Abstract
Background:
Although immobility is a common risk factor for venous thromboembolism (VTE) in medical inpatients, lack of a consistent definition of this term may limit accurate assessment of VTE risk for thromboprophylaxis.
Objective:
To examine various definitions of immobility used in recent pharmacological thromboprophylaxis clinical trials.
Data Sources:
PubMed and relevant references from articles/reviews from 2008 to 2016 were searched. Randomized controlled trials (RCTs) and other clinical studies involving adult hospitalized medical patients in acute care hospital settings that used the term immobility were selected. Two investigators independently abstracted data in duplicate, and accuracy was checked by a third investigator.
Results:
Twenty-one clinical studies were included. There was heterogeneity among individual VTE risk factors, with respect to the definition of immobility in medical inpatients in these trials. Thirteen studies utilized objective criteria to define “immobility” including duration (12 studies) and distance or time walked (6 studies). In contrast, 7 studies focused principally on subjective definitions (ie, describing the nature of immobility rather than specifying its quantitative measurement). Three RCTs vaguely defined the level of patient’s immobility after hospitalization.
Conclusion:
Despite the well-known effectiveness of pharmacological thromboprophylaxis for the prevention of VTE in acutely ill medical patients, there is no current consensus on how to define immobility. The heterogeneous nature of definitions of immobility has led to uncertainty about the importance of immobility in VTE risk assessment models. Although clinical studies have incorporated varying definitions of immobility into their inclusion criteria, immobility as a specific VTE risk factor has not been clearly defined.
Keywords: immobilization, hospitalization, pulmonary embolism, venous thromboembolism, venous thrombosis
Introduction
Venous thromboembolism (VTE) represents different clinical manifestations of the same disease process, comprising deep vein thrombosis (DVT) and pulmonary embolism (PE). VTE is one of the most common causes of mortality in hospitalized patients.1 Although the prognosis of VTE is worse in hospitalized medical patients compared to surgical patients, less is understood about VTE in hospitalized medical patients compared to surgical patients. Uncertainties remain regarding the risk assessment and prevention of VTE in medical patients due to the complexity of patient populations and heterogeneity among the available studies.
Common risk factors for VTE in medical patients include, among others, a history of VTE, obesity, advanced age, immobility, malignancy, and heart failure.2,3 Medical patients often have a history of reduced mobility upon admission due to the morbidity associated with their underlying illness(es). Proper identification of VTE risk factors facilitates appropriate and timely initiation of thromboprophylactic therapy for reducing the incidence of VTE during hospitalization and beyond.
Immobility is a common risk factor for VTE, and prolonged immobility reduces blood flow and leads to the development of venous stasis. Venous stasis, along with endothelial injury and hypercoagulability, is also involved in and contributes to the pathophysiology of venous thrombosis.4 Patients with prolonged bed rest (>14 days) have a 5-fold increase in risk of DVT,5 and a recent meta-analysis of epidemiological studies demonstrated a 2- to 3-fold increase in VTE risk in medical patients with reduced mobility.6 More recently, a validation study for a risk assessment model (RAM) of VTE identified immobility as an important predictor for the development of VTE.7 Currently, the American College of Chest Physicians (ACCP) recommends thromboprophylaxis for medical patients at high risk of VTE according to the Padua score.8 The Padua prediction score is a RAM that includes 11 risk factors used to identify medical patients at high risk for VTE. This detailed assessment, which highlights the importance of immobility,9 has been suggested as the best model to assess the risk of VTE in medical inpatients.10
Although immobility is a well-recognized risk factor for VTE, a consistent definition of this term has not been agreed upon in medical inpatients. Many major clinical trials of VTE prophylaxis in medical patients have included immobility as a risk factor or in their inclusion criteria, but few have specifically investigated the influence of ambulatory status on the efficacy and safety of the VTE prophylaxis. Most importantly, the lack of an easy to use and inconsistent definition of immobility has caused ambiguous awareness among clinicians, observation prejudice, improper diagnostic models for risk assessment, and difficulties in reproducing or validating previous studies. Furthermore, inconsistent definitions of immobility make it difficult to investigate the extent to which medical patients with reduced mobility actually benefit from pharmacological prophylaxis.
Previously, Emed et al conducted a systematic review investigating various definitions of immobility in studies of thromboprophylaxis in hospitalized medical patients prior to 2008.11 The authors concluded that there was a marked lack of consistency in how the concept of immobility was defined and utilized in thromboprophylactic Randomized controlled trial (RCTs) in medical inpatients. The goal of our systematic review, which was conducted using studies performed from 2008 to 2016, was to evaluate various definitions applied to the assessment of immobility in more recent pharmacological thromboprophylaxis studies in hospitalized medical patients to investigate whether clinicians and researchers have adopted more objective criteria in terms of mobility status following the 2008 report by Emed et al.
Methods
Data Sources and Searches
We systematically searched the PubMed database. The following key terms were used in the literature search: [“medical patients” or “medicine patients” or “medical inpatients” or “wards” or “medical floor”] and [“VTE” or “DVT” or “PE” or “thrombosis” or “thromboembolism” or “venous thrombosis” or “deep vein thrombosis” or “pulmonary embolism”] and [“prophylaxis” or “prevention” or “antithrombotic therapy” or “antithrombotic measures”] and [“randomized controlled trial” or “clinical trial”]. In addition to searching the database, the reference lists of all included studies, meta-analyses, and reviews were manually searched.
Study Selection
We reviewed published studies that met the following inclusion criteria: (1) the study population consisted of adult, hospitalized medical patients in acute care hospital settings; (2) RCTs or other clinical trials related to medical inpatient’s mobility; (3) published in peer-reviewed journals between 2008 and 2016 (to retrieve the most up-to-date evidence); and (4) written in English. Reports were excluded if they (1) were conducted in outpatient clinics, nursing homes, patient homes, other nonacute health-care settings, inpatient rehabilitation units, or the emergency department or (2) included a pediatric population. Reviews, editorials, comments, and letters were excluded from this analysis.
Data Extraction
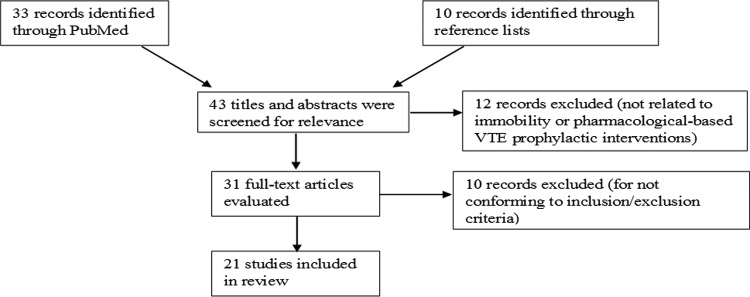
Potentially relevant studies included 33 records identified in the PubMed database. Ten additional records were identified from the reference lists of meta-analyses and reviews. Titles and abstracts were screened for relevance to medical inpatient’s mobility and pharmacological-based VTE prophylactic interventions by the authors. This resulted in an initial selection of 31 articles. These 31 studies underwent full-text review to determine whether they met the inclusion criteria. After full-text review, 10 studies were excluded for not conforming to the inclusion/exclusion criteria. Specific examples of exclusions included 1 article that exclusively described a study protocol with no data or results12 and 1 study that enrolled healthy volunteers as opposed to patients with underlying medical conditions13 (Figure 1).
Figure 1.
Flowchart of literature review and analysis.
Quality Assessment
To manage the risk of bias across studies (ie, publication bias, selective reporting), all 22 studies were reviewed independently by the 3 authors. Two authors (FY, LNB) reviewed the articles to ensure that they met inclusion criteria and abstracted the data in duplicate, and they were checked for accuracy by the third author (SHY).
Results
Study Characteristics
After review, 21 studies were selected for inclusion in this systematic review. A summary of findings under the emergent themes is provided (Table 1). We identified 11 studies that were either RCTs or subgroup analyses and 10 additional cohort and other clinical studies. There were 6 international RCTs including the Apixaban Dosing to Optimize Protection from Thrombosis (ADOPT) trial, Extended-Duration Venous Thromboembolism Prophylaxis in Acutely Ill Medical Patients With Recently Reduced Mobility (EXCLAIM) trial, the study to Evaluate the Mortality Reduction of Enoxaparin in Hospitalized Acutely Ill Medical Receiving Enoxaparin (LIFENOX), CERToparIn For thromboprophYlaxis in medical patients (CERTIFY) trial, Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban with Enoxaparin (MAGELLAN) trial, and The Strategies to Enhance Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients (SENTRY) trial.
Table 1.
The Definition of Immobility as Described in the Included Studies in Medical Inpatients.
| Author, Year (Reference) | Study Name | Study Design | Definition of Immobility | Duration of Immobility | Measures | |
|---|---|---|---|---|---|---|
| 1 | Germini et al, 201614 | Quasi-RCT | Reduced mobility | Not stated | Subjective | |
| 2 | a. Cohen et al, 201315 | MAGELLAN | RCT | a. Complete immobilization: the patient is totally confined by his or her illness to bed or chair. The patient may be allowed to use a bedside commode or with assistance may be allowed bathroom privileges b. Decreased mobility: immobilization caused by the illness requiring the patient to remain in bed or chair more than 50% of the time during daytime hours c. Ongoing decreased mobility: immobilization caused by the illness requiring the patient to remain in bed or chair during daytime hours more than was normal and usual for the patient prior to hospitalization | ≥1 day during the hospitalization ≥4 days after randomization in any type of care setting | Objective (days) |
| b. Cohen et al., 201416 | Subanalysis of the MAGELLAN trial | RCT | ||||
| 3 | Nendaz et al, 201417 | ESTIMATE | Cohort study | Complete bed rest or inability to walk for >30 min/d | >3 days | Objective (days and walking time) |
| 4 | a. Spyropoulos et al, 201118 | IMPROVE | Case–control cohort study | Confinement to a bed or chair >24 hours | ≥7 days | Objective (days) |
| b. Rosenberg et al, 201419 | IMPROVE | Case–control cohort study | Bed rest or hospital stay | ≥7 days | Objective (days) | |
| 5 | a. Hull et al, 201020 | EXCLAIM | RCT | Reduced mobility before enrollment Level 1 mobility: total bed rest or being sedentary without bathroom privileges Level 2 mobility: total bed rest or being sedentary with bathroom privileges | ≤3 days recent reduced mobility ≥3 days anticipated reduced mobility | Objective (days) |
| b. Turpie et al, 201221 | Subgroup analysis of the EXCLAIM trial | RCT | ||||
| c. Yusen et al, 201322 | Subgroup analysis of the EXCLAIM trial | RCT | ||||
| 6 | Pai et al, 201323 | SENTRY | Cluster RCT | Confined to bed or needs assistance to ambulate | Not stated | Subjective |
| 7 | Ageno et al, 201224 | FONDAIR | Cohort study | Remain in bed | ≥4 days | Objective (days) |
| 8 | Bergese et al, 201225 | DESIR-ABLE | Open-label single-arm study | Medically ill with prolonged immobility | Not stated | Subjective |
| 9 | Schellong et al, 201026 | CERTAIN | Randomized, open-label study | Significant recent decrease in mobility (completely bedridden or only able to walk short distances with the support of a nurse) | Not stated | Subjective |
| 10 | a. Riess et al, 201027 | CERTIFY | RCT | Significant decrease in mobility (bedridden or only able to walk short distances) | ≥4 days | Objective (days) |
| b. Tebbe et al, 201128 | Subgroup analysis of the CERTIFY trial | RCT | ||||
| 11 | a. Cohen et al, 200829 | ENDORSE | Multinational cross-sectional study | Long-term immobility (before hospitalization). Immobile with bathroom privileges or complete immobilization (during hospitalization) | Not stated | Subjective |
| b. Ongen et al, 201130 | ENDORSE—Turkish Arm | |||||
| c. Nendaz et al, 201031 | IMPART—part of ENDORSE study | <30-minute walk per day | Not stated | Objective (walking time) | ||
| 12 | Goldhaber et al, 201132 | ADOPT | RCT | All patients had to be moderately or severely restricted in their mobility Moderately restricted mobility allowed for walking within the hospital room or to the bathroom Severely restricted mobility was defined as being confined to bed or to a chair at the bedside | Not stated | Subjective |
| 13 | Rodríguez-Mañas, et al, 201033 | ANCIANOS | Cohort study | Bedridden | ≥4 days | Objective (days) |
| 14 | Kakkar et al, 201134 | LIFENOX | RCT | We did not collect data on mobility status, an important determinant of the risk of venous thromboembolism | -- |
Abbreviations: ADOPT, Apixaban Dosing to Optimize Protection from Thrombosis; ANCIANOS, Thromboprophylaxis with the low-molecular-weight heparin bemiparin sodium in elderly medical patients in usual clinical practice; CERTAIN, certoparin in acutely ill medical patients; CERTIFY, CERToparIn For thromboprophYlaxis in medical patients; DESIR-ABLE, multicenter trial of desirudin for the prophylaxis of thrombosis: an alternative to heparin-based anticoagulation; ENDORSE, venous thromboembolism risk and prophylaxis in the acute hospital care setting; ESTIMATE, Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland; EXCLAIM, Extended-Duration Venous Thromboembolism Prophylaxis in Acutely Ill Medical Patients With Recently Reduced Mobility; FONDAIR, Fondaparinux 1.5 mg for the prevention of VTE in medical patients with renal insufficiency; IMPART, adequacy of venous thromboprophylaxis in acutely ill medical patients; IMPROVE, International Medical Prevention Registry on Venous Thromboembolism; LIFENOX, Study to Evaluate the Mortality Reduction of Enoxaparin in Hospitalized Acutely Ill Medical Receiving Enoxaparin; MAGELLAN, Prevention of Venous Thromboembolism in Hospitalized Acutely Ill Medical Patients Comparing Rivaroxaban with Enoxaparin; VTE, venous thromboembolism; RCT, randomized controlled trial; SENTRY, Strategies to Enhance Venous Thromboembolism Prophylaxis in Hospitalized Medical Patients.
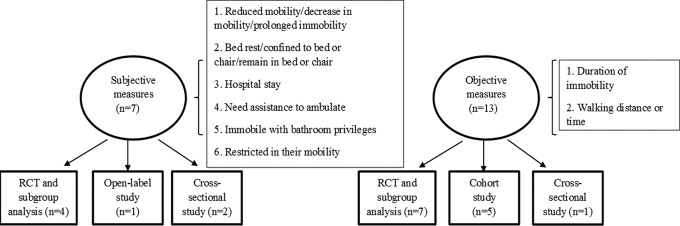
Variability in Definitions
Immobility was defined subjectively or objectively within these studies (Figure 2, Table 2). For subjective definitions, the word immobility was used but not clearly defined. The most frequent definitions included “reduced mobility” or “prolonged immobility” or “confined to/remain in bed” or “immobile with bathroom privileges.” For example, in the quasi-RCT study conducted by Germini et al, immobility was simply defined as reduced mobility.14 Similarly, vague definitions of immobility or mobility were found in the SENTRY RCT, which considered mobility based on an order for bed rest or if chart notes indicated that the patient could not ambulate without support.23 The multicenter trial of desirudin for the prophylaxis of thrombosis: an alternative to heparin-based anticoagulation (DESIR-ABLE) study recruited patients with prolonged immobility,25 and in the venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE) study, 28% of its enrolled patients were immobile with bathroom privileges.29 In addition, the term “hospital stay” was sometimes used interchangeably with “bed rest.”19
Figure 2.
Characteristics of studies included in this review in terms of the definition of immobility.
Table 2.
Concept of Immobility as Defined in the Included Studies in Medical Inpatients.
| Concept of Immobility | Number of Studies [Reference(s)] |
|---|---|
| Nature of Immobility | |
| Reduced mobility/decrease in mobility | 4 studies14-16,20 |
| Bed rest/confined to bed or chair/remain in bed or chair | 16 studies12,16–24,26–28,30,32,33 |
| Hospital stay | 1 study19 |
| Needs assistance to ambulate | 2 studies23,26 |
| Prolonged immobility | 1 study25 |
| Immobile with bathroom privileges | 5 studies20–22,29,30 |
| Restricted in their mobility | 1 study32 |
| Level of immobility | |
| Level 1: total bed rest or being sedentary without bathroom privileges/level 2: with bathroom privileges | 3 studies20–22 |
| Complete immobilization or decreased mobility | 7 studies15,16,26–30 |
| Moderately or severely restricted mobility | 1 study32 |
| Duration of immobility | |
| Not stated | 8 studies14,23,25,26,29–32 |
| ≥1 day | 2 studies15,16 |
| >3 days | 4 studies17,20–22 |
| ≥4 days | 6 studies15,16,24,27,28,33 |
| ≥7 days | 2 studies18,19 |
| Walking distance or time | |
| Inability to walk for >30 min/d | 1 study17 |
| Within the hospital room or to the bathroom | 1 study32 |
| Short distances | 3 studies26–28 |
| <30-minute walk per day | 1 study31 |
Selected studies dichotomized the concept of immobility by assigning different levels. For example, the ADOPT RCT enrolled patients with moderate (allowed to walk within the hospital room or to the bathroom) or severe (confined to bed or to a chair at the bedside) reductions in mobility.32 In the EXCLAIM study and its subgroup analyses, the researchers categorized reduced mobility into 2 levels—level 1 immobility (total bed rest or being sedentary without bathroom privileges) or level 2 immobility (total bed rest or being sedentary with bathroom privileges).20–22
In contrast, 13 studies objectively specified the duration of immobility/mobility rather than stating a level or degree of immobility. For example, in the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) study, immobility was defined as hospitalization or bed rest >7 days.18,19 In the study by Nendaz et al, immobility duration was required to be >3 days (complete bed rest or inability to walk for >30 min/d).17 In the fondaparinux 1.5 mg for the prevention of VTE in medical patients with renal insufficiency (FONDAIR) cohort study, patients were expected to remain in bed for at least 4 days.24 In the CERTIFY RCT, an expected reduction in mobility for at least 4 days was required.27,28
Six studies used walking distance or time as an attempt to objectively quantify immobility.17,26–28,31,32 For example, immobility was defined as “only able to walk short distances” in the certoparin in acutely ill medical patients (CERTAIN) and CERTIFY studies26–28 and as “<30-minute walk per day” in the adequacy of venous thromboprophylaxis in acutely ill medical patients (IMPART) study.31
Other Study Characteristics
Among the 20 studies that included the concept of ambulation in their design, 12 studies used mobility status as inclusion criteria and 8 studies considered reduced mobility as a VTE risk factor. However, only 2 studies reported the impact of mobility on VTE events, namely IMPROVE and the EXCLAIM trial. The IMPROVE trial studied the impact of immobility on the incidence of VTE versus non-VTE events and concluded that immobilization was an independent risk factor for VTE.18,19 The EXCLAIM trial was unique in that it was the first study that delineated more than 1 level of immobility and compared efficacy and safety outcomes in hospitalized medical patients with differing levels of mobility.20
Discussion
This study, conducted with hospitalized medical patients, was based on a systemic review of the literature using the PubMed database. We examined how immobility was defined in RCTs and other clinical trials for pharmacological-based VTE thromboprophylaxis and found that the definition of immobility is heterogeneous among studies and not consistently reported.
The definition of immobility spans a very broad spectrum. There are many factors that may determine how immobility contributes to the magnitude of VTE risk (ie, the degree [level] and/or duration/distance of immobility). Immobility consists of a continuum from being fully bedridden to having reduced mobility. In general, when immobility is present (regardless of definition), even subtle reductions in mobility along this continuum may increase the incidence of VTE events. However, the definition of immobility differs among clinical trials, making detailed comparisons among the studies difficult. Qualitatively, immobility has been defined as reduced mobility, prolonged immobility, confined to bed or chair or hospital stay, and so on. Three RCTs, namely ADOPT,32 MAGELLAN,15 and EXCLAIM,20 stratified patients prior to randomization according to their level of mobility. Apixaban Dosing to Optimize Protection from Thrombosis trial classified its patients into level 1 or level 2, MAGELLAN divided its patients into complete immobilization or decreased mobility groups, and EXCLAIM used “moderately or severely restricted mobility” to enroll patients. However, the criteria used to delineate these levels/groups/categories in these studies were not clearly defined. Interestingly, even within the same study, immobility was defined differently between the original and post hoc studies. For example, the ENDORSE study and 1 of its subanalyses included patients who were immobile with bathroom privileges or complete immobilization,29,30 whereas another ENDORSE subanalysis defined immobility as <30-minute walk per day.31
In contrast, selected studies focused on quantitative assessment, including duration rather than subjectively describing characteristics of immobility. To date, the most comprehensive definition of immobility was in the MAGELLAN RCT and its subgroup analysis.15,16 Patients included in this trial were expected to be “completely immobilized (totally confined to bed or chair, may use a beside commode, or may have bathroom privileges) ≥1 day during the hospitalization” or “decreased in mobility (immobilization caused by the illness requiring the patient to remain in bed or chair more than 50% of the time during daytime hours) ≥4 days after randomization.” This MAGELLAN protocol also specified that patients with ongoing deceased mobility (ie, “immobilization caused by the illness requiring the patient to remain in bed or chair during daytime hours more than was normal and usual for the patient prior to hospitalization”) would be recruited. However, the actual duration of decreased mobility was not reported in the published manuscript.
As inclusion criteria for mobility status differed across individual studies and were not precise, it is important to recognize that the predictive value of immobility was not equivalent. While many studies included a subjective definition of immobility in their inclusion criteria, 13 studies in our review consisted of clinical trials that utilized an objective measurement of the duration of immobility. However, only 2 of the 13 reported the actual days of immobility in the published manuscript.24,27 Thus, the extent to which immobility truly increases the risk of VTE in hospitalized medical patients is unknown. The absence of a consistent case definition of immobility also complicates evaluation of the efficacy of VTE prophylaxis in clinical trial results. It has been shown that the risk of thrombosis is also affected by prehospitalization and posthospitalization mobility status, and that risk persists for a minimum of 3 months following hospital discharge.35 Although the EXCLAIM trial demonstrated a beneficial effect of longer treatment duration within an older population, it did not follow up on the mobility status of its enrolled patients. In assessing whether prophylaxis is indicated, physicians should consider the nature and duration of an individual’s immobile state.
Immobility, with its various definitions, has been considered a risk factor for VTE based on recommendations from the ACCP. However, evidence that immobility increases a patient’s risk for VTE is mostly derived from studying nonambulatory patients. The association of mobility to VTE risk depends upon the study design and the RAM used, and many RAMs have not been compared or validated for the definition of immobility. The evidence supporting their findings is often weak and conflicting. For example, immobility was proposed as 1 of the 7 risk factors in the IMPROVE study but was not found to be associated with risk of VTE in their study population.18,19 Both IMPROVE studies have weaknesses in their study design. First, neither of these studies directly evaluated the patient’s actual duration of immobility/length of hospital stay. Second, the characteristics of immobility were not recorded, and/or the authors did not present them in the published manuscript (ie, were the patients confined to bed, a chair, or other assistive devices or were they allowed to use the bathroom or walk in their room?). Finally, the authors did not report the degree or the magnitude of immobility in their studies (ie, were the patients completely or partially immobilized?).
Although no explicit definition of immobility was provided for many of the included studies, only 2 studies included in our systematic review recognized the unclear definition of immobility as a limitation. In the SENTRY study, immobility was “ambiguously defined and inconsistently documented by health-care providers” which may “have resulted in an incomplete picture of patient’s thrombosis and bleeding risks.”23 The LIFENOX RCT reported that although mobility was an important VTE risk factor, “they did not collect data on mobility status.”34 Although it was not included in our systematic review because the authors defined “ambulation” as opposed to “immobility,” a post hoc analysis of the prophylaxis in MEDical patients with ENOXaparin (MEDENOX) study acknowledged that “no study has compared or validated the different definitions of ambulation.”36
Although nearly all trials we included in this review acknowledged that immobility is a common risk factor for VTE, there remains significant ambiguity regarding the level of risk that immobility poses on VTE outcomes. It should be noted that these studies are in contrast to the results from a recent historical cohort study that found bedridden patients with prolonged immobilization (>3 months) to be no more prone to VTE than mobile patients.37 These findings may be explained by the fact that many other factors may confound the relationship between immobility and VTE, including patient’s age, obesity, underlying diseases, the starting time of pharmacological prophylaxis after patient’s hospitalization, and so on. Thus, high-quality studies using a consistent, global definition of immobility status are needed to better identify the cumulative weight of immobility when combined with other risk factors for determining VTE outcomes.
Limitations of RCTs
Examination of the findings from 11 RCTs and its subanalysis revealed that pharmacological intervention is superior to placebo for VTE prophylaxis in medical inpatients. However, immobility was not consistently reported in these studies, which introduced a number of notable limitations. First, no detailed information about the duration of immobility was reported. For example, the EXCLAIM study was the main RCT that examined the effects of immobility status on VTE prophylaxis. This study mentioned that patients were “likely to have reduced mobility for at least 3 days after enrollment,” but they did not report how many days patients actually experienced reduced mobility after hospitalization.20 A similar issue was identified in the ADOPT study, which dictated that patients had “an expected hospital stay of at least 3 days” but the actual length of the patient’s hospital stay was not reported.32 Second, while some trials stratified the patients into 2 or 3 groups based on the level of mobility, the degree of mobility varied between different studies. In EXCLAIM, level 2 immobility was defined as “mobility restriction with bathroom privileges,” whereas ADOPT defined moderately restricted mobility as “the ability to walk within the hospital room or to the bathroom.” Without further details, such as the walking distance within the hospital room or to the bathroom, the average number of bathroom trips, and so on, it is difficult to compare results from these 2 studies from an immobility/mobility status standpoint. Finally, another confounding factor that is not well understood is the nature and magnitude of the association between VTE and prolonged immobility. The criteria for prolonged immobility differ based on each study design. It has been established that the total time spent immobile may contribute to the risk of VTE, but the extent to which prolonged immobility during hospitalization and/or prehospitalization or posthospitalization affects the risk of VTE in medical patients with comorbid conditions (ie, respiratory failure, heart failure, advanced age, obesity, etc) is not known.
Conclusion
Despite the well-documented efficacy of thromboprophylaxis for the prevention of VTE in acutely ill medical patients, no current worldwide consensus on how to define immobility in these patients exists. This reflects the heterogeneous nature of immobility and uncertainty about the prevalence of immobility in VTE risk assessment. In summary, the majority of studies included in our systematic review failed to provide an unambiguous definition of immobility. As a consequence, the assessment of immobility remains unreliable as a concise and clear definition of immobility is lacking. Our review suggests that discrepancies in clinical studies could be due to the heterogeneity of the definition of immobility. Several explanations could account for this strong heterogeneity—(1) the various definitions and the lack of documentation of immobility, (2) the various methodologies implemented on this topic, and (3) the variability and complexity of the included medical inpatients, in terms of underlying disease(s) and comorbid factors. These discrepancies highlight difficulties in analyzing and extrapolating data from these clinical studies in this context. In addition, data from current studies lack clarity, making it difficult to determine when VTE prophylaxis should be administered in a patient population with reduced mobility.
Future Directions
Although the necessity of clearly defining immobility in RCTs was previously reported in the review by Emed et al, progress toward a consistent and universal definition of this term to date remains limited. The pervasiveness of this problem is not limited to research as clinical care of patients is hindered by unclear definitions and inconsistent reporting as well. Future studies, particularly RCTs, are needed to clearly define relationships between immobility, VTE risk, and prophylaxis for medical inpatients, and these studies would benefit from having an objective, reliable, and generalizable definition of immobility. One pivotal finding of this review is the lack of a description of how immobility was defined in the included studies, making it difficult to compare results across studies. Since there is no acceptable consensus on the definition of immobility, we recommend the use of both qualitative and quantitative measures to assess immobility in order to standardize assessments and maximize reliability. This is a necessary step before meaningful VTE prophylaxis recommendations for medical inpatients with impaired immobility can be defined.
Footnotes
Authors’ Note: F. Ye, L. N. Bell, and S. H. Yale contributed to the concept and design, analysis and/or interpretation of data, critical writing or revising the intellectual content, and final approval of the version to be published. J. Mazza and A. Lee contributed to the analysis and/or interpretation of data, critical writing or revising the intellectual content, and final approval of the version to be published.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ramagopalan SV, Wotton CJ, Handel AE, Yeates D, Goldacre MJ. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dobesh P. The importance of appropriate prophylaxis for the prevention of venous thromboembolism in at-risk medical patients. Int J Clin Pract. 2010;64(11):1554–1562. [DOI] [PubMed] [Google Scholar]
- 3. Haas S, Spyropoulos AC. Primary prevention of venous thromboembolism in long-term care: identifying and managing the risk. Clin Appl Thromb Hemost. 2008;14(2):149–158. [DOI] [PubMed] [Google Scholar]
- 4. Schina MJ, Neumyer MM, Healy DA, et al. Influence of age on venous physiologic parameters. J Vasc Surg. 1993;18(5):749–752. [DOI] [PubMed] [Google Scholar]
- 5. Weill-Engerer S, Meaume S, Lahlou A, et al. Risk factors for deep vein thrombosis in inpatients aged 65 and older: a case-control multicenter study. J Am Geriatr Soc. 2004;52(8):1299–1304. [DOI] [PubMed] [Google Scholar]
- 6. Pottier P, Hardouin JB, Lejeune S, Jolliet P, Gillet B, Planchon B. Immobilization and the risk of venous thromboembolism. A meta-analysis on epidemiological studies. Thromb Res. 2009;124(4):468–476. [DOI] [PubMed] [Google Scholar]
- 7. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947–954.e942. [DOI] [PubMed] [Google Scholar]
- 8. Espitia O, Fouassier M, Hardouin JB, et al. Thrombin generation assay in hospitalized nonsurgical patients: a new tool to assess venous thromboembolism risk? Clin Appl Thromb Hemost. 2017;23(1):45–51. [DOI] [PubMed] [Google Scholar]
- 9. Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457. [DOI] [PubMed] [Google Scholar]
- 10. Kahn SR, Lim W, Dunn AS, et al. ; American College of Chest Physicians. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Emed JD, Morrison DR, Des Rosiers L, et al. Definition of immobility in studies of thromboprophylaxis in hospitalized medical patients: a systematic review. J Vasc Nurs. 2010;28(2):54–66. [DOI] [PubMed] [Google Scholar]
- 12. Cohen AT, Spiro TE, Büller HR, et al. Extended-duration rivaroxaban thromboprophylaxis in acutely ill medical patients: MAGELLAN study protocol. J Thromb Thrombolysis. 2011;31(4):407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waha JE, Goswami N, Schlagenhauf A, et al. Effects of exercise and nutrition on the coagulation system during bedrest immobilization. Medicine (Baltimore). 2015;94(38):e1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Germini F, Agnelli G, Fedele M, et al. Padua prediction score or clinical judgment for decision making on antithrombotic prophylaxis: a quasi-randomized controlled trial. J Thromb Thrombolysis. 2016;42(3):336–339. [DOI] [PubMed] [Google Scholar]
- 15. Cohen AT, Spiro TE, Büller HR, et al. ; MAGELLAN Investigators. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513–523. [DOI] [PubMed] [Google Scholar]
- 16. Cohen AT, Spiro TE, Spyropoulos AC, et al. ; MAGELLAN Study Group. D-dimer as a predictor of venous thromboembolism in acutely ill, hospitalized patients: a subanalysis of the randomized controlled MAGELLAN trial. J Thromb Haemost. 2014;12(4):479–487. [DOI] [PubMed] [Google Scholar]
- 17. Nendaz M, Spirk D, Kucher N, et al. Multicentre validation of the Geneva Risk Score for hospitalised medical patients at risk of venous thromboembolism. Explicit ASsessment of Thromboembolic RIsk and Prophylaxis for Medical PATients in SwitzErland (ESTIMATE). Thromb Haemost. 2014;111(3):531–538. [DOI] [PubMed] [Google Scholar]
- 18. Spyropoulos AC, Anderson FA, Fitzgerald G, et al. ; IMPROVE Investigators. Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest. 2011;140(3):706–714. [DOI] [PubMed] [Google Scholar]
- 19. Rosenberg D, Eichorn A, Alarcon M, McCullagh L, McGinn T, Spyropoulos AC. External validation of the risk assessment model of the International Medical Prevention Registry on Venous Thromboembolism (IMPROVE) for medical patients in a tertiary health system. J Am Heart Assoc. 2014;3(6):e001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hull RD, Schellong SM, Tapson VF, et al. ; EXCLAIM (Extended Prophylaxis for Venous ThromboEmbolism in Acutely Ill Medical Patients With Prolonged Immobilization) study. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153(1):8–18. [DOI] [PubMed] [Google Scholar]
- 21. Turpie AG, Hull RD, Schellong SM, et al. ; EXCLAIM Investigators. Venous thromboembolism risk in ischemic stroke patients receiving extended-duration enoxaparin prophylaxis: results from the EXCLAIM study. Stroke. 2013;44(1):249–251. [DOI] [PubMed] [Google Scholar]
- 22. Yusen RD, Hull RD, Schellong SM, et al. Impact of age on the efficacy and safety of extended-duration thromboprophylaxis in medical patients. Subgroup analysis from the EXCLAIM randomised trial. Thromb Haemost. 2013;110(6):1152–1163. [DOI] [PubMed] [Google Scholar]
- 23. Pai M, Lloyd NS, Cheng J, et al. Strategies to enhance venous thromboprophylaxis in hospitalized medical patients (SENTRY): a pilot cluster randomized trial. Implement Sci. 2013;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ageno W, Riva N, Noris P, et al. ; FONDAIR study group. Safety and efficacy of low-dose fondaparinux (1.5 mg) for the prevention of venous thromboembolism in acutely ill medical patients with renal impairment: the FONDAIR study. J Thromb Haemost. 2012;10(11):2291–2297. [DOI] [PubMed] [Google Scholar]
- 25. Bergese SD, Minkowitz HS, Arpino PA, et al. Multicenter trial of desirudin for the prophylaxis of thrombosis: an alternative to heparin-based anticoagulation (DESIR-ABLE). Clin Appl Thromb Hemost. 2013;19(4):418–423. [DOI] [PubMed] [Google Scholar]
- 26. Schellong SM, Haas S, Greinacher A, et al. An open-label comparison of the efficacy and safety of certoparin versus unfractionated heparin for the prevention of thromboembolic complications in acutely ill medical patients: CERTAIN. Expert Opin Pharmacother. 2010;11(18):2953–2961. [DOI] [PubMed] [Google Scholar]
- 27. Riess H, Haas S, Tebbe U, et al. A randomized, double-blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non-surgical patients: CERTIFY Study. J Thromb Haemost. 2010;8(6):1209–1215. [DOI] [PubMed] [Google Scholar]
- 28. Tebbe U, Schellong SM, Haas S, et al. Certoparin versus unfractionated heparin to prevent venous thromboembolic events in patients hospitalized because of heart failure: a subgroup analysis of the randomized, controlled CERTIFY study. Am Heart J. 2011;161(2):322–328. [DOI] [PubMed] [Google Scholar]
- 29. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387–394. [DOI] [PubMed] [Google Scholar]
- 30. Ongen G, Yılmaz A, Cirak AK, et al. Venous thromboembolism risk and thromboprophylaxis among hospitalized patients: data from the Turkish arm of the ENDORSE study. Clin Appl Thromb Hemost. 2011;17(5):539–545. [DOI] [PubMed] [Google Scholar]
- 31. Nendaz MR, Chopard P, Lovis C, et al. Adequacy of venous thromboprophylaxis in acutely ill medical patients (IMPART): multisite comparison of different clinical decision support systems. J Thromb Haemost. 2010;8(6):1230–1234. [DOI] [PubMed] [Google Scholar]
- 32. Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. ; ADOPT Trial Investigators. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 33. Rodríguez-Mañas L, Gómez-Huelgas R, Veiga-Fernández F, Ruiz GM, González JM; ANCIANOS Investigators. Thromboprophylaxis with the low-molecular-weight heparin bemiparin sodium in elderly medical patients in usual clinical practice: the ANCIANOS study. Clin Drug Investig. 2010;30(5):337–345. [DOI] [PubMed] [Google Scholar]
- 34. Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF; LIFENOX Investigators. Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med. 2011;365(26):2463–2472. [DOI] [PubMed] [Google Scholar]
- 35. Engbers MJ, Blom JW, Cushman M, Rosendaal FR, van Hylckama Vlieg A. The contribution of immobility risk factors to the incidence of venous thrombosis in an older population. J Thromb Haemost. 2014;12(3):290–296. [DOI] [PubMed] [Google Scholar]
- 36. Amin AN, Girard F, Samama MM. Does ambulation modify venous thromboembolism risk in acutely ill medical patients? Thromb Haemost. 2010;104(5):955–961. [DOI] [PubMed] [Google Scholar]
- 37. Gatt ME, Paltiel O, Bursztyn M. Is prolonged immobilization a risk factor for symptomatic venous thromboembolism in elderly bedridden patients? Results of a historical-cohort study. Thromb Haemost. 2004;91(3):538–543. [DOI] [PubMed] [Google Scholar]