Abstract
Background:
Obstructive sleep apnea syndrome (OSAS) is an independent risk factor for cardiovascular disease (CVD). Although monocyte to high-density lipoprotein cholesterol ratio (MHR) is increasingly being implicated in cardiovascular morbidity and mortality, no study has attempted to determine the role of MHR in cardiovascular morbidity of patients with OSAS. We aimed to investigate the association between MHR and CVD in patients with OSAS and the relationship between severity of OSAS, polysomnographic parameters, and MHR.
Methods:
In this cohort study, patients who had undergone a full-night polysomnography for the diagnosis of OSAS were recruited. Included patients were grouped according to the apnea–hypopnea index (AHI) as mild (5-15), moderate (15-30), and severe (>30) OSAS. Patients with AHI < 5 served as the control group. The presence of heart failure, coronary artery disease, or arrhythmia was defined as CVD.
Results:
A total of 1050 patients were included (131 controls, 222 mild, 228 moderate, and 469 severe OSAS). The severe group had higher MHR compared with the control and other OSAS groups (9.99, 12.11, 13.65, and 20.67 in control, mild, moderate, and severe OSAS groups, respectively, P < .001). The MHRs were significantly correlated with AHI, oxygen desaturation index, and minimum O2 saturation values (P < .001). Values of MHR were significantly higher in patients with CVD compared with those without (P < .001). Multiple regression analysis demonstrated that MHR is an independent predictor of CVD.
Conclusion:
The MHR is strongly associated with CVD and the severity of OSAS and might be used as a biomarker to predict CVD in patients with OSAS.
Keywords: monocyte/HDL cholesterol ratio, cardiovascular disease, obstructive sleep apnea syndrome, monocyte, HDL cholesterol
Introduction
Obstructive sleep apnea syndrome (OSAS), characterized by repetitive complete or partial upper airway collapses occurring during sleep, is a common disorder affecting 3% to 7% of the middle-aged population.1 Obstructive sleep apnea syndrome significantly increases the risk of cardiovascular diseases (CVDs) including hypertension, heart failure, arrhythmias, and coronary artery diseases.2–4 Chronic intermittent hypoxia, sympathetic activation, inflammation, oxidative stress, and endothelial dysfunction are frequently seen in sleep apnea syndrome and may constitute etiologic mechanisms linking OSAS to CVD.5,6 Macrophages and monocytes are the most important cell types for the secretion of proinflammatory and pro-oxidant cytokines at the site of inflammation.7 Monocytes are the cells found prominently in the development of atherosclerotic lesions.8 In addition, high-density lipoprotein cholesterol (HDL-C) has been shown to defend endothelial cells against the unfavorable effects of low-density lipoprotein (LDL) and to prohibit oxidation of LDL molecules.9 Thus, it was thought that HDL-C exhibits both anti-inflammatory and antioxidant actions. Recently, monocyte count to HDL-C ratio (MHR) has emerged as a new cardiovascular prognostic marker.10–13 Although this association is shown in the general population, none of the previous studies have ever investigated the association between MHR and CVD in patients with OSAS.
Based on this information, the aim of the study was to evaluate the association of MHR with the severity of the disease—defined as apnea–hypopnea index (AHI)—and cardiovascular events in patients with OSAS.
Materials and Methods
Study Population
This was a retrospective multicenter study. Patients who had undergone a sleep study at the sleep study centers of Gaziosmanpasa University School of Medicine, Kahramanmaras Elbistan State Hospital, and Istanbul Medeniyet University School of Medicine between May 2013 and July 2016 based on the clinical suspicion that they may have OSAS were recruited to the study. Polysomnographic (PSG) evaluation of all patients was performed. Based on their AHI scores, patients were categorized as OSAS negative (control) group (AHI < 5), mild (AHI: 5-15), moderate (AHI: 15-30), and severe OSAS (AHI > 30) groups, according to the American Academy of Sleep Medicine (AASM) task force criteria.14
Patients younger than 18 years of age and with central sleep apnea syndrome, upper airway-resistant syndrome, narcolepsy, and movement disorders were excluded from the study. Patients with liver or kidney disease, chronic alcoholism, malignancy, recent infection, hyperthyroidism and hypothyroidism, inflammatory connective tissue disorders, inflammatory bowel disease, hypoxemic lung disease—including chronic obstructive pulmonary diseases, interstitial lung disease, asthma—and hematologic disease (leukemia, anemia, or myelodysplastic syndrome) were also excluded. Data related to the demographic characteristics, sleep patterns, medical history, including cardiovascular and metabolic diseases, medication use, and habits were retrieved using a standardized questionnaire before the sleep study. Routine blood tests were performed. As for the purpose of this study, the term CVD referred only to the presence of heart failure, coronary artery disease, or arrhythmia. Diagnosis of CVD had been made by an expert cardiologist (by using electrocardiogram, echocardiography, and coronary angiography), medical history and as a medical treatment, the patients were receiving 1 or more than 1 antiaggregant, anti-ischemic agent, β-blocker, angiotensin-converting enzyme inhibitor, angiotensin-receptor blocker, calcium channel blockers, and thiazide and thiazide-like diuretics. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by the local ethics committee.
Sleep Study
Overnight PSG was performed in all patients using a 55-channel polysomnography (Alice Sleepware; Philips Respironics, Pennsylvania), which included the following: electrooculograms (2 channels), electroencephalograms (4 channels), electromyograms of the submental muscles (1 channel), anterior tibialis muscle of both legs (2 channels), electrocardiograms, airflow measurements (with oronasal thermistor and nasal cannula pressure transducer), body position sensor that discerns changes in the body position during sleep, and a snore sensor for the detection of snoring vibrations. Respiratory efforts of the chest and abdominal muscles (2 channels) were recorded using piezoelectric belts and arterial oxyhemoglobin saturation (Sao 2: 1 channel) by pulse oximetry with a finger probe. The recordings were scored according to the standard criteria of AASM. Apnea was defined as a ≥90% decrease in the airflow amplitude persisting for at least 10 seconds relative to the baseline amplitude. The AASM has provided 2 definitions for hypopnea. The recommended one is a ≥30% decrease in the airflow amplitude relative to the baseline values associated with ≥4% oxygen desaturation, all sustaining for at least 10 seconds. Alternative definition is expressed as a ≥50% decrease in the airflow amplitude relative to the baseline values associated with a ≥3% oxygen desaturation or arousal from sleep, all sustaining for at least 10 seconds. In our study, hypopnea was determined according to the alternative definition.15 The AHI was calculated as the number of apneic plus hypopneic episodes per hour of sleep. Patients with AHI ≥5 events/hour were diagnosed as having OSAS. Oxygen desaturation index (ODI) was defined as the total number of measurements of oxyhemoglobin desaturation of ≥4% within ≥10 seconds to <3 minutes from the baseline, divided by the total sleep time.
Laboratory Measurements
Blood samples were obtained from the patients in the morning, after 12 hours of fasting, for the measurement of plasma glucose, total serum cholesterol, triglyceride, and HDL and LDL cholesterols. All variables were analyzed by Roche Cobas autoanalyzer (USA). We collected the blood samples in tubes containing ethylenediaminetetraacetic acid for the measurements of the monocyte, leukocyte, hemoglobin, and platelet counts. The samples were analyzed within 60 minutes after collection using a CELLDYN 3700 automated hematology analyzer (Abbott Laboratories, Abbott Park, Illinois).
Statistical Analysis
Data were presented as mean ± standard deviation, median, interquartile range, or count, percentage. Two independent sample t tests and 1-way analysis of variances or Kruskal Wallis test were used to compare the continuous data between or among groups. For multiple comparisons, Tukey honest significant difference or Bonferroni-corrected Mann-Whitney U test was used. Chi-square test was used to compare the categorical data between or among groups. Receiver operating characteristic (ROC) curve analysis was used to determine the cutoff value of MHR in predicting significant CVD classification. OptimalCutpoints and pROC packages in R programming language were used for cutoff and performance values. A P value <.05 was considered significant. Other analyses were performed using SPSS 19 (IBM SPSS Statistics 19, SPSS Inc, an IBM Co, Somers, New York). The ROC curves were constructed using MedCalc (MedCalc 11.3, Mariakerke, Belgium).
Results
One thousand fifty participants took part in the study. The clinical characteristics, laboratory parameters, and PSG findings are shown in Table 1. Of the participants, 695 (66%) were men and 355 (34%) were women. Mean age and body mass index (BMI) of the patients were 49.44 ± 11.63 years and 31.98 ± 6.36 kg/m2, respectively. A total of 919 (87%) patients were classified as having OSAS, whereas 131 (12%) patients with an AHI < 5 constituted the control group. Two hundred twenty-two (21%), 228 (22%), and 469 (45%) participants were categorized as the mild, moderate, and severe OSAS groups, respectively. The rates of CVD, hypertension, and diabetes mellitus were significantly different among groups (P < .05; Table 1).
Table 1.
Demographic, Clinical, Laboratory, and Polysomnographic Findings of the Study Population.a
| Control Group (n = 131) | Mild OSAS (n = 222) | Moderate OSAS (n = 228) | Severe OSAS (n = 469) | P | |
|---|---|---|---|---|---|
| Age (years) | 43.31 ±11.75b | 46.86 ± 10.83c | 50.3 ± 11.32d | 51.96 ± 11.27d | <.001 |
| Gender, male, n (%) | 60 (45.8)b | 153 (68.9)c | 161 (70.6)c | 321 (68.4)c | <.001 |
| BMI (kg/m2) | 29.97 ± 6.67b | 30.29 ± 5.7b | 31.19 ± 5.08b | 33.73 ± 6.67c | <.001 |
| Hypertension, n (%) | 24 (18.3)b | 42 (18.9)b | 76 (33.3)c | 226 (48.2)d | <.001 |
| Diabetes mellitus, n (%) | 11 (8.4)b | 27 (12.2)b | 41 (18)b,c | 114 (24.3)c | <.001 |
| CVD, n (%) | 4 (3.1)b | 22 (9.9)b,c | 40 (17.5)c | 195 (41.6)d | <.001 |
| Laboratory findings | |||||
| Total WBC (103/mL) | 7.33 ± 1.86b | 7.87 ± 1.85b | 7.71 ± 2.06b | 8.34 ± 2.03c | <.001 |
| Monocytes (×103/μL) | 0.46 ± 0.14b | 0.52 ± 0.16c | 0.56 ± 0.16c | 0.83 ± 0.18d | <.001 |
| Hemoglobin (g/dL) | 13.89 ± 1.62b | 14.61 ± 1.64c | 14.49 ± 1.68c | 14.48 ± 1.71c | .001 |
| Platelets (×103/μL) | 251.62 ± 62.75 | 255.86 ± 60.94 | 258.37 ± 77.28 | 254 ± 64.25 | 0.785 |
| MHR | 9.99 ± 3.98b | 12.11 ± 4.97c | 13.65 ± 5.16d | 20.67 ± 7.57e | <.001 |
| Total cholesterol (mg/dL) | 177.05 ± 42.14b | 181.69 ± 50.58b | 182.84 ± 46.01b | 198.01 ± 47.14c | .001 |
| Triglycerides (mg/dL) | 154.26 ± 70.69b | 182.04 ± 98.5c | 190.35 ± 78.85c | 212.78 ± 106.39d | <.001 |
| LDL (mg/dL) | 127.19 ± 33.99b | 135.94 ± 36.33b,c | 138.94 ± 39.08c,d | 144.82 ± 42.67d | <.001 |
| HDL (mg/dL) | 49.34 ± 11.66b | 47.21 ± 17.36b | 43.8 ± 13.4c | 43.16 ± 12.52c | <.001 |
| Glucose (mg/dL) | 100.06 ± 25.12b | 103.99 ± 21.93b | 107.2 ± 30.85b | 118.68 ± 38.78c | <.001 |
| Polysomnographic findings | |||||
| SE (%) | 82.44 ± 10.05 | 84.05 ± 10 | 83.03 ± 10.25 | 82.53 ± 10.1 | .284 |
| AHI events/hour | 2.61 ± 1.35b | 9.51 ± 2.8c | 21.27 ± 3.89d | 57.28 ± 22.28e | <.001 |
| Minimum O2 sat (%) | 89.74 ±.5.3b | 85.43 ± 5.6c | 81.47 ±.8.61d | 71.16 ± 14.23e | <.001 |
| Desaturation (%) | 0 (0-0.1)b | 0.2 (0-0.9)c | 1.05 (0.3-3.5)d | 9.2 (2.6-3.4)e | <.001 |
| ODI | 1.7 (0.8-3.4)b | 7 (4.5-10)c | 17.65 (12.95-22.1)d | 48.35 (31.1-74.15)e | <.001 |
Abbreviations: AHI, apnea hypopnea index; BMI, body bass index; CVD, cardiovascular disease; desaturation (%), sleep time of Spo 2 < 90%; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MHR, monocyte to high-density lipoprotein ratio; ODI, oxygen desaturation index; O2 saturation, oxygen saturation; OSAS, obstructive sleep apnea syndrome; SE, sleep efficiency; WBC, white blood cell.
aEach different superscripts (b, c, d, e) indicate statistical significance.
Laboratory parameters, glucose, total serum cholesterol, triglyceride, HDL and LDL cholesterols, monocyte, leukocyte, hemoglobin, and MHR differed significantly among the 4 groups (P < .05; Table 1). The MHR value showed a significant increase in parallel with the severity of OSAS.
Polysomnographic study results are shown in Table 1. The API, ODI, minimum oxygen saturation, and desaturation levels (%) were significantly different between groups (P < .05).
Correlations between PSG and laboratory parameters were evaluated. The MHRs were positively correlated with AHI (Figure 1), ODI values, and desaturation percentages and negatively correlated with minimum O2 saturation values (P < .05; Table 2).
Figure 1.
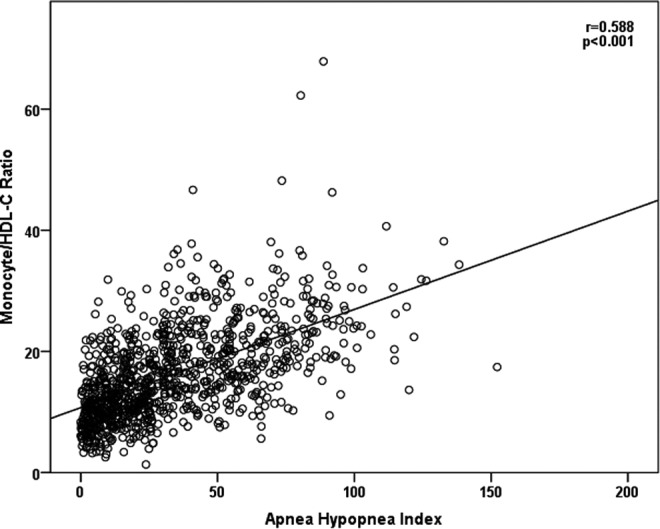
Correlation between monocyte to high-density lipoprotein cholesterol ratio (MHR) and apnea–hypopnea index (AHI).
Table 2.
Correlations Between Polysomnographic Parameters and Monocyte to High-Density Lipoprotein Cholesterol Ratio.
| MHR | ||
|---|---|---|
| r | P | |
| AHI events/hour | .588 | <.001 |
| Minimum O2 saturation (%) | −.390 | <.001 |
| Desaturation (%) | .334 | <.001 |
| ODI | .545 | <.001 |
Abbreviations: AHI, apnea–hypopnea index; MHR, monocyte to high-density lipoprotein cholesterol ratio; ODI, oxygen desaturation index.
The cases were divided into 2 groups as those with (n = 261) or without (n = 789) CVD. Mean MHRs were 21.02 and 14.34 in patients with and without CVD, respectively (P < .001; Figure 2).
Figure 2.
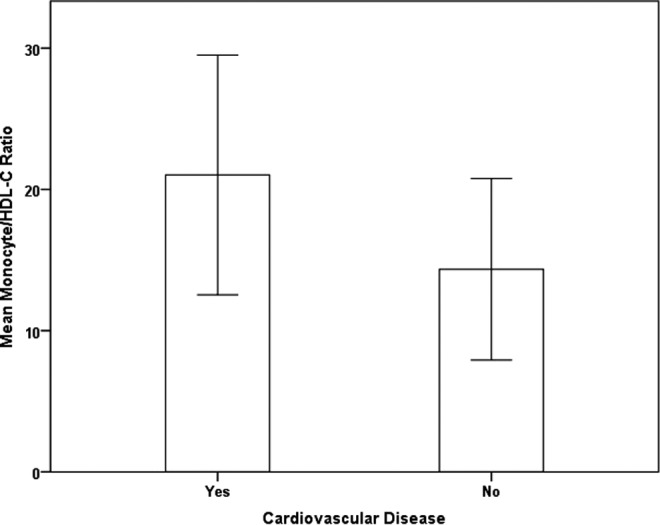
Monocyte to high-density lipoprotein cholesterol ratio (MHRs) in patients with and without cardiovascular disease in the study groups.
All potential determinants for the CVD were further investigated in an univariate screening procedure (Table 3). Subsequently, all parameters related to the CVD, with a significance level below 0.1, were introduced in a stepwise multiple regression analysis. The final regression model included age, BMI, hypertension, diabetes mellitus, LDL cholesterol, AHI, and MHR values; the independent predictors of CVD were MHR, age, and AHI in patients with OSAS (Table 4). The MHR was independently associated with CVD even after adjusted for these variables (odds ratio = 1.066, 95% confidence interval: 1.038-1.094, P < .0001).
Table 3.
Univariate Analysis Predicting Cardiovascular Diseases in Patients With Obstructive Sleep Apnea Syndrome.a
| Odds Ratio | 95% Confidence Interval | P | |
|---|---|---|---|
| Age (years) | 1.052 | 1.038-1.066 | <.001 |
| Gender | 1.065 | 0.793-1.429 | 0.677 |
| BMI (kg/m2) | 1.065 | 1.042-1.089 | <.001 |
| AHI events/h | 1.044 | 1.037-1.050 | <.001 |
| MHR | 1.132 | 1.108-1.157 | <.001 |
| Hypertension | 2.612 | 1.960-3.481 | <.001 |
| Diabetes mellitus | 2.428 | 1.744-3.381 | <.001 |
| LDL cholesterol (mg/dL) | 1.005 | 1.001-1.008 | .010 |
Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; LDL, low-density lipoprotein; MHR, monocyte to high-density lipoprotein cholesterol ratio.
aSignificant values are given in bold.
Table 4.
Risk Factors for Cardiovascular Diseases in Patients With Obstructive Sleep Apnea Syndrome.a
| Odds Ratio | 95% Confidence Interval | P | |
|---|---|---|---|
| Age (years) | 1.048 | 1.031-1.066 | <.001 |
| BMI (kg/m2) | 1.007 | 0.98-1.036 | .614 |
| AHI events/hour | 1.033 | 1.026-1.041 | <.001 |
| MHR | 1.064 | 1.036-1.093 | <.001 |
| Hypertension | 1.185 | 0.819-1.715 | .369 |
| Diabetes mellitus | 1.304 | 0.861-1.973 | .210 |
| LDL cholesterol (mg/dL) | 1.000 | 0.996-1.004 | .913 |
Abbreviations: AHI, apnea–hypopnea index; BMI, body mass index; MHR, monocyte to high-density lipoprotein cholesterol ratio.
aSignificant values are given in bold.
The ROC analysis was performed to determine the cutoff value of MHR (>14.73) in predicting significant CVD classification. We found sensitivity value as 77.9%, specificity value as 59.3%, and area under the ROC curve value as 0.746 (P < .001; Figure 3).
Figure 3.
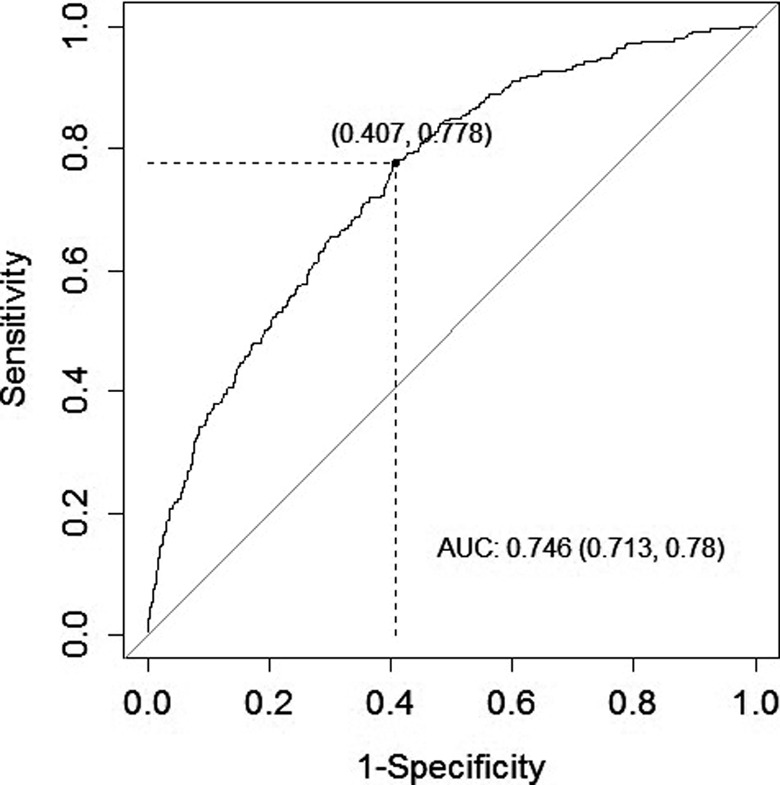
Mean Monocyte to high-density lipoprotein cholesterol ratio (MHRs) cut-off value for to demonstrate the presence of cardiovascular disease, and related ROC curve.
Discussion
The salient findings of the present study are (1) MHRs increase as the severity of OSAS increases and (2) MHRs are significantly higher in patients with CVD compared with those without CVD in patients with OSAS, and MHR is an independent predictor for CVD in OSAS.
There is much evidence that patients with OSAS have an increased risk for cardiovascular and cerebrovascular diseases.16,17 Intermittent episodes of hypoxia as a result of transient cessation of breathing during sleep are major physiologic characteristics of OSAS, which resemble symptoms of ischemia–reperfusion injury. Intermittent episodes of nocturnal hypoxemia induce formation of oxygen-free radicals leading to a state of low-grade circulation and inflammation.18 Inflammation is one of the fundamental factors contributing to the onset and progression of atherosclerosis.19 Increased concentrations of mediators or markers of inflammation predict subsequent atherosclerotic CVD.20 Recently, C-reactive protein (CRP), platelet/lymphocyte ratio (PLR), and neutrophil/lymphocyte ratio (NLR) have been used as inflammatory markers and have been shown to be independent risk factors for atherosclerosis.21–23 Several recent studies showed that CRP, NLR, and PLR have also been increased in OSAS.24–26 It has been known that monocyte activation plays an important role in chronic inflammation and CVD (such as heart failure and atherosclerosis), in which monocytes and differentiated macrophages can modulate inflammatory cytokines and tissue remodeling. Monocyte adherence to endothelial cell surface and extravasation to the damaged tissue induce the production of several cytokines—including tumor necrosis factor-α, interleukin (IL)-1, and IL-6 (potent inflammatory cytokines)—platelet-derived endothelial cell growth factor, transforming growth factor-α and -β, macrophage colony-stimulating factor, and insulin-like growth factor.27 Thereafter, monocytes differentiate into the macrophages that ingest oxidized LDL cholesterol and form the dangerous foamy cells.28 Contrarily, HDL molecules counteract the migration of macrophages and promote efflux of oxidized cholesterol from these cells. Recent studies also indicate a role of HDL in controlling the monocyte activation, adhesiveness, and inflammation and in controlling the proliferation of progenitor cells that give rise to monocytes.29,30 Besides its anti-inflammatory and antioxidative effects, HDL molecules also enhance vasorelaxation and increase endothelial nitric oxide synthase expression.31 Therefore, monocytes exert a proinflammatory and pro-oxidant effects, but HDL-C functions as a reversal factor during those processes. In our study, significantly higher monocyte counts were detected in patients with OSAS when compared with the control group. In the severe OSAS group, monocyte counts were found to be higher than those observed in cases with moderate and mild OSAS. This finding might explain the reason of higher incidence of cardiovascular events in severe OSAS.
One may hypothesize that an increased MHR may be a predictor for the development and progression of cardiovascular events. The MHR is a newly introduced inflammatory marker. Its relation to CVDs has been examined in a few studies. Kanbay et al reported that increased MHR was an independent predictor of major cardiovascular events during follow-up in patients with chronic kidney disease.32 Kundi et al reported that MHR is significantly and independently related to the presence and severity of coronary ectasia.33 Çiçek and colleagues showed that admission of MHR is associated independently and significantly with short- and long-term mortality in patients with ST-segment elevation myocardial infarction who undergo successful primary percutaneous coronary intervention.34 Recently, Canpolat et al indicated that MHR was an independent predictor of atrial fibrillation recurrence after cryoballoon-based catheter ablation and was associated significantly with the presence of slow coronary flow.35 This is the first study that showed the association between MHR and the severity of OSAS and presence of CVD in patients with OSAS even after adjusted for well-known risk factor for CVD in multivariate analysis. We also showed a positive correlation between MHR and the severity of hypoxemia defined by ODI.
Our study yielded important findings in a relatively larger cohort that emphasized the importance of MHR in the prediction of CVD in patients with OSAS. However, there are some limitations of our study that have to be mentioned. First, we did not follow the patients prospectively and did not investigate the effect of continuous positive airway pressure treatment on MHRs. Second, serum HDL-C level and monocyte count in CBC are subject to changes with time in a given patient; therefore, one-time measurements may not completely reflect the real trend of the studied parameters. Trend in a time interval would be a better indicator. Third, although hs-CRP was studied as an inflammatory marker, oxidative stress biomarkers were not assessed.
In conclusion, MHR is strongly associated with the severity of OSAS and CVD in patients with OSAS. The MHR might be used as a biomarker to predict CVD in patients with OSAS. Further prospective studies are warranted to evaluate the prognostic value of MHR for the development of new cardiovascular events in patients with OSAS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Durán J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 pt 1):685–689. [DOI] [PubMed] [Google Scholar]
- 2. Weiss JW, Launois SH, Anand A, Garpestad E. Cardiovascular morbidity in obstructive sleep apnea. Prog Cardiovasc Dis. 1999;41(5):367–376. [DOI] [PubMed] [Google Scholar]
- 3. Yang WS, Lee WJ, Funahashi T, et al. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab. 2001;86(8):3815–3819. [DOI] [PubMed] [Google Scholar]
- 4. Shetty GK, Economides PA, Horton ES, Mantzoros CS, Veves A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care. 2004;27(10):2450–2457. [DOI] [PubMed] [Google Scholar]
- 5. Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat Rev Cardiol. 2010;7(12):677–685. [DOI] [PubMed] [Google Scholar]
- 6. Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Postgrad Med J. 2009;85(1010):693–698. [DOI] [PubMed] [Google Scholar]
- 7. Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80(5):1156–1164. [DOI] [PubMed] [Google Scholar]
- 8. Weber C, Shantsila E, Hristov M, et al. Role and analysis of monocyte subsets in cardiovascular disease. Joint consensus document of the European Society of Cardiology (ESC) Working Groups “Atherosclerosis & Vascular Biology” and “Thrombosis.” Thromb Haemost. 2016;116(4):626–637. [DOI] [PubMed] [Google Scholar]
- 9. Li XP, Zhao SP, Zhang XY, Liu L, Gao M, Zhou QC. Protective effect of high density lipoprotein on endothelium-dependent vasodilatation. Int J Cardiol. 2000;73(3):231–236. [DOI] [PubMed] [Google Scholar]
- 10. Canpolat U, Aytemir K, Yorgun H, et al. The role of preprocedural monocyte-to-high-density lipoprotein ratio in prediction of atrial fibrillation recurrence after cryoballoon-based catheter ablation. Europace. 2015;17(12):1807–1815. [DOI] [PubMed] [Google Scholar]
- 11. Cetin MS, Ozcan Cetin EH, Kalender E, et al. Monocyte to HDL cholesterol ratio predicts coronary artery disease severity and future major cardiovascular adverse events in acute coronary syndrome. Heart Lung Circ. 25(11):1077-1086. [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Li S, Guo YL, et al. Is monocyte to HDL ratio superior to monocyte count in predicting the cardiovascular outcomes: evidence from a large cohort of Chinese patients undergoing coronary angiography. Ann Med. 2016;48(5):305–312. [DOI] [PubMed] [Google Scholar]
- 13. Cetin EH, Cetin MS, Canpolat U, et al. Monocyte/HDL-cholesterol ratio predicts the definite stent thrombosis after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Biomark Med. 2015;9(10):967–977. [DOI] [PubMed] [Google Scholar]
- 14. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 15. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification. 1st ed Westchester, NY: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 16. Peker Y, Hedner J, Kraiczi H, et al. Respiratory disturbance index: an independent predictor of mortality in coronary artery disease. Am J Respir Crit Care Med. 2000;162(1):81–86. [DOI] [PubMed] [Google Scholar]
- 17. Dyken ME, Somers VK, Yamada T, et al. Investigating the relationship between stroke and obstructive sleep apnea. Stroke. 1996;27(3):401–407. [DOI] [PubMed] [Google Scholar]
- 18. Takama N, Kurabayasi M. Influence of untreated sleep disordered breathing on the long-term prognosis of patients with cardiovascular disease. Am J Cardiol. 2009;103(5):730–734. [DOI] [PubMed] [Google Scholar]
- 19. Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12(2):171–172. [DOI] [PubMed] [Google Scholar]
- 20. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. [DOI] [PubMed] [Google Scholar]
- 21. Taniguchi H, Momiyama Y, Ohmori R, et al. Associations of plasma C-reactive protein levels with the presence and extent of coronary stenosis in patients with stable coronary artery disease. Atherosclerosis. 2005;178(1):173–177. [DOI] [PubMed] [Google Scholar]
- 22. Kurtul A, Murat SN, Yarlioglues M, et al. Association of platelet-to-lymphocyte ratio with severity and complexity of coronary artery disease in patients with acute coronary syndromes. Am J Cardiol. 2014;114(7):972–978. [DOI] [PubMed] [Google Scholar]
- 23. Kurtul S, Sarli B, Baktir AO, et al. Neutrophil to lymphocyte ratio predicts SYNTAX score in patients with non-ST segment elevation myocardial infarction. Int Heart J. 2015;56(1):18–21. [DOI] [PubMed] [Google Scholar]
- 24. Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C-reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005;46(5):801–809. [DOI] [PubMed] [Google Scholar]
- 25. Altintas N, Çetinoğlu E, Yuceege M, et al. Neutrophil-to-lymphocyte ratio in obstructive sleep apnea; a multicenter, retrospective study. Eur Rev Med Pharmacol Sci. 2015;19(17):3234–3240. [PubMed] [Google Scholar]
- 26. Koseoglu HI, Altunkas F, Kanbay A, Doruk S, Etikan I, Demir O. Platelet-lymphocyte ratio is an independent predictor for cardiovascular disease in obstructive sleep apnea syndrome. J Thromb Thrombolysis. 2015;39(2):179–185. [DOI] [PubMed] [Google Scholar]
- 27. Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130(2):147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghattas A, Griffiths HR, Devitt A, Lip GY, Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J Am Coll Cardiol. 2013;62(17):1541–1551. [DOI] [PubMed] [Google Scholar]
- 29. Murphy AJ, Chin-Dusting JP, Sviridov D, Woollard KJ. The anti inflammatory effects of high density lipoproteins. Curr Med Chem. 2009;16(6):667–675. [DOI] [PubMed] [Google Scholar]
- 30. Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37(7):710–718. [DOI] [PubMed] [Google Scholar]
- 31. Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144(1):165–172. [DOI] [PubMed] [Google Scholar]
- 32. Kanbay M, Solak Y, Unal HU, et al. Monocyte count/HDL cholesterol ratio and cardiovascular events in patients with chronic kidney disease. Int Urol Nephrol. 2014;46(8):1619–1625. [DOI] [PubMed] [Google Scholar]
- 33. Kundi H, Gok M, Kiziltunc E, et al. Relation between monocyte to high-density lipoprotein cholesterol ratio with presence and severity of isolated coronary artery ectasia. Am J Cardiol. 2015;116(11):1685–1689. [DOI] [PubMed] [Google Scholar]
- 34. Çiçek G, Kundi H, Bozbay M, et al. The relationship between admission monocyte HDL-C ratio with short-term and long-term mortality among STEMI patients treated with successful primary PCI. Coron Artery Dis. 2016;27(3):176–184. [DOI] [PubMed] [Google Scholar]
- 35. Canpolat U, Çetin EH, Cetin S, et al. Association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin Appl Thromb Hemost. 2016;22(5):476–482. [DOI] [PubMed] [Google Scholar]


