the angular velocity of the disk (ω = 2πN, where N is the electrode rotating speed); B, the Levich slope; n, the transferred electron number; F, the Faraday constant (96 485 C mol–1); C0, the bulk concentration of anthraquinone derivatives; D0, the diffusion coefficient of anthraquinone derivatives in the electrolyte, and υ, the kinematic viscosity.27Table 2 shows the experimentally obtained values. From Table 2, it can be observed that the sulfonate groups occupy different positions on the anthraquinone skeleton, and the diffusion coefficients of two anthraquinone derivatives differ by about 1.5 times. However, its kinetic current densities differ by a factor of eight. Therefore, in the CV diagram, the reason why the peak current density of the 2-AQS is higher than that of the 1-AQS can be attributed to the difference in electrochemical activity of each derivative.
Table 2. Electrochemical Properties of the Redox Couple Determined from Rotating Disk Electrode Experiments.
| sample | Ik × 10–4 (A cm–2) φ = 0.35 V vs SCE | D (cm2 s–1) | υ (cm2 s–1) |
|---|---|---|---|
| sodium 9,10-anthraquinone-1-sulfonate | 6.89 × 10–6 | 3.40 × 10–6 | 0.013496 |
| sodium 9,10-anthraquinone-2-sulfonate | 2.21 × 10–5 | 3.85 × 10–6 | 0.013572 |
Figure 3a–d shows some parts of the representative cyclic voltammograms of the seven pretreated GF electrodes in the five anthraquinone derivative solutions. The electrochemical data from the cyclic voltammograms of all the pretreated GF electrodes are listed in Tables S3–S7. By comparing the CV data (in Tables S3–S7), it can be found that the performances of the seven pretreated GF electrodes are better than that of the pristine GF. These data are larger than that reported by Hukinson et al.9 using the glass carbon electrode. In our work, the highest current density is ∼17.5 mA·cm–2, which is close to the performance (about 20 mA·cm–2) of the CV curve reported by Shah et al. for all vanadium RFBs.28 Therefore, we believe that the treated GF has a significant potential to be applied in AORFBs. In addition, the first circle CV curve completely coincided with the 20th circle CV curve, which indicates that the surface functional groups and surface topography of the GF electrode exhibit certain stability. However, long-term stability also requires supplementary work for verification. In the different anthraquinone derivatives, the order of the catalytic activity of the pretreated GF is different. Among the above five solutions, the air-heat-treated GF electrode showed the best catalytic activity. By comparing the electrochemical activities and reversibilities for the same kind of GF electrodes, such as the air-heat-treated GF electrode, in the five kinds of anthraquinone derivative solutions, the observed electrochemical activity in descending order was 2-AQS > 1-AQS > 2,7-AQDS > AR ≈ 1,5-AQDS. The reversibility of the redox reaction of 1,5-AQDS and AR solutions on the air-heat-treated GF electrode is poor. To further verify the conclusions from the cyclic voltammograms and discuss the electrochemical reaction mechanism of the pretreated GF electrode in different anthraquinone derivative solutions, EIS was also carried out.
Figure 3.
Cyclic voltammograms (a) A-550 °C-3 h, (b) S-24 h, (c) S1N3-24 h, (d) 24 h-550-3 h, (e) 550-2-24 h, (f) 700–10 h, (g) V-550 °C-3 h, (h) M-550 °C-3 h at the scan rate of 10 mV s–1.
3.2.2. Electrochemical Impedance Spectroscopy
As shown in Figure 4, it can be found that the Nyquist plot measured at the open-circuit
potential consists of two parts: a large semicircle in the high-frequency
range and a straight line in the low-frequency range. The semicircle
in high-frequency ranges indicates that the charge-transfer step takes
place at this potential. A straight line showing a typical diffusion
process gradually appears in the low-frequency range. This behavior
demonstrates that the electrode reaction is mix-controlled by the
charge-transfer step and diffusion step in the solution. From the
Bode diagrams of the pristine GF, two peaks located at log f ≈ 0.04 Hz and log f ≈ 2.0
Hz in the φ – log f plot can be observed.
This result means that the two-time constants should be included in
the equivalent circuits of the pristine GF. Batchelor-McAuley et al.
investigated the quantitative characteristics of the redox reaction
of 2,6-AQDS and 2-AQS over the full pH range and in the presence of
variable amounts of Na+ and K+ ions.29 They suggested that the electrochemical reduction
mechanism for the anthraquinone redox system is a CECE type mechanism,
where C means protonation and E is electron transfer.
Therefore, the electron-transfer process of 2-AQS should be divided into two steps. In the first step, a C=O unit on a special ring structure receives an electron, and in the second step, another C=O unit gains an electron.30Figure 4g shows the equivalent circuits for the measured EIS plots, where R1 accounts for the uncompensated solution resistance from the reference electrode to the working electrode, and R2 and CPE1 (constant phase element) are ascribed to the charge-transfer resistance and double-layer capacity of the first reduction step for 2-AQS on the pristine GF electrode surface, respectively. R3 and CPE2 are the charge-transfer resistance and double-layer capacity of the second reduction step for 2-AQS, respectively. W is the Warburg impedance because of ion diffusion in solution inside the GF. It can be found that the simulated curves (lines in Figure 4a–f) are consistent with the measured data. From the fitted data in Table 3, it is found that the total resistance of the GF decreases after different heat treatments. The total resistance of the air-heat-treated 550 °C-3 h GF samples is the smallest, which is in agreement with the observation that the peak current density in the cyclic voltammogram is the largest. Sun et al.31 believed that the catalytic effect of heat-treated GF electrodes on vanadium ions is mainly due to the C–OH content. This attribution means that the increase in the C–OH content can improve the hydrophilicity of the GF samples32 and promote the reaction with the anthraquinone derivative, where the specific reaction process is shown in Scheme 1.
Table 3. Simulated Parameters of EIS Spectra for GFs by Heat Treatment in 2-AQS Solution.
| CPE1 |
||||
|---|---|---|---|---|
| samples | R1/(Ω) | Y/(S·s–n) | n (0 < n < 1) | R2/(Ω) |
| pristine GF | 0.5485 | 0.000124 | 0.9227 | 3.502 |
| A-550 °C-3 h | 1.265 | 0.02144 | 0.9015 | 3.206 |
| M-550 °C-3 h | 0.6272 | 0.03363 | 0.7768 | 2.453 |
| V-550 °C-3 h | 0.7399 | 0.08575 | 0.9999 | 7.489 |
| CPE2 |
|||||
|---|---|---|---|---|---|
| samples | Y/(S·s–n) | n (0 < n < 1) | R3/(Ω) | W/(S·s0.5) | (R2 + R3)/(Ω) |
| pristine GF | 0.02663 | 0.8669 | 127.1 | 0.1086 | 130.6 |
| A-550 °C-3 h | 0.02527 | 0.9214 | 35.23 | 0.1872 | 38.44 |
| M-550 °C-3 h | 0.02081 | 0.9527 | 44.13 | 0.2733 | 46.59 |
| V-550 °C-3 h | 0.5377 | 0.8001 | 87.4 | 0.2140 | 94.89 |
Scheme 1. Specific Reaction Process of C–OH Functional Groups for Promoting the Reaction with the Anthraquinone Derivative.
Basically, the increase in the C–OH content on the GF can help attract water molecules with the presence of battery electrolyte solution, and wet the uncoated part of the GF electrode.33 This generates a required hydrophilic environment, presumably in the form of a double-layer structure34 with inner-layer water molecules adsorbed onto the uncoated area of the GF fiber surfaces. After replacing the water molecules on the electrode surface, the organic molecules are easily attached to the electrode surface. This will reduce the electron transfer resistance, and increase the reaction rate with H+, thus increasing the catalytic activity of the electrode. We suggested that the electron transfer in electrochemical reactions can be carried out in two ways. Figure 4h shows that the electrons of anthraquinone molecules can be obtained by the connecting bonds between carbon atoms (A), or by the electron tunneling effect of the second layer of graphite laminates (B). Generally, the distance limit of the electron tunneling effect is 1 nm, which is higher than the distance between the graphite layers (0.355 nm). When the sp3 bonding is not favorable for electron transfer in the redox reaction process, the electrons required for the electrochemical reaction of anthraquinone can be obtained by the electron tunneling effect. Therefore, beyond that, we believed that in addition to a small fraction of organic molecules that react with C–OH on the GF, most of the organic molecules experienced catalysis directly via the GF electrodes. This means that the electron transferability of the electrode can affect the reaction rate of the organic molecules,35,36 and subsequently increased the electrocatalytic activity of the GF to the anthraquinone derivatives. Therefore, the abovementioned two aspects are the main reasons for the improvement in the catalytic performance of the GF after heat treatment.
In this work, further inspection shows that the Bode plots shown in Figure 5 do not agree with the Bode plots shown in Figure 4. This result demonstrates that the reaction mechanism of 2,7-AQDS is different from that of 2-AQS (Figure S3). The response reaction rate of this side of the C=O unit is much slower than that of the other side of the C=O unit. Therefore, in the equivalent circuit diagram, there should be two parallel time constants, which represent the reaction process of C=O on either side of the benzene ring. For this reason, the equivalent circuit diagram shown in Figure 5g is used for fitting. The simulated curves show consistent results with the measured data, which indicated that the equivalent circuits shown in Figure 5g are suitable for describing the electrochemical reaction processes of the four samples (A-500 °C-3 h, A-550 °C-3 h, A-600 °C-3 h, M-550 °C-3 h). In the equivalent circuit diagram (Figure 5g), the sum of R2 and R3 is considered as the total resistance of the electrode. Table 4 shows the fitted values of the electrochemical impedance spectrum. It can be seen that the GF sample subjected to air-heat treatment at 550 °C for 3 h exhibits the smallest electrical resistance and the best electrochemical performance.
Figure 5.
EIS plots of three samples (a–f) and equivalent circuits (g,h) used to simulate the EIS plots(f), points: tested data, line: simulated results. The electrolyte solution contains 2,7-AQDS.
Table 4. Simulated Parameters of EIS Spectra for GFs by Heat Treatment in 2,7-AQDS Solution.
| CPE1 |
||||
|---|---|---|---|---|
| samples | R1/(Ω) | Y/(S·s–n) | n (0 < n < 1) | R2/(Ω) |
| A-500 °C-3 h | 1.077 | 0.3474 | 0.4559 | 2.050 |
| A-550 °C-3 h | 0.8636 | 0.3391 | 0.4475 | 0.1631 |
| A-600 °C-3 h | 1.221 | 0.274 | 0.4521 | 0.3742 |
| M-550 °C-3 h | 0.6944 | 1.393 | 0.9577 | 2.704 |
| CPE2 |
||||
|---|---|---|---|---|
| samples | Y/(S·s–n) | n (0 < n < 1) | R3/(Ω) | (R2 + R3)/(Ω) |
| A-500 °C-3 h | 1.327 | 0.9766 | 4.611 | 6.661 |
| A-550 °C-3 h | 1.148 | 0.9994 | 0.9038 | 1.067 |
| A-600 °C-3 h | 0.9335 | 0.9967 | 5.502 | 5.876 |
| M-550 °C-3 h | 0.3667 | 0.5410 | 1.305 | 4.009 |
To further investigate whether the reaction mechanism of the GF electrode in different anthraquinone derivatives is identical, the A-550 °C-3 h electrodes were chosen, and their electrochemical impedance measurements in five anthraquinone derivative solutions were obtained. Figure 6 shows that the Bode diagrams and the suitable equivalent circuits in the five solutions are different. The results obtained by the CVs and EIS indicate that the number and positions of the substitutional functional groups on the anthraquinone backbones affect not only the reaction rate but also the reaction mechanism. Er et al.37 reported that the effects of −CHO, −CN, −COOH, −COOCH3, and −NO2 groups on increasing the redox potential (E0) are increased when these groups are substituted for the quinone hydrogens adjacent to the ketone units. The functionalization of quinones with electron-withdrawing groups, such as −SO3H, −PO3H2, and −NO2, exhibits an opposite effect and results in high E0 values. The differences in the reaction mechanisms are precisely due to the number and locations of the substituents.
Figure 6.
EIS plots of A-550 °C-3 h GF electrode and equivalent circuits used to simulate the EIS plots, points: tested data, line: simulated results. (a,b) 2-AQS, (c,d) 1-AQS, (e,f) AR, (g,h) 2,7-AQDS, (i,j) 1,5-AQDS.
4. Conclusions
In this study, five types of anthraquinone derivatives were used as electrolyte solutions. The modified GF samples were used as the electrodes for CV and EIS. The effects of the substituents and substitutions of the five anthraquinone derivatives on the electrochemical properties of the cells were explored. The electrolyte solutions containing the anthraquinone derivatives exhibited good overall reversibility; however, disodium 9,10-anthraquinone-1,5-disulfonate and alizarin red exhibited poor reversibility. The result of electrochemical measurements showed that the electrocatalytic activities of the anthraquinone derivatives containing one sulfonate are better than those of the anthraquinone derivatives containing two sulfonates, which are superior to that of alizarin red. According to the redox reaction process using the anthraquinone derivatives, two C=O groups on the benzene rings in the anthraquinones react with two H+ in the solution to generate two C–OH groups. The change in the type, number, and position of the functional group of the anthraquinone derivatives exerts a certain influence on the reaction process. When the number of functional groups is increased or the substitution position is closer to the C=O groups, a more pronounced hindrance in the C=O reaction on the benzene ring is observed, and thus, the electrocatalytic activity and reversibility decrease. On the basis of all the test results of the five derivatives, the 2-AQS electrolyte solution is the best electrolyte in terms of both the reversibility and electrochemical performance.
With respect to the surface treatment of GF, the effects of different heat treatment conditions on the electrocatalytic activities of the GF electrodes were explored, and air, vacuum, and a mixed air-vacuum were used to generate the three types of GF modification methods. With respect to acid-treatment methods, the electrocatalytic performance of the GF changed by soaking it in pure concentrated sulfuric acid and by mixing concentrated sulfuric acid with concentrated nitric acid at different volume ratios and then soaking in the mixed liquor. Furthermore, the two methods of mixed acid and mixed heating treatments were combined to explore the influence of the mixed treatments on the electrocatalytic performance of the GF. Finally, the surface of the GF was modified by nitrogen doping. The results show that all the surface-treatment methods of GFs are beneficial for improving their electrochemical performance. Among the samples, the air-heat-treated GF samples exhibited the best electrochemical performance. Studies of the morphology and crystal structure of the GF samples after heat treatment with air showed that the graphite surface of the GF sample was reduced and became smoother after air-heat treatment, and that the lattice parameters of the carbon fiber were reduced. This result shows that the air-heat-treatment method will exfoliate the outer layer of the GF fiber at the pores and destruction sites, which can improve the catalytic activity of the GF electrode to some extent. According to the analysis of the XPS results, the heat treatment process improves the surface of the GF by increasing the content of C–OH functional groups. C=O groups are beneficial for improving the electron-transfer capabilities of the GF, and the C–OH groups are beneficial for improving the catalytic performance of the oxidation–reduction reaction with the hydrazine derivative electrolyte. The results of all the tests show that the physical properties and electrochemical catalytic performances of the GF samples are the best after air-heat treatment at 550 °C for 3 h.
Acknowledgments
This work was supported by Tianjin Natural Science Foundation (grant no 16JCYBJC21100 and 16JCQNJC06000).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01103.
XRD, SEM, the molecular structures of the five anthraquinone derivatives, and electrochemical reaction equation and the analytic result of CV (PDF)
Author Contributions
These authors contributed equally. The paper was written through contributions of all the authors. All the authors have given approval to the final version of the paper.
The authors declare no competing financial interest.
Supplementary Material
References
- Chen H.; Lu Y.-C. A High-Energy-Density Multiple Redox Semi-Solid-Liquid Flow Battery. Adv. Energy Mater. 2016, 6, 1502183. 10.1002/aenm.201502183. [DOI] [Google Scholar]
- Takechi K.; Kato Y.; Hase Y. A Highly Concentrated Catholyte Based on a Solvate Ionic Liquid for Rechargeable Flow Batteries. Adv. Mater. 2015, 27, 2501–2506. 10.1002/adma.201405840. [DOI] [PubMed] [Google Scholar]
- Gerhardt M. R.; Tong L.; Gómez-Bombarelli R.; Chen Q.; Marshak M. P.; Galvin C. J.; Aspuru-Guzik A.; Gordon R. G.; Aziz M. J. Anthraquinone Derivatives in Aqueous Flow Batteries. Adv. Energy Mater. 2017, 7, 1601488. 10.1002/aenm.201601488. [DOI] [Google Scholar]
- Winsberg J.; Hagemann T.; Janoschka T.; Hager M. D.; Schubert U. S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chem., Int. Ed. 2017, 56, 686–711. 10.1002/anie.201604925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K.; Fang Q.; Gu S.; Li S. F. Y.; Yan Y. Nonaqueous Redox-Flow Batteries: Organic Solvents, Supporting Electrolytes, and Redox Pairs. Energy Environ. Sci. 2015, 8, 3515–3530. 10.1039/c5ee02341f. [DOI] [Google Scholar]
- Zhang J.; Corman R. E.; Schuh J. K.; Ewoldt R. H.; Shkrob I. A.; Zhang L. Solution Properties and Practical Limits of Concentrated Electrolytes for Nonaqueous Redox Flow Batteries. J. Phys. Chem. C 2018, 122, 8159–8172. 10.1021/acs.jpcc.8b02009. [DOI] [Google Scholar]
- Ding Y.; Zhang C.; Zhang L.; Zhou Y.; Yu G. Molecular Engineering of Organic Electroactive Materials for Redox Flow Batteries. Chem. Soc. Rev. 2018, 47, 69–103. 10.1039/c7cs00569e. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Tong L.; Tabor D. P.; Beh E. S.; Goulet M.-A.; De Porcellinis D.; Aspuru-Guzik A.; Gordon R. G.; Aziz M. J. Alkaline Benzoquinone Aqueous Flow Battery for Large-Scale Storage of Electrical Energy. Adv. Energy Mater. 2018, 8, 1702056. 10.1002/aenm.201702056. [DOI] [Google Scholar]
- Huskinson B.; Marchak M. P.; Sch C.; Er S.; Gerhardt M. R.; Galvin C. J.; Chen X.; Aspuru-Guzik A.; Gordon R. G.; Aziz M. J. A Metal-Free Organic-Inorganic Aqueous Flow Battery. Nature 2014, 505, 195–198. 10.1038/nature12909. [DOI] [PubMed] [Google Scholar]
- Lin K.; Rafael G.; Beh E. S.; Tong L.; Chen Q.; Valle A.; Aspuru-Guzik A.; Aziz M. J.; Gordon R. G. A Redox-Flow Battery with an Alloxazine-Based Organic Electrolyte. Nat. Energy 2016, 1, 16102. 10.1038/nenergy.2016.102. [DOI] [Google Scholar]
- Lin K.; Chen Q.; Gerhardt M. R.; Tong L.; Kim S. B.; Eisenach L.; Valle A. W.; Hardee D.; Gordon R. G.; Aziz M. J.; Marshak M. P. Alkaline quinone flow battery. Science 2015, 349, 1529–1532. 10.1126/science.aab3033. [DOI] [PubMed] [Google Scholar]
- Gerhardt M. R.; Tong L.; Gómez-Bombarelli R.; Chen Q.; Marshak M. P.; Galvin C. J.; Aspuru-Guzik A.; Gordon R. G.; Aziz M. J. Anthraquinone Derivatives in Aqueous Flow Batteries. Adv. Energy Mater. 2017, 7, 1601488. 10.1002/aenm.201601488. [DOI] [Google Scholar]
- Hofmann J. D.; Pfanschilling F. L.; Krawczyk N.; Geigle P.; Hong L.; Schmalisch S.; Wegner H. A.; Mollenhauer D.; Janek J.; Schröder D. Quest for Organic Active Materials for Redox Flow Batteries: 2,3-Diaza-anthraquinones and Their Electrochemical Properties. Chem. Mater. 2018, 30, 762–774. 10.1021/acs.chemmater.7b04220. [DOI] [Google Scholar]
- Yang B.; Hoober-Burkhardt L.; Krishnamoorthy S.; Murali A.; Prakash G. K. S.; Narayanan S. R. An Inexpensive Aqueous Flow Battery for Large-Scale Electrical Energy Storage Based on Water-Soluble Organic Redox couples. J. Electrochem. Soc. 2016, 163, A1442–A1449. 10.1149/2.1371607jes. [DOI] [Google Scholar]
- Xu Y.; Wen Y.; Cheng J.; Yanga Y.; Xie Z.; Cao G.. Novel organic redox flow batteries using soluble quinonoid compounds as positive materials. 2009 World Non-Grid-Connected Wind Power and Energy Conference, 2009.
- Chen Q.; Gerhardt M. R.; Hartle L.; Aziz M. J. Cycling analysis of a quinone-bromide redox flow battery. J. Electrochem. Soc. 2016, 163, A5010–A5013. 10.1149/2.0021601jes. [DOI] [Google Scholar]
- Hosseini M. G.; Mousavihashemi S.; Murcia-López S.; Flox C.; Andreu T.; Morante J. R. High-power positive electrode based on synergistic effect of N- and WO3-decorated carbo n felt for vanadium redox flow batteries. Carbon 2018, 136, 444–453. 10.1016/j.carbon.2018.04.038. [DOI] [Google Scholar]
- He Z.; Jiang Y.; Meng W.; Jiang F.; Zhou H.; Li Y.; Zhu J.; Wang L.; Dai L. HF/H2O2 treated graphite felt as the positive electrode for vanadium redox flow battery. Appl. Surf. Sci. 2017, 423, 111–118. 10.1016/j.apsusc.2017.06.154. [DOI] [Google Scholar]
- Lee W.; Jo C.; Youk S.; Shin H. Y.; Lee J.; Chung Y.; Kwon Y. Mesoporous tungsten oxynitride as electrocatalyst for promoting redox reactions of vanadium redox couple and performance of vanadium redox flow battery. Appl. Surf. Sci. 2018, 429, 187–195. 10.1016/j.apsusc.2017.07.022. [DOI] [Google Scholar]
- Mazúr P.; Mrlík J.; Beneš J.; Pocedič J.; Vrána J.; Dundálek J.; Kosek J. Performance evaluation of thermal treated graphite felt electrode for vanadium redox flow battery and their four-point single cell characterization. J. Power Sources 2018, 380, 105–114. 10.1016/j.jpowsour.2018.01.079. [DOI] [Google Scholar]
- Li Y.; Liu Z.; Wu Y.; Chen J.; Zhao J.; Jin F.; Na P. Carbon dots-TiO2 nanosheets composites for photoreduction of Cr(VI) under sunlight illumination: Favorable role of carbon dots. Appl. Catal. B Environ. 2018, 224, 508–517. 10.1016/j.apcatb.2017.10.023. [DOI] [Google Scholar]
- Park J. E.; Kim M.-J.; Lim M. S.; Kang S. Y.; Kim J. K.; Oh S.-H.; Her M.; Cho Y.-H.; Sung Y.-E. Graphitic carbon nitride-carbon nanofiber as oxygen catalyst in anion-exchange membrane water electrolyzer and rechargeable metal–air cells. Appl. Catal., B 2018, 237, 140–148. 10.1016/j.apcatb.2018.05.073. [DOI] [Google Scholar]
- Li X.; Fang Y.; Lin X.; Tian M.; An X.; Fu Y.; Li R.; Jin J.; Ma J. MOF derived Co3O4 nanoparticles embedded in N-doped mesoporous carbon layer/MWCNT hybrids: extraordinary bi-functional electrocatalysts for OER and ORR. J. Mater. Chem. A 2015, 3, 17392–17402. 10.1039/c5ta03900b. [DOI] [Google Scholar]
- Liu X.; Zhou W.; Yang L.; Li L.; Zhang Z.; Ke Y.; Chen S. Nitrogen and sulfur codoped porous carbon derived from human hair as highly efficient metal-free electrocatalysts for hydrogen evolution reactions. J. Mater. Chem. A 2015, 3, 8840–8846. 10.1039/c5ta01209k. [DOI] [Google Scholar]
- González Z.; Álvarez P.; Blanco C.; Vega-Díaz S.; Tristán-López F.; Rajukumar L. P.; Cruz-Silva R.; Elías A. L.; Terrones M.; Menéndez R. The influence of carbon nanotubes characteristics in their performance as positive electrodes in vanadium redox flow batteries. Sustainable Energy Technol. Assess. 2015, 9, 105–110. 10.1016/j.seta.2014.12.008. [DOI] [Google Scholar]
- Jeong S.; An S.; Jeong J.; Lee J.; Kwon Y. Effect of mesocelluar carbon foam electrode material on performance of vanadium redox flow battery. J. Power Sources 2015, 278, 245–254. 10.1016/j.jpowsour.2014.12.074. [DOI] [Google Scholar]
- Yang X.; Hu X.; Wang X.; Fu W.; He X.; Asefa T. Metal-organic framework-derived Fe3C@NC nanohybrids as highly-efficient oxygen reduction electrocatalysts in both acidic and basic media. J. Electroanal. Chem. 2018, 823, 755–764. 10.1016/j.jelechem.2018.07.024. [DOI] [Google Scholar]
- Shah A. B.; Wu Y.; Joo Y. L. Direct addition of sulfur and nitrogen functional groups to graphite felt electrodes for improving all-vanadium redox flow battery performance. Electrochim. Acta 2019, 297, 905–915. 10.1016/j.electacta.2018.12.052. [DOI] [Google Scholar]
- Batchelor-McAuley C.; Li Q.; Dapin S. M.; Compton R. G. Voltammetric Characterization of DNA Intercalators across the Full pH Range: Anthraquinone-2,6-disulfonate and Anthraquinone-2-sulfonate. J. Phys. Chem. B 2010, 114, 4094–4100. 10.1021/jp1008187. [DOI] [PubMed] [Google Scholar]
- Damjanovic A.; Dey A.; Bockris J. O. M. Kinetics of oxygen evolution and dissolution on platinum electrodes. Electrochim. Acta 1966, 11, 791–814. 10.1016/0013-4686(66)87056-1. [DOI] [Google Scholar]
- Sun B.; Skyllas-Kazacos M. Chemical modification of graphite electrode materials for vanadium redox flow battery application-part II. Acid treatments. Electrochim. Acta 1992, 37, 2459–2465. 10.1016/0013-4686(92)87084-d. [DOI] [Google Scholar]
- Sun B.; Skyllas-Kazacos M. Modification of graphite electrode materials for vanadium redox flow battery application- Part I. Thermal treatment. Electrochim. Acta 1992, 37, 1253–1260. 10.1016/0013-4686(92)85064-r. [DOI] [Google Scholar]
- Chou Y.-S.; Jeng K.-T.; Yen S.-C. Characterization and electrochemical properties of graphite felt-based electrode modified using an ionomer impregnation approach for vanadium redox flow battery. Electrochim. Acta 2017, 251, 109–118. 10.1016/j.electacta.2017.08.064. [DOI] [Google Scholar]
- Llobet E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators, B 2013, 179, 32–45. 10.1016/j.snb.2012.11.014. [DOI] [Google Scholar]
- Chen P.; McCreery R. L. Control of electron transfer kinetics at glassy carbon electrodes by specific surface modification. Anal. Chem. 1996, 68, 3958–3965. 10.1021/ac960492r. [DOI] [Google Scholar]
- McCreery R. L.; McDermott M. T. Comment on electrochemical kinetics at ordered graphite electrodes. Anal. Chem. 2012, 84, 2602–2605. 10.1021/ac2031578. [DOI] [PubMed] [Google Scholar]
- Er S.; Suh C.; Marshak M. P.; Aspuru-Guzik A. Computational design of molecules for an all-quinone redox flow battery. Chem. Sci. 2015, 6, 885–893. 10.1039/c4sc03030c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.