Abstract
Background.
Robotic lobectomy has been described for non-small cell lung cancer (NSCLC). Our objectives were to (1) evaluate the use of robotic lobectomy over time, (2) identify factors associated with its use, and (3) assess outcomes after robotic lobectomy compared with other surgical approaches.
Methods.
Stage I to IIIA NSCLC patients were identified from the National Cancer Data Base (2010 to 2012). Trends in robotic lobectomy were assessed over time, and multivariable logistic regression models were developed to identify factors associated with its use. Propensity-matched cohorts were constructed to compare post-operative outcomes after robotic lobectomy with thoracoscopic and open lobectomy.
Results.
Lobectomy was performed in 62,206 patients by open (n = 45,527), thoracoscopic (n = 12,990), or robotic (n = 3,689) procedures at 1,215 hospitals. Between 2010 and 2012, robotic lobectomy significantly increased, from 3.0% to 9.1% (p < 0.001). Academic (odds ratio, 1.55; 95% confidence interval, 1.04 to 2.33) and high-volume hospitals (odds ratio, 1.49; 95% confidence interval, 1.04 to 2.14) were associated with increased use of robotic lobectomy. Length of stay was shorter in robotic lobectomy compared with open lobectomy (6.1 vs 6.9 days; p < 0.001). Fewer lymph nodes (9.9 vs 10.9; p < 0.001) and 12 or more nodes were examined less frequently (32.0% vs 35.6%; p = 0.005) in robotic resections than in thoracoscopic resections. There was no difference between robotic and open or robotic and thoracoscopic lobectomy patients in margin positivity, 30-day readmission, and deaths at 30 and 90 days.
Conclusions.
Robotic lobectomies have significantly increased in stage I to IIIA NSCLC patients, with outcomes similar to other approaches. Additional studies are needed to determine if this technology offers potential advantages compared with video-assisted thoracoscopic operations.
Minimally-invasive techniques in lung cancer resection have been well-described [1–3]. Studies of open operations compared with video-assisted thoracoscopic surgery (VATS) have firmly established the latter as a safe and favorable approach for use in pulmonary resections [4, 5]. Consequently, the proportion of patients undergoing VATS has significantly increased in recent years [6].
Use of robotic technology in the surgical treatment of non-small cell lung cancer (NSCLC) is increasingly being reported [2, 3]. Proponents of robotic lung resection have noted several benefits, including high-definition threedimensional visualization of relevant anatomy, improved maneuverability and articulation in confined spaces, such as in mediastinal lymph node dissection, and accessibility for learners in teaching cases [2, 7]. However, several disadvantages to robotic surgery have also been raised, including its steep learning curve, increased operative times, higher costs, and concerns regarding the absence of haptic feedback, with some evidence suggesting higher iatrogenic complications [2, 3, 8].
Several studies have examined outcomes after robotic lung resection. However, studies are limited because of factors such as reports from single institutions that include data from few surgeons, reliance on administrative rather than clinically-collected data, small sample sizes resulting in underpowered analyses, and the absence of oncologically-relevant outcomes data [7, 9–11]. Moreover, national data evaluating the use of robotic lung resection in stage I to IIIA patients in the current era are lacking.
Our objectives in this study were to (1) evaluate the use of robotic lobectomy over time, (2) identify patient-, tumor-, and hospital-level factors associated with its use, and (3) assess postoperative outcomes after robotic lobectomy compared with other surgical approaches.
Material and Methods
This study was reviewed and considered exempt by the Northwestern University Institutional Review Board.
Data Source and Study Population
The National Cancer Data Base (NCDB) is an oncology registry that longitudinally collects demographic variables, tumor-related data, treatment information, and clinical outcomes for patients with neoplastic diseases [12, 13]. Data are clinically abstracted by trained and audited registrars [12]. Participating hospitals are provided cancer-specific data for the purposes of quality. More than 1,500 facilities participate in the NCDB, capturing more than 70% of all newly diagnosed cancer cases in the United States [12].
Patients aged 18 and older who underwent operations for stage I to IIIA NSCLC between 2010 and 2012 were identified. Pathologic stage was preferentially used in patients unless unavailable, in which case clinical stage was used. The study excluded patients in whom a VATS or robotic operation was attempted but were subsequently converted to an open operation (VATS, 18.9%; robotic, 10.3%). As a sensitivity test, these patients were included in an additional propensity-matched analysis. Patients were included only if they underwent a lobectomy or bilobectomy in a NCDB-participating hospital during the entirety of this 3-year period.
Study Variables
Population area was ascertained by linking patient ZIP codes with the 2000 United States Census Bureau data [13]. Comorbidities evaluated included chronic obstructive pulmonary disease, congestive heart failure, diabetes mellitus, hypertension, and peripheral vascular disease. Tumor-specific variables assessed included tumor size, anatomic location, histology, and stage. Hospitals were categorized into community, academic, National Cancer Institute (NCI)-designated, or “other” according to their NCDB assignment during accreditation. In addition, we assigned hospitals into quartiles by their surgical lobectomy volume during the 3-year study period.
Several outcomes were assessed. Mean hospital length of stay (LOS), prolonged LOS (defined as >14 hospital days), and lymph node sampling have previously been evaluated in studies assessing robotic lung resection [3, 7, 10, 14, 15]. Margin positivity, with positive microscopic (R1) or macroscopic (R2) disease, is another oncologic quality measure used in studies evaluating surgical resection [2, 16]. We also assessed 30-day unplanned hospital readmission, and deaths at 30 days and 90 days.
Statistical Analysis
Baseline characteristics were compared using χ2 testing for categoric variables and t tests for continuous variables. Use of different surgical approaches over time was assessed with the Cochran-Armitage trend test.
In addition, we sought to identify patient-, tumor-, and hospital-level factors associated with robotic operations. We developed multivariable logistic regression models with robust cluster-corrected SEs to account for clustering of patients within hospitals. The dependent variable evaluated was robotic lobectomy, and the covariates included in these models were based on similar studies evaluating adoption of new technology [16, 17].
In unmatched analyses, χ2 testing was used for categoric variables and the Wilcoxon rank sum test was used for continuous variables. Propensity-score matching is a method used in observational studies to reduce treatment-selection bias inherent in these study designs [1, 3, 18]. In this approach, a multivariable logistic regression model including potentially confounding covariates was developed with the dependent variable being use of a robotic approach [18]. Covariates chosen for inclusion were based on clinical relevance and prior studies and included age, sex, race, population area, history of chronic obstructive pulmonary disease, congestive heart failure, diabetes, hypertension, peripheral vascular disease, tumor size, anatomic location, histology, stage, hospital type, and hospital surgical volume [3, 19]. Propensity scores were derived for the probability of undergoing a robotic operation. Propensity-score nearest-neighbor matching was then used, using the logit of the propensity score with a caliper of 0.2 multiplied by the SD [18, 19]. Propensity-matched data were assessed using the McNemar test for categorical data and the Wilcoxon signed rank test for continuous data. An additional sensitivity analysis was also performed in which VATS or robotic patients who had conversion to an open procedure were included (Supplemental Table). All p values reported are two-sided, with statistical significance considered to be 0.05. Analyses were performed in SAS 9.3 software (SAS Institute Inc, Cary, NC).
Results
Between 2010 and 2012, 62,206 patients from 1,215 hospitals underwent lobectomy for stage I to IIIA NSCLC. Lobectomy was most often performed using an open approach (45,527 [73.2%]), followed by VATS (12,990 [20.9%]) and then robotic (3,689 [5.9%]). Patient demographic and clinical characteristics are presented in Table 1.
Table 1.
Baseline Characteristics in Open, Video-Assisted Thoracic Surgery, and Robotic Lobectomy Patients (N = 62,206)
| Open (n = 45,527) |
VATS (n = 12,990) |
Robotic (n = 3,689) |
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | SD | Mean | SD | Mean | SD | p Valuea | p Valueb |
| No. | % | No. | % | No. | % | |||
| Age, y | 67.0 | 10.1 | 67.3 | 10.1 | 67.6 | 9.7 | <0.001 | 0.145 |
| Female sex | 23,701 | 52.1 | 7,219 | 55.6 | 2,027 | 54.9 | 0.001 | 0.499 |
| Race | ||||||||
| White | 38,666 | 84.9 | 11,188 | 86.1 | 2,997 | 81.2 | <0.001 | <0.001 |
| Black | 4,075 | 9.0 | 978 | 7.5 | 362 | 9.8 | ||
| Other | 2,786 | 6.1 | 824 | 6.3 | 330 | 8.9 | ||
| Population areac | ||||||||
| Metropolitan | 35,108 | 77.1 | 10,558 | 81.3 | 3,060 | 82.9 | <0.001 | 0.021 |
| Nonmetropolitan or unspecified | 10,419 | 22.9 | 2,432 | 18.7 | 629 | 17.1 | ||
| Comorbidities | ||||||||
| COPD | 15,941 | 35.0 | 4,310 | 33.2 | 1,337 | 36.2 | 0.133 | 0.001 |
| Congestive heart failure | 1,227 | 2.7 | 319 | 2.5 | 100 | 2.7 | 0.955 | 0.382 |
| Diabetes | 6,973 | 15.3 | 1,964 | 15.1 | 605 | 16.4 | 0.079 | 0.057 |
| Hypertension | 19,954 | 43.8 | 6,010 | 46.3 | 1,736 | 47.1 | <0.001 | 0.394 |
| Peripheral vascular disease | 2,607 | 5.7 | 717 | 5.5 | 244 | 6.6 | 0.026 | 0.012 |
| Tumor size | ||||||||
| <3.0 cm | 25,210 | 55.4 | 8,252 | 63.5 | 2,414 | 65.4 | <0.001 | 0.001 |
| 3.0–4.9 cm | 12,638 | 27.8 | 3,314 | 25.5 | 955 | 25.9 | ||
| 5.0–6.9 cm | 4,772 | 10.5 | 961 | 7.4 | 228 | 6.2 | ||
| ≥7.0 cm | 2,907 | 6.4 | 463 | 3.6 | 92 | 2.5 | ||
| Tumor locationc | ||||||||
| Lower lobe | 14,579 | 32.0 | 4,314 | 33.2 | 1,292 | 35.0 | <0.001 | 0.141 |
| Middle lobe | 2,754 | 6.0 | 910 | 7.0 | 264 | 7.2 | ||
| Upper lobe | 26,570 | 58.4 | 7,483 | 57.6 | 2,047 | 55.5 | ||
| Other or unspecified | 1,624 | 3.6 | 283 | 2.2 | 86 | 2.3 | ||
| Histology | ||||||||
| Adenocarcinoma | 26,229 | 57.6 | 8,275 | 63.7 | 2,298 | 62.3 | <0.001 | 0.145 |
| Squamous | 12,852 | 28.2 | 2,978 | 22.9 | 888 | 24.1 | ||
| Carcinoid | 2,647 | 5.8 | 788 | 6.1 | 249 | 6.7 | ||
| Other | 3,799 | 8.3 | 949 | 7.3 | 254 | 6.9 | ||
| AJCC stage | ||||||||
| IA | 18,385 | 40.4 | 6,129 | 47.2 | 1,818 | 49.3 | <0.001 | 0.127 |
| IB | 9,991 | 21.9 | 3,000 | 23.1 | 845 | 22.9 | ||
| IIA | 6,664 | 14.6 | 1,631 | 12.6 | 433 | 11.7 | ||
| IIB | 4,822 | 10.6 | 1,008 | 7.8 | 255 | 6.9 | ||
| IIIA | 5,665 | 12.4 | 1,222 | 9.4 | 338 | 9.2 | ||
| Hospital type | ||||||||
| Community | 30,228 | 66.4 | 6,732 | 51.8 | 1,830 | 49.6 | <0.001 | <0.001 |
| Academic | 11,144 | 24.5 | 3,686 | 28.4 | 1,236 | 33.5 | ||
| NCI designated | 3,968 | 8.7 | 2,558 | 19.7 | 622 | 16.9 | ||
| Other | 187 | 0.4 | 14 | 0.1 | 1 | 0.0 | ||
| Hospital volume | ||||||||
| Quartile 1 | 1,759 | 3.9 | 287 | 2.2 | 25 | 0.7 | <0.001 | <0.001 |
| Quartile 2 | 5,812 | 12.8 | 1,074 | 8.3 | 232 | 6.3 | ||
| Quartile 3 | 11,751 | 25.8 | 2,403 | 18.5 | 749 | 20.3 | ||
| Quartile 4 | 26,205 | 57.6 | 9,226 | 71.0 | 2,683 | 72.7 | ||
Open and robotic lobectomy.
VATS and robotic lobectomy.
Population area unknown in 2,077 (3.3%) and tumor location unknown in 1,824 (2.8%) patients.
AJCC — American Joint Committee on Cancer; COPD — chronic obstructive pulmonary disease; NCI — National Cancer Institute; VATS — video-assisted thoracic surgery.
Use of Robotic Lobectomy in NSCLC
The number of hospitals performing at least 1 robotic lobectomy increased by 66.7% during the study period, from 153 (12.6%) in 2010 to 255 (21.0%) in 2012 (Table 2). During this period, hospitals that performed at least 1 robotic lobectomy also significantly increased their median case volume, from 2 (interquartile range, 1 to 4) in 2010 to 4 (interquartile range, 1 to 9) in 2012 (p < 0.001). Between 2010 and 2012, the percentage of robotic lobectomies significantly increased from 3.0% to 9.1% (p < 0.001; Fig 1). During the same period, VATS lobectomies significantly increased from 16.9% to 24.0% (p < 0.001) as did the overall percentage of minimally- invasive lobectomies (19.9% to 33.1%; p < 0.001).
Table 2.
Number of Hospitals Performing at Least One Robotic Lobectomy Over Time
| Variable | 2010 (n = 1,215) |
2011 (n = 1,215) |
2012 (n = 1,215) |
|---|---|---|---|
| Hospitals, No. (%) | 153 (12.6) | 203 (16.7) | 255 (21.0) |
| Volume of robotic cases, median (IQR) | 2 (1–4) | 3 (1–7) | 4 (1–9) |
IQR = interquartile range.
Fig 1.
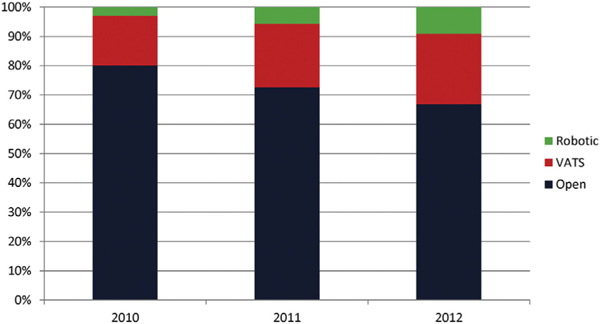
Use of open, video-assisted thoracic surgery (VATS), and robotic lobectomy (2010 to 2012).*Significant increase in robotic lobectomy from 3.0% to 9.1% between 2010 and 2012 (p < 0.001).
Factors Associated With the Use of Robotic Lobectomy in NSCLC
In multivariable analysis, older age (66 to 75 years: odds ratio [OR], 1.16; 95% confidence interval [CI], 1.02 to 1.33; >75 years: OR, 1.20; 95% CI, 1.03 to 1.41 vs ≤55 years) and living in a metropolitan area (OR, 1.29; 95% CI, 1.05 to 1.58) were associated with an increased likelihood to undergo robotic lobectomy (Table 3). Patients with larger tumors were less likely to undergo robotic lobectomy (5.0 to 6.9 cm: OR, 0.63; 95% CI, 0.53 to 0.76; ≥7.0 cm: OR, 0.46; 95% CI, 0.35 to 0.60; vs <3.0 cm), as were patients with higher staged disease (IIA: OR, 0.85; 95% CI, 0.75 to 0.96; IIB: OR, 0.81; 95% CI, 0.69 to 0.96; IIIA: OR, 0.80; 95% CI, 0.69 to 0.93; vs stage IA). Undergoing the operation at an academic or NCI-designated center (OR, 1.55; 95% CI, 1.03 to 2.33) and an operation at the highest hospital volume quartile (OR, 1.48; 95% CI, 1.03 to 2.12) were both associated with increased use of robotic operations (Table 3).
Table 3.
Factors Associated With the Use of Robotic Lobectomy in Stage I to IIIA Non-Small Cell Lung Cancer Patients
| OR (95% CI) | |||
|---|---|---|---|
| Characteristic | Unadjusted | Adjusted | p Valuea |
| Sex (male) | 0.92 (0.86–0.98) | 0.98 (0.91–1.06) | 0.637 |
| Age (years) | |||
| ≤55 | Referent | ||
| 56–65 | 1.02 (0.91–1.15) | 1.04 (0.92–1.18) | 0.504 |
| 66–75 | 1.13 (1.01–1.25) | 1.16 (1.02–1.33) | 0.024 |
| >75 | 1.13 (1.00–1.27) | 1.20 (1.03–1.41) | 0.022 |
| Race | |||
| White | Referent | ||
| Black | 1.19 (1.07–1.33) | 1.14 (0.94–1.39) | 0.191 |
| Other | 1.52 (1.35–1.71) | 1.44 (0.92–2.26) | 0.115 |
| Population area | |||
| Nonmetropolitan or unspecified | Referent | ||
| Metropolitan | 1.37 (1.25–1.50) | 1.29 (1.05–1.58) | 0.013 |
| Comorbidities | |||
| COPD | 1.07 (1.00–1.15) | 1.14 (1.02–1.28) | 0.018 |
| Congestive heart failure | 1.03 (0.84–1.26) | 1.02 (0.82–1.26) | 0.880 |
| Diabetes | 1.09 (1.00–1.19) | 1.05 (0.95–1.16) | 0.347 |
| Hypertension | 1.11 (1.04–1.19) | 1.05 (0.94–1.16) | 0.395 |
| Peripheral vascular disease | 1.18 (1.03–1.35) | 1.18 (1.03–1.35) | 0.016 |
| Tumor size | |||
| <3.0 cm | Referent | ||
| 3.0–4.9 cm | 0.83 (0.77–0.90) | 0.87 (0.75–1.01) | 0.061 |
| 5.0–6.9 cm | 0.55 (0.48–0.63) | 0.63 (0.53–0.76) | <0.001 |
| ≥7.0 cm | 0.38 (0.31–0.47) | 0.46 (0.35–0.60) | <0.001 |
| Tumor location | |||
| Lower lobe | Referent | ||
| Middle lobe | 1.05 (0.92–1.21) | 0.95 (0.79–1.14) | 0.591 |
| Upper lobe | 0.88 (0.82–0.95) | 0.85 (0.77–0.94) | 0.001 |
| Other or unspecified | 0.66 (0.53–0.83) | 0.70 (0.54–0.92) | 0.001 |
| Histology | |||
| Adenocarcinoma | Referent | ||
| Squamous | 0.84 (0.78–0.91) | 0.91 (0.83–1.00) | 0.049 |
| Carcinoid | 1.09 (0.95–1.25) | 1.03 (0.89–1.20) | 0.652 |
| Other | 0.80 (0.70–0.92) | 0.91 (0.78–1.07) | 0.255 |
| AJCC stage | |||
| IA | Referent | ||
| IB | 0.88 (0.81–0.95) | 0.98 (0.88–1.09) | 0.724 |
| IIA | 0.70 (0.63–0.78) | 0.85 (0.75–0.96) | 0.009 |
| IIB | 0.59 (0.52–0.67) | 0.81 (0.69–0.96) | 0.015 |
| IIIA | 0.66 (0.59–0.75) | 0.80 (0.69–0.93) | 0.004 |
| Hospital type | |||
| Community/other | Referent | ||
| Academic or NCI designated | 1.77 (1.65–1.89) | 1.55 (1.03–2.33) | 0.037 |
| Hospital volume | |||
| Quartile 1, 2, and 3 | Referent | ||
| Quartile 4 (highest volume) | 1.74 (1.61–1.87) | 1.48 (1.03–2.12) | 0.033 |
P value from multivariable regression model.
AJCC = American Joint Committee on Cancer; CI = confidence interval; COPD = chronic obstructive pulmonary disease; NCI = National Cancer Institute; OR = odds ratio.
Unmatched Analyses of Postoperative Outcomes
In unmatched analyses, patients undergoing open lobectomy had a significantly higher mean LOS (7.4 [SD 6.7] days vs 6.1 [SD, 5.50] days; p < 0.001) and a higher proportion with prolonged LOS (8.0% vs 5.9%; p < 0.001) compared with robotic patients (Table 4). The rate of prolonged LOS was significantly lower in VATS patients compared with robotic lobectomy patients (4.8% vs 5.9%; p — 0.007). VATS also had higher mean lymph node counts (10.8 [SD, 8.6] nodes vs 9.9 [SD, 7.3] nodes; p < 0.001) and a higher proportion of patients with 12 or more lymph nodes examined (35.4% vs 32.0%; p < 0.001). Margin positivity was significantly higher in those undergoing an open operation (5.1% vs 3.4%; p < 0.001) than a robotic operation. The unplanned readmission rate was higher after VATS lobectomy (5.0% vs 4.1%; p — 0.018) than after robotic lobectomy. Robotic lobectomy had lower 30-day (1.7% vs 2.4%; p — 0.006) and 90-day (3.0% vs 4.8%; p < 0.001) mortality rates compared with open lobectomy, although both were similar to VATS lobectomy (Table 4).
Table 4.
Comparison of Outcomes in Unmatched Open, Video-Assisted Thoracic Surgery, and Robotic Lobectomy Patients
| Outcome | Open | VATS | Robotic | p Valuea | p Valueb |
|---|---|---|---|---|---|
| Hospital LOS, mean (SD), d | 7.4 (6.7) | 6.0 (6.3) | 6.1 (5.5) | <0.001 | 0.025 |
| Prolonged LOS, No. (%) | 3,467/43,405 (8.0) | 600/12,604 (4.8) | 210/3,570 (5.9) | <0.001 | 0.007 |
| Lymph nodes examined, mean (SD), No. | 9.6 (7.4) | 10.8 (8.6) | 9.9 (7.3) | 0.003 | <0.001 |
| ≥12 lymph nodes examined, No. (%) | 12,582/42,601 (29.5) | 4,199/11,877 (35.4) | 1,096/3,423 (32.0) | 0.002 | <0.001 |
| Positive surgical margin, No. (%) | 2,333/45,527 (5.1) | 500/12,990 (3.8) | 125/3,689 (3.4) | <0.001 | 0.194 |
| 30-day unplanned readmission, No. (%) | 2,011/45,342 (4.4) | 651/12,969 (5.0) | 150/3,680 (4.1) | 0.308 | 0.018 |
| 30-day mortality, No. (%) | 1,070/43,791 (2.4) | 189/12,432 (1.5) | 58/3,429 (1.7) | 0.006 | 0.474 |
| 90-day mortality, No. (%) | 2,013/42,334 (4.8) | 349/11,943 (2.9) | 97/3,238 (3.0) | <0.001 | 0.826 |
Open and robotic lobectomy.
VATS and robotic lobectomy.
LOS = length of stay; VATS = video-assisted thoracic surgery.
Propensity-Matched Analyses of Postoperative Outcomes
In matched analyses, the mean LOS was significantly lower in those undergoing robotic (6.1 [SD, 5.5] days vs 6.9 [SD, 5.7] days; p < 0.001) compared with open lobectomy (Table 5). There was no significant difference in the rates of prolonged LOS between open and robotic lobectomy (6.8% vs 5.9%; p — 0.123), although VATS patients had a significantly lower rate of prolonged LOS (4.6% vs 5.9%; p — 0.013). The mean number of examined lymph nodes was similar between open and robotic lobectomy (9.9 [SD, 7.5] nodes vs 9.9 [SD, 7.3] nodes; p — 0.746), with VATS having a significantly higher mean lymph node count (10.9 [SD, 8.8] nodes; p < 0.001). Compared with robotic lobectomy, a significantly higher proportion of VATS patients also had 12 or more lymph nodes examined (35.6% vs 32.0%; p — 0.005). There were no significant differences in margin positivity, 30-day unplanned readmission, and deaths at 30 days and 90 days when robotic patients were compared with open and VATS lobectomy patients (Table 5). In sensitivity analyses that included VATS and robotic patients who were converted to an open approach, the results were unchanged with the exception of prolonged LOS after VATS and robotic lobectomy, which was no longer statistically significant (Supplemental Table).
Table 5.
Comparison of Outcomes in Propensity-Matched Open, Video-Assisted Thoracic Surgery, and Robotic Lobectomy Patients
| Outcome | Open | VATS | Robotic | p valuea | p valueb |
|---|---|---|---|---|---|
| Hospital LOS, mean (SD), d | 6.9 (5.7) | 5.9 (6.1) | 6.1 (5.5) | <0.001 | 0.019 |
| Prolonged LOS, No. (%) | 238/3,514 (6.8) | 165/3,588 (4.6) | 210/3,570 (5.9) | 0.123 | 0.013 |
| Lymph nodes examined, mean (SD), No. | 9.9 (7.5) | 10.9 (8.8) | 9.9 (7.3) | 0.746 | <0.001 |
| ≥12 lymph nodes examined, No. (%) | 1,074/3,432 (31.3) | 1,205/3,381 (35.6) | 1,096/3,423 (32.0) | 0.699 | 0.005 |
| Positive surgical margin, No. (%) | 132/3,689 (3.6) | 126/3,689 (3.4) | 125/3,689 (3.4) | 0.656 | 0.948 |
| 30-day unplanned readmission, No. (%) | 146/3,674 (4.0) | 170/3,682 (4.6) | 150/3,680 (4.1) | 0.810 | 0.258 |
| 30-day mortality, No. (%) | 64/3,530 (1.8) | 51/3,553 (1.4) | 58/3,429 (1.7) | 0.389 | 0.545 |
| 90-day mortality, No. (%) | 117/3,405 (3.4) | 95/3,401 (2.8) | 97/3,238 (3.0) | 0.091 | 0.877 |
Open and robotic lobectomy.
VATS and robotic lobectomy.
LOS = length of stay; VATS = video-assisted thoracic surgery.
Comment
In this study of patients with stage I to IIIA NSCLC, we found a significant increase in the use of robotic lobectomy in recent years, with several predictive factors associated with its use. To our knowledge, this study presents the largest series of patients who underwent robotic operations for NSCLC to date. Our study suggests outcomes after robotic lobectomy are comparable to those after VATS and may be a valuable alternative in offering patients a minimally-invasive surgical option.
Use and Predictors of Robotic Lobectomy in NSCLC
In a multistate study, Kent and colleagues [3] reported an increase in the use of robotic resection from 0.2% in 2008 to 3.4% in 2010. Our study similarly found that 3.0% of all lobectomies were performed robotically in 2010, with a threefold increase in use by 2012. This significant increase in robotic lobectomies likely reflects several factors. First, several studies published during this period have supported the use of a robotic approach in NSCLC, possibly leading to increased acceptance among surgeons [2, 9–11, 20].
Second, technology diffusion within an industry, such as surgery, often follows a sigmoidal growth curve, with significant increases in use as early adopters champion potential benefits [21].
However, this enthusiasm for robotic surgery should be tempered by several studies that have reported higher costs with robotics compared with VATS [8, 22, 23]. Given the steep learning curve and associated increased operative times with robotic resection, the true value of this surgical approach has yet to be determined [22, 23].
Studies evaluating predictors of robotic resections in NSCLC are lacking. However, reports in rectal and prostate cancer have found that academic hospitals, highvolume centers, and urban locations were associated with an increased likelihood of robotic surgery [16, 24]. Our study similarly found that treatment in a populated area was associated with robotic surgery. Evidence suggests that hospitals are more likely to purchase robotic technology if located in a region where this is already offered [25]. This technology may serve as a marketing tool to bolster a hospital’s reputation as it competes for a limited set of patients [21, 25]. This may be particularly true of academic hospitals that place a high premium on innovation in an effort to be on the forefront of medicine.
Outcomes After Robotic Lobectomy Compared With VATS and Open Lobectomy
Similar to our findings, prior studies have found reduced LOS with robotic lobectomy in NSCLC compared with open lobectomy, with comparable lymph node sampling and deaths at 30 days [1–3,15,26]. Our study further adds to the literature, with findings of equivalent outcomes for these two surgical approaches with regard to margin positivity, hospital readmission, and deaths at 90 days.
In comparing VATS and robotic lobectomy, LOS and complication rates appear to be similar between these groups, although some evidence suggests a higher rate of intraoperative bleeding in robotic operations [1,3, 8,20,23,27]. We also did not find a meaningful difference in LOS between VATS and robotic patients, though VATS patients had lower rates of prolonged LOS. We found lymph node sampling was slightly better in VATS patients than in robotic patients. Others have reported conflicting results regarding the rate of pathologic nodal upstaging, a quality metric used as a proxy for meticulous nodal evaluation, between VATS and robotic lobectomy patients [15, 27]. These disparate findings may be related to varying levels of surgeon experience and differences in study populations. Similar to prior publications, we also did not find a difference in mortality rates when VATS and robotic lobectomy were compared [1, 3, 20, 27].
Our study should be considered with several limitations. First, we were unable to assess important factors such as individual surgeon experience with robotic surgery, cost data, and operative details, such as thoracotomy size and technique, because these variables are not collected by the NCDB.
Second, as with any large data set, there may be coding inaccuracies potentially affecting our results.
Third, NCDB-participating institutions must be Commission on Cancer accredited to submit data. Consequently, our data may be vulnerable to selection bias, although we note this registry includes hospitals diverse in geographic location and size [13].
Conclusions
Use of robotic lobectomy in stage I to IIIA NSCLC disease has significantly increased in recent years. Several factors were associated with the use of this technology and include older age, metropolitan area, smaller tumors with lower staged disease, and treatment at an academic/ NCI-designated center or high-volume hospital. Overall, results after robotic lobectomy, particularly outcomes such as margin positivity, unplanned readmission, and deaths at 30 days and 90 days, are similar to open and VATS lobectomy. Given the reported increased costs associated with robotic operations, additional studies are needed to determine whether this approach offers potential advantages over VATS as surgeons consider incorporating this technology into their own practices.
Supplementary Material
Acknowledgments
Dr Rajaram is supported by the Agency for Healthcare Research and Quality #T32HS000078 and the American College of Surgeons Clinical Scholars in Residence Program.
Footnotes
DISCUSSION
DR ELIZABETH A. DAVID (Sacramento, CA): Good afternoon. Thank you. Very nice paper. I have a couple of questions for you. One thing that jumped out to me about your data was the high percentage of open lobes, and I just wondered if you had any comment.
And then, secondly, I think the subtle differences that you showed between video-assisted thoracic surgery (VATS) and robotics are probably due more to the learning curve that is going on in our community and just wondered if you had any thoughts about that?
DR RAJARAM: Thank you for the questions. Certainly the proportion of individuals who underwent an open surgery rather than a minimally invasive one is higher than some data have shown for STS database analyses.
But I think this also just underscores the type of individuals performing lobectomies nationally. Oftentimes it is not necessarily thoracic or specialty trained surgeons. Our results are actually in line with data from the Nationwide Inpatient Sample, which have also shown about 60% to 70% of individuals undergoing an open resection. We did try and get somewhat at the learning curve within this analysis. It is hard to do that without individual surgeon experience being tracked within the database itself. But certainly given the large increase, the threefold increase in utilization of robotic surgery, we do feel that probably some of this is at least related to just familiarity with that technology.
DR FRANK A. BACIEWICZ, JR (Detroit, MI): One thing that I thought was interesting was that over the course of the study, it looked like the number of open cases stayed about the same, whereas the increase in the minimally invasive was due to an increase in robotics rather than thoracoscopic. I do not know if that was what you presented.
The question I had was, did you look at operative time for these various operations? In other words, did the robotic operation take longer than the VATS, and did that factor into any of your data, because at least in my experience, even after doing this for a number of years, the robotic operation still takes me longer than to do than an open approach. So I wondered if you factored that into your data?
DR RAJARAM: Thank you for the questions. VATS increased by about 50% over the time period from about 16% to 17% to about 24%, so there was a significant increase. But it was not as dramatic as robotic surgery, which went from 3% to 9%. So both modalities did increase over that time period with a reduction of open lobectomies. And, unfortunately, operative time isn’t captured within this data set, so that is not something that we could evaluate.
DR JAMES R. JETT (Denver, CO): A question about lymph nodes. A clarification first. You had 12 lymph nodes. Was that your median number of lymph nodes? Is that why you chose that number of 12?
DR RAJARAM: The greater than 12 nodes was a quality metric, which has been used within the literature, and a parameter that we wanted to evaluate. The median lymph node count was 8 or 9 depending on the type of surgical approach.
DR JETT: I think the Chamberlain lecture used 10 as the number of lymph nodes, did they not? But the question is this: Can you not tell from the database what are N1 and N2 nodes? You said that was a limitation of your study. I mean, that is a huge limitation.
DR RAJARAM: Yes, we would agree with that. So it is captured somewhat, but there is a lot of missing data, unfortunately, and not reliable enough to use for the time period that we used, especially on a national level when we are looking at all different types of centers rather than just high-volume centers.
DR JOHN A. HOWINGTON (Evanston, IL): Again, great presentation. I am happy you are doing the work. Just a confirmation: Over the period of time, the rate of thoracoscopic lobectomy went from 17% to 24%, so it is growing?
DR RAJARAM: Yes.
DR HOWINGTON: Okay. And then you mentioned that the rate of prolonged hospitalization, so beyond 14 days I think was your definition...
DR RAJARAM: Yes.
DR HOWINGTON: … was significantly higher with robotic lobectomy compared to thoracoscopic?
DR RAJARAM: Yes.
DR HOWINGTON: Do you know, what was the driver for that, was it prolonged air leak or otherwise?
DR RAJARAM: No. Unfortunately, those types of morbidities are not captured within the database. One of the interesting things looking at this is that readmission was similar between those two groups. So if that is a proxy for inpatient complications, we would expect there to be a difference with readmission as well, but that is not something we saw.
DR HOWINGTON: Thank you.
DR DAVID T. COOKE (Sacramento, CA): In your study, you obviously had an increase of robotic use over time. Does the database offer you the level of granularity to determine if centers are going from no minimally invasive technique to robotics, or are they transitioning from VATS to robotics?
DR RAJARAM: That is not something we specifically looked at. We did look at the number of centers overall performing robotic surgery. Of the 1,215 hospitals, about 153 within the first year did a robotic lobectomy. By the last year, about 250 hospitals performed robotic lobectomy. So there was about a two-thirds increase. Additionally, the median case volume doubled over that time period at those hospital centers doing robotic lobectomy. But we did not specifically look to see if there was being a trade-off effect with thoracoscopic versus robotic.
DR MITCHELL J. MAGEE (Dallas, TX): I applaud you for utilizing this rich resource of clinical information, and I think it is very valuable information in terms of real-world practices and sort of getting a 50,000-foot view of what is going on nationally. But just a word of caution: As I showed in my own comparison, there are some inconsistencies and some discrepancies. And I think particularly as it pertains to approach, I think with robotics, it is a little bit easier to define whether you are robotic or you are open. But oftentimes the coders as they are rating it as a VATS vs an open, you may start out as a VATS. You may convert. You may have an incision where you spread the ribs or you do not. And I think there is a fine line in terms of interpreting and defining whether it is a VATS or an open case, and how it is coded in the registry is going to vary a fair amount depending on how well educated the registrars are.
DR RAJARAM: Sure thing. There is definitely some variation there. I would agree with that.
DR ERIC L. GROGAN (Nashville, TN): And one final question before we move on. How did you deal with missing data in this data set? Did you use multiple imputation? Did you exclude and just use patients that had a full complete data set? Tell us how you dealt with that.
DR RAJARAM: Sure. We only included individuals with stage I to IIIA disease who underwent surgery, which helped eliminate a lot of actual patients who had missing data otherwise. And then, actually, the only parameters that had significant missing data over a percent or so was tumor location and whether they were in a metropolitan region or not, and for those individuals we excluded.
DR GROGAN: So the downside to not imputing, even on the front end, is that if you are going with a database that has a complete group of data only, you may be selecting for higher-quality institutions that do better data submission. So that is just one of the cautions for only selecting for complete cases.
Dr DeCamp discloses a financial relationship with PneumRx, Pulmonx, Holaira, and Soffio Medical.
References
- 1.Adams RD, Bolton WD, Stephenson JE, Henry G, Robbins ET, Sommers E. Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann Thorac Surg 2014;97:1893–8; discussion 1899–900. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio RJ, Bryant AS, Minnich DJ. Starting a robotic program in general thoracic surgery: why, how, and lessons learned. Ann Thorac Surg 2011;91:1729–37. [DOI] [PubMed] [Google Scholar]
- 3.Kent M, Wang T, Whyte R, Curran T, Flores R, Gangadharan S. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg 2014;97:236–42; discussion 242–4. [DOI] [PubMed] [Google Scholar]
- 4.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl): e278S–313S. [DOI] [PubMed] [Google Scholar]
- 5.Phillips JD, Merkow RP, Sherman KL, DeCamp MM, Bentrem DJ, Bilimoria KY. Factors affecting selection of operative approach and subsequent short-term outcomes after anatomic resection for lung cancer. J Am Coll Surg 2012;215:206–15. [DOI] [PubMed] [Google Scholar]
- 6.Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366–78. [DOI] [PubMed] [Google Scholar]
- 7.Nasir BS, Bryant AS, Minnich DJ, Wei B, Cerfolio RJ. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203–8; discussion 208–9. [DOI] [PubMed] [Google Scholar]
- 8.Paul S, Jalbert J, Isaacs AJ, Altorki NK, Isom OW, Sedrakyan A. Comparative effectiveness of robotic-assisted vs thoracoscopic lobectomy. Chest 2014;146:1505–12. [DOI] [PubMed] [Google Scholar]
- 9.Giulianotti PC, Buchs NC, Caravaglios G, Bianco FM. Robot-assisted lung resection: outcomes and technical details. Interact Cardiovasc Thorac Surg 2010;11:388–92. [DOI] [PubMed] [Google Scholar]
- 10.Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6: 355–60. [DOI] [PubMed] [Google Scholar]
- 11.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383–9. [DOI] [PubMed] [Google Scholar]
- 12.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American College of Surgeons. National Cancer Data Base. Available at https://www.facs.org/quality-programs/cancer/ncdb. Accessed Dec 28, 2015.
- 14.Wright CD, Gaissert HA, Grab JD, O’Brien SM, Peterson ED, Allen MS. Predictors of prolonged length of stay after lobectomy for lung cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk- adjustment model. Ann Thorac Surg 2008;85:1857–65; discussion 1865. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JL, Louie BE, Cerfolio RJ, et al. The prevalence of nodal upstaging during robotic lung resection in early stage non-small cell lung cancer. Ann Thorac Surg 2014;97:1901–6; discussion 1906–7. [DOI] [PubMed] [Google Scholar]
- 16.Speicher PJ, Englum BR, Ganapathi AM, Nussbaum DP, Mantyh CR, Migaly J. Robotic low anterior resection for rectal cancer: a national perspective on short-term oncologic outcomes. Ann Surg 2015;262:1040–5. [DOI] [PubMed] [Google Scholar]
- 17.Chan JK, Gardner AB, Taylor K, et al. The centralization of robotic surgery in high-volume centers for endometrial cancer patients—a study of 6560 cases in the U.S. Gynecol Oncol 2015;138:128–32. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27:2037–49. [DOI] [PubMed] [Google Scholar]
- 19.Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER- Medicare database. BMJ 2014;349:g5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louie BE, Farivar AS, Aye RW, Vallieres E. Early experience with robotic lung resection results in similar operative out-comes and morbidity when compared with matched videoassisted thoracoscopic surgery cases. Ann Thorac Surg 2012;93:1598–605. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy DP, DeCamp MM. Robotic lobectomy and the principles of technology diffusion. Chest 2014;146: 1425–6. [DOI] [PubMed] [Google Scholar]
- 22.Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000–7. [DOI] [PubMed] [Google Scholar]
- 23.Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929–37. [DOI] [PubMed] [Google Scholar]
- 24.Chang SL, Kibel AS, Brooks JD, Chung BI. The impact of robotic surgery on the surgical management of prostate cancer in the USA. BJU Int 2015;115:929–36. [DOI] [PubMed] [Google Scholar]
- 25.Barbash GI, Friedman B, Glied SA, Steiner CA. Factors associated with adoption of robotic surgical technology in US hospitals and relationship to radical prostatectomy procedure volume. Ann Surg 2014;259:1–6. [DOI] [PubMed] [Google Scholar]
- 26.Farivar AS, Cerfolio RJ, Vallieres E, et al. Comparing robotic lung resection with thoracotomy and video-assisted thor- acoscopic surgery cases entered into the society of thoracic surgeons database. Innovations (Phila) 2014;9:10–5. [DOI] [PubMed] [Google Scholar]
- 27.Yang CF, Sun Z, Speicher PJ, et al. Use and outcomes of minimally invasive lobectomy for stage I non-small cell lung cancer in the National Cancer Data Base. Ann Thorac Surg 2016;101:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


