Abstract
Introduction:
Estimation of residual rivaroxaban plasma concentrations may be requested before invasive procedures and some patients at high thromboembolic risk will have a bridging therapy with heparins when rivaroxaban is interrupted.
Objective:
The objective of this study was to assess the performance of the STA-Liquid Anti-Xa assay (STA LAX) and the low and normal procedures of the Biophen Direct Factor Xa Inhibitors (DiXaI) assay, in patients with and without bridging with low-molecular-weight heparins (LMWHs).
Materials and Methods:
Seventy-nine blood samples were collected from 77 patients on rivaroxaban at CTROUGH or before an invasive procedure. Rivaroxaban plasma concentrations were estimated using Biophen DiXaI, Biophen DiXaI LOW, and STA LAX and compared to liquid chromatography coupled with mass spectrometry (LC-MS/MS) measurements. Stratifications were performed according to heparin bridging.
Results:
The Biophen DiXaI LOW and STA LAX showed better correlation with LC-MS/MS measurements than Biophen DiXaI in patients not bridged with LMWH (R: 0.97, 0.96, and 0.91, respectively). However, the performance of Biophen DiXaI LOW and STA LAX decreased when residual LMWH activity was present (R: 0.18 and 0.19 respectively) demonstrating that these tests are not specific to rivaroxaban.
Conclusion:
In patients not bridged with LMWH, we suggest to use the Biophen DiXaI LOW and STA LAX for the estimation of rivaroxaban concentrations <50 ng/mL. These results should be confirmed on a larger cohort of patients. Patients bridged with LMWH have inaccurate estimates of low levels of rivaroxaban and the 3 assays studied should not be used to estimate if it is safe to perform a procedure.
Keywords: anticoagulants, drug monitoring, heparin, low-molecular-weight, perioperative period, rivaroxaban
Introduction
The management of rivaroxaban in the perioperative context requires specific expertise in both clinical and laboratory aspects.1 In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) trial, treatment interruptions occurred in 33% of participants, and 45% of the interruptions were in patients treated with rivaroxaban. Approximately 8% of these patients were bridged, and a low-molecular-weight heparin (LMWH) was used in 98% of these patients. Surgical procedures accounted for 39% of the treatment interruptions in the rivaroxaban arm.2 A similar pattern was also reported from the Dresden registry in which 27% of the participants underwent a surgical procedure, of whom 30% were bridged with a LMWH after the preprocedural interruption of their direct oral anticoagulant (DOAC).3 The median duration of DOAC interruption before surgery was 2 days.3 However, a prospective observational study on the periprocedural management of patients treated with dabigatran etexilate or rivaroxaban showed that 2 days of interruption may not be sufficient to eliminate the entire residual effect of these drugs. Indeed, in 14% of patients, although DOACs had been discontinued for 48 hours, DOAC concentrations remained above 30 ng/mL which was defined as the safe threshold for invasive procedures.4 Thus, some situations will require an assessment of the residual effect of rivaroxaban to guide therapeutic decisions, for example, before selecting fibrinolytic treatment in a patient on rivaroxaban presenting with an acute ischemic stroke.5
Specific coagulation assays are now available that can reliably estimate rivaroxaban plasma concentrations within its therapeutic range.6–10 Nevertheless, as shown for dabigatran,11 2 previous studies have demonstrated the need for coagulation assays sensitive to low (<30 ng/mL) plasma concentrations of rivaroxaban.12,13 These studies used blood samples collected in real-life conditions, but none of them dealt with the problem of measuring plasma rivaroxaban concentrations in patients bridged with heparins when rivaroxaban is stopped.
Some tests used to estimate the plasma concentration of rivaroxaban are also used to estimate heparin concentrations by applying appropriate calibrators.14 The STA-Liquid Anti-Xa assay from Diagnostica Stago (Diagnostica Stago, Asnière, France) is a global anti-Xa assay designed to estimate low and on-therapy plasma concentrations of rivaroxaban and to measure LMWH anti-Xa activity. As a consequence, the presence of heparin when estimating rivaroxaban concentrations might affect the result and vice versa.
To avoid this interference, Samama et al have developed an optimized chromogenic assay (Biophen Direct Factor Xa Inhibitors (DiXaI) from Hyphen BioMed, Neuville-Sur-Oise, France). This test uses a specific buffer allowing the measurement of the sole anti-Xa activity from rivaroxaban even in the presence of heparins or fondaparinux.15 Its limit of quantitation (LOQ) of around 50 ng/mL is not appropriate for the perioperative setting, and an adaptation of the procedure is proposed to allow the estimation of lower rivaroxaban plasma concentrations.
We hypothesized that low residual heparin plasma concentrations found on the day of the invasive procedure may interfere with some assays used to quantify rivaroxaban.14
The objective of this study was to assess the performance (limit of detection, LOQ, and accuracy) of the low and the high procedure of the Biophen DiXaI assay and the STA-Liquid Anti-Xa assay from Diagnostica Stago (Diagnostica Stago, Asnière, France), using real-life samples from patients with or without bridging with LMWH.
Materials and Methods
The study was performed in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the CHU UCL Namur, Yvoir, Belgium. Written informed consent was obtained from each donor.
Clinical Samples
Eighty-six plasma samples from 84 patients on rivaroxaban were collected from April 2014 until the end of July 2015 in the CHU UCL Namur, Yvoir, Belgium.
The inclusion criteria were patients receiving rivaroxaban for secondary prevention of thromboembolic events in atrial fibrillation or treatment of venous thromboembolism, and collection of blood samples at trough concentrations or just before an invasive procedure.
The only exclusion criterion was the absence of result for 1 of the 3 chromogenic assays. Seven blood samples were excluded from the study, which was realized on the remaining 79 blood samples collected from 77 patients.
We aimed at collecting at least 30 samples from patients bridged and not bridged with LMWH. Blood was taken by venipuncture in the antecubital vein using a 21-gauge needle (Terumo) or through a peripheral venous catheter (BD Insyte-W, 18- or 16-gauge) and collected into 0.109 M sodium citrate (9:1 v/v) tubes (Venosafe, Terumo, Heverlee, Belgium).
Platelet-poor plasma (PPP) was obtained from the supernatant fraction after double centrifugation at 1500g for 15 minutes at room temperature. Samples were then aliquoted and frozen immediately at −80°C. Plasma sample aliquots were thawed and heated to 37°C for at least 5 minutes before running the experiments.
We measured the creatinine clearance with the Cockcroft-Gault equation, using the actual body weight.
Distribution of Collected Blood Samples Following LMWH Presence and Last Administration
Some blood samples came from patients bridged with LMWH after cessation of rivaroxaban. As residual heparin might interfere with the estimation of plasma concentrations of rivaroxaban by anti-Xa assays, we divided patients into 3 groups (Figure 1).
Figure 1.
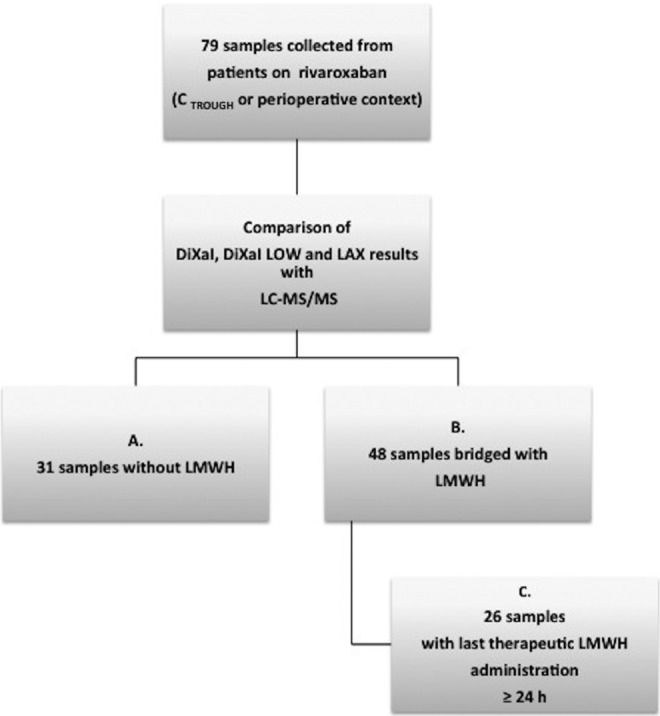
Distribution of the collected blood samples following LMWH presence and last administration. The 79 blood samples included in the analyses were collected from patients receiving rivaroxaban at trough concentrations (CTROUGH) or before an invasive procedure. For these samples, an estimation of rivaroxaban plasma concentrations with 3 chromogenic assays (Biophen DiXaI, Biophen DiXaI LOW, and STA LAX) was performed and compared to measurements obtained by LC-MS/MS. Stratifications were performed according to heparin bridging. LMWH indicates low-molecular-weight heparin; LC-MS/MS, liquid chromatography coupled with mass spectrometry.
Group A represents the patients who were not bridged with LMWH (n = 31). These blood samples were collected from patients at CTROUGH (n = 28; timing of last administration between 21 and 48 hours) outside a perioperative context or within a perioperative context without the use of heparin bridging (n = 3; timing of last administration between 42 and 72 hours).
Group B represents the patients who were bridged with LMWH (n = 48; timing of last administration between 65 and 158 hours).
Group C was a subdivision of group B and included patients bridged with therapeutic LMWH whose last administration of LMWH was ≥24 hours ago (n = 26). The group C was designed to analyze the effect of low LMWH levels (last administration of LMWH was ≥24 hours) on rivaroxaban estimation.
This stratification of the time interval between blood sampling and last LMWH administration is based on the international recommendations for safe hemostasis before an invasive procedure (eg, neuraxial anesthesia), when therapeutic LWMH bridging is established preoperatively.16–18 Therapeutic LMWH was defined as enoxaparin 1 mg/kg twice a day or nadroparin calcium 171 international units (IU) anti-Xa/kg once a day.
Estimation of the Anti-Xa Activity of LMWH Administered ≥24 Hours Ago
To assess the impact of residual anti-Xa activity from LMWH on the estimation of rivaroxaban concentrations, samples from group C were used (n = 26). In these samples, the residual rivaroxaban plasma concentrations were below the LOQ of the LC-MS/MS (ie, 1.0 ng/mL). Thus, residual LMWH anti-Xa activity can be measured without interference from the anti-Xa activity of any residual rivaroxaban.
Normal Pooled Plasma
Normal pooled plasma (NPP) was obtained from the platelet poor plasma (PPP) of 49 healthy individuals. The exclusion criteria for the recruitment of healthy individuals were thrombotic or hemorrhagic events, pregnancy, or use of antiplatelet, anticoagulant agent or any drug potentially affecting platelet or coagulation factors within 2 weeks of blood sampling. Blood was taken by venipuncture in the antecubital vein using a 21-gauge needle (Terumo). The sample preparation steps were the same as for the clinical samples.
Chromogenic Anti-Xa Assays
Biophen Direct Factor Xa Inhibitors
We incubated 200 µL of diluted plasma (1:50 with the Tris-NaCl-EDTA buffer at pH 7.85) with 75 µL of human FXa (Hyphen BioMed) for 120 seconds at 37°C, then 75 µL of a specific FXa substrate [CS-11(65)] (Hyphen BioMed) were added to start the reaction on a STA-R Evolution coagulometer (Diagnostica Stago). The concentrations of calibrators for the normal therapeutic range of rivaroxaban (Biophen Rivaroxaban Plasma Calibrator, Hyphen BioMed) were 50, 250, and 500 ng/mL in the initial samples after reconstitution.
The low procedure (Biophen DiXaI LOW) was the same as the normal one except that plasma was diluted 1:8 in the buffer and the calibration was performed with standards for low plasma concentrations of rivaroxaban (Biophen Rivaroxaban Calibrator Low). The standard rivaroxaban concentrations were 0, 52, and 110 ng/mL.
STA-Liquid Anti-Xa
We mixed 30 μL of diluted plasma (1:4 in STA-Owren-Koller) with 150 μL of chromogenic substrate (CBS 02.44 consisting of MAPA-glycine-arginyl-p-nitroanilide, HCl) and incubated this for 240 seconds. We then added 150 μL of bovine FXa (prewarmed at 37°C), starting the reaction on a STA-R Evolution coagulometer (Diagnostica Stago). The concentrations of calibrators (STA-Rivaroxaban Calibrator, Diagnostica Stago) were 0, 94, 236, and 472 ng/mL in the initial samples after reconstitution.
Anti-Xa measurement of LMWH
The anti-Xa activity of LMWH was measured with the Biophen Heparin Liquid Reagent Technology (LRT) assay (Hyphen BioMed). We mixed 50 μL of diluted plasma (1:2 in STA-Owren-Koller) with 125 μL of prewarmed specific chromogenic substrate of factor Xa (SXa-11) and incubated this for 240 seconds at 37°C. We then added 125 µL of bovine FXa (prewarmed at 37°C) and measured the color development. Results were expressed in OD/min. Calibration curves were realized with Biophen Heparin Calibrators (Hyphen BioMed) (5 plasma samples with LMWH concentrations ranging from 0.0 to 1.6 IU/mL). The LOQ of the anti-Xa activity is 0.05 UI/mL, according to the manufacturer.
Liquid Chromatography Coupled With Tandem Mass Spectrometry
All chemical reagents and solvents were used as obtained from commercial sources (Sigma Aldrich, Acros, Biosolve). Rivaroxaban and [13C6]-rivaroxaban-d5 were purchased from Alsachim (Illkirch Graffenstaden, France). The LC-MS/MS method was adapted from the procedures described by Douxfils et al19 and Rohde.20 All LC-MS/MS measurements were carried out on an Ultra Performance Liquid Chromatography (UPLC; Acquity H-Class system, Waters, Milford Massachussetts, USA) coupled to a tandem-quadrupole mass spectrometer (Xevo TQ-S; Waters). Chromatographic separation was achieved using a Waters Cortecs UPLC C18 column (2.1 × 100 mm, 1.6 μm) at a temperature of 40°C and a flow rate of 500 μL/min. The mobile phase was composed of ammonium formate buffer (A; 10 mM, pH 3) and acetonitrile (B). Gradient started at 20% B and linearly ramped up to 55% B in 4 minutes. The proportion of B was then set to 90% for 1 minute before returning to 20% B. All experiments were carried out with an injection volume of 2 μL, the autosampler was set to 10°C. The MS/MS detection was done with electrospray ionization (ESI) in the positive mode and multiple reaction monitoring (MRM). The capillary voltage was set to 0.5 kV, the source block temperature was 150°C and the nitrogen desolvation gas was heated to 600°C with a flow rate of 1000 L/h. Four transitions were monitored: 436.00 > 145.05 with cone voltage at 50.0 V and collision energy at 30.0 eV (rivaroxaban, quantitative ion product); 436.00 > 231.20 with cone voltage at 50.0 V and collision energy at 20.0 eV (rivaroxaban, qualitative ion product); 442.00 > 145.05 with cone voltage at 50.0 V and collision energy at 30.0 eV ([13C6]-rivaroxaban, quantitative ion product); 442.00 > 237.20 with cone voltage at 50.0 V and collision energy at 20.0 eV ([13C6]-rivaroxaban, qualitative ion product). The dwell time was set to 0.038 seconds. Preparation of the sample was realized according the following procedure: 50 μL of plasma was added to an Ostro plate (Waters); 350 μL of 1% formic acid containing internal standard (2.0 ng/mL) was mixed with each sample for protein precipitation. The plate was then placed on a vacuum manifold (Waters) and the samples were collected in a Waters 2 mL square collection plate. Weighted (1/x) linear calibration was done using calibration standards prepared by spiking blank plasma at 1, 3, 6, 10, 50, 125, 250, and 500 ng/mL. Validation standards were prepared by spiking blank plasma at 1, 3, 9, 200, and 400 ng/mL. Validation with the QCs on 3 different days showed repeatability relative standard deviation (RSD) below 5.0%, an intermediate precision RSD below 9.8%, and a relative bias below 11.1%. The limit of detection (LOD) and the LOQ were 0.5 and 1.0 ng/mL, respectively. The method was validated according to FDA guidelines.21
Statistical Analyses
GraphPad Prism version 6.0c for MacOSx (GraphPad Software, San Diego, California; www.graphpad.com) was used for the statistical analyses. Due to the fact that little is known about the combined impact of Xa-Inhibitors and LMWHs on Xa-Assays, this study is of exploratory nature and not formally powered. Results for the rivaroxaban anti-Xa assays (Biophen DiXaI, Biophen DiXaI LOW, and STA LAX) and those obtained using LC-MS/MS were compared by Spearman correlation, linear regression, and Bland-Altman analyses. The coefficients of correlation of group A were compared to those of group B and C. A P value lower than .05 was considered as statistically significant. Bland-Altman was calculated as the difference (A − B)/average, where A was the result of the corresponding coagulation test and B, the result of the LC-MS/MS. Limits of agreement of the Bland-Altman analyses were calculated as the mean difference – or + 1.96 × standard deviation for the 5th and the 95th limit of agreement, respectively. The lower LOD was calculated as ([3 × standard deviation of Y0]/slope) and the lower LOQ was calculated as ([10 × standard deviation of Y0]/slope). The standard deviation of Y0 was calculated by running the tests 10 times with NPP. For STA LAX, the calibration curve is better defined by a second order polynomial relation. Therefore, the 3 first points of the calibration curve (value of the intrapolation: 8.6, 85.2, and 233.9 ng/mL) that can be fitted by a linear regression (R2 ≥ 0.98) were taken to calculate the LOD and LOQ.
Results
Plasma concentrations of rivaroxaban in the blood samples ranged from 0.0 to 108.2 ng/mL according to LC-MS/MS. Table 1 shows the statistical characteristics of the rivaroxaban plasma concentrations of the different groups. All the patients had a creatinine clearance estimated with the Cockcroft-Gault equation above 50 mL/min.
Table 1.
Statistical Characteristics, Spearman Correlation, Linear Regression, and Bland-Altman Analyses of the Rivaroxaban Plasma Concentrations in the Different Groups.a
| Biophen DiXaI | Biophen DiXaI LOW | STA-Liquid Anti-Xa | |
|---|---|---|---|
| Group A: Patients not bridged (n = 31) | |||
| Range of rivaroxaban plasma concentration, ng/mL | 2.1 to 108.2 | ||
| Median, ng/mL | 32.6 | ||
| Interquartile range, ng/mL | 13.4 to 71.30 | ||
| Bland-Altman | |||
| Mean bias, ng/mL | 8.8 | 4.7 | −1.5 |
| SD, ng/mL | 16.8 | 8.4 | 10.7 |
| 95% Limits of agreement, ng/mL | −24.1 to 41.8 | −11.8 to 21.2 | −22.4 to 19.5 |
| R square | 0.82 | 0.94 | 0.95 |
| Spearman r | 0.91 | 0.97 | 0.96 |
| 95% CI | 0.81 to 0.96 | 0.94 to 0.99 | 0.92 to 0.98 |
| Group B: Patients bridged with LMWH (n = 48) | |||
| Range of rivaroxaban plasma concentration, ng/mL | <1.0 to 10.1 | ||
| Median, ng/mL | 1.0 | ||
| Interquartile range, ng/mL | 0.0 to 1.0 | ||
| Bland-Altman | |||
| Mean bias, ng/mL | 12.4 | 13.5 | 20.9 |
| SD, ng/mL | 9.7 | 14.5 | 12.6 |
| 95% Limits of agreement, ng/mL | −6.6 to 31.5 | −14.8 to 41.8 | −3.7 to 45.5 |
| R square | 0.05 | 0.01 | 0.00 |
| Spearman r | 0.26 | 0.18 | 0.19 |
| 95% CI | −0.03 to 0.52 | −0.11 to 0.45 | −0.11 to 0.45 |
| Group C: Patients bridged with therapeutic LMWH with a last administration of LMWH ≥24 hours (subgroup of group B; n = 26) | |||
| Range of rivaroxaban plasma concentration, ng/mL | <1.0 to 10.1 | ||
| Median, ng/mL | 0.5 | ||
| Interquartile range, ng/mL | 0.0 to 1.0 | ||
| Bland-Altman | |||
| Mean bias, ng/mL | 11.4 | 10.2 | 17.6 |
| SD, ng/mL | 10.4 | 7.0 | 6.6 |
| 95% Limits of agreement, ng/mL | −8.9 to 31.7 | −3.6 to 24.0 | 4.7 to 30.6 |
| R square | 0.01 | 0.10 | 0.10 |
| Spearman r | 0.04 | 0.25 | 0.29 |
| 95% CI | −0.37 to 0.43 | −0.16 to 0.59 | −0.12 to 0.62 |
Abbreviations: CI, confidence interval; LC-MS/MS, liquid chromatography coupled with mass spectrometry; LMWH, low-molecular-weight heparin; SD, standard deviation.
aBland-Altman was Calculated as the difference (A − B)/average, where A was the result of the corresponding coagulation test and B, the result of the LC-MS/MS. Limits of agreement of the Bland-Altman analyses were calculated as the mean difference − or + 1.96 × standard deviation for the 5th and the 95th limit of agreement, respectively.
Spearman and Linear Correlations
For group A, the Spearman r was 0.91, 0.97, and 0.96 for Biophen DiXaI, Biophen DiXaI LOW, and STA LAX, respectively. The linear regression gave an R square of 0.82, 0.94, and 0.95 for Biophen DiXaI, Biophen DiXaI LOW, and STA LAX, respectively (Table 1).
The difference in correlation with the LC-MS/MS measurement of Biophen DiXaI LOW and Biophen DiXaI was clinically significant (95% confidence interval [CI] of Spearman coefficient: 0.81-0.96 vs 0.94-0.99, respectively; Table 1). For STA LAX and Biophen DiXaI, this difference tended toward significance (95% CI of Spearman coefficient: 0.92-0.98 vs 0.81-0.96, respectively).
For groups B and C, the Spearman coefficient and the linear regression showed only a weak correlation between chromogenic assays and LC-MS/MS measurements.
For Biophen DiXaI, Biophen DiXaI LOW, and STA LAX, the coefficients of correlation obtained with group A are significantly higher than the coefficients of correlation obtained with group B (eg, for Biophen DiXaI the 95% CI was 0.81-0.96 for group A vs −0.03 to 0.52 for group B). The same results were obtained when comparing the coefficients of correlation of groups A and C (Table 1 ).
Bland-Altman Analyses
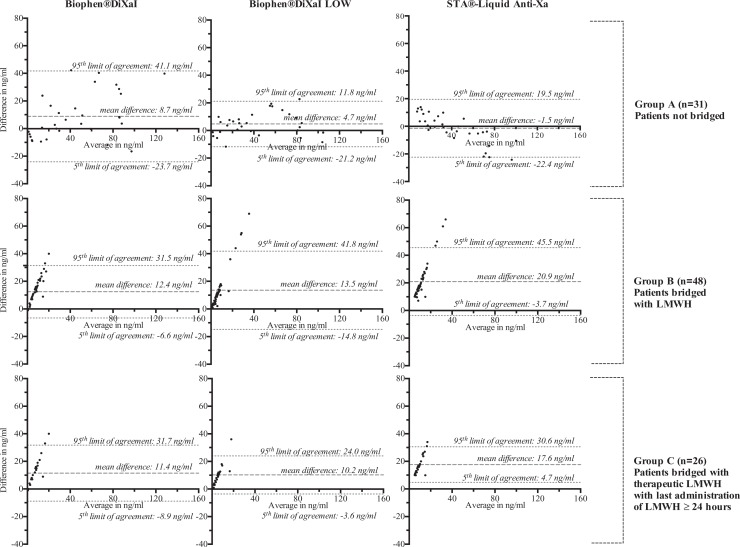
Figure 2 and Table 1 present the Bland-Altman analyses.
Figure 2.
Bland-Altman analyses of the estimation of rivaroxaban plasma concentrations of Biophen DiXaI, Biophen DiXaI LOW, and STA LAX with LC-MS/MS measurements. LC-MS/MS indicates liquid chromatography coupled with mass spectrometry.
In the absence of bridging (group A), the mean difference between specific assays and LC-MS/MS was less than 10 ng/mL.
In bridged patients (group B), the mean difference between Biophen DiXaI LOW, STA LAX, and LC-MS/MS increased (from 4.7 to 13.5ng/mL for Biophen DiXaI LOW and from −1.5 to 20.9 ng/mL for STA LAX) whereas it remains similar from Biophen DiXaI (8.8 ng/mL for group A and 12.4 ng/mL for group B).
Limit of Detection and Quantitation
The LOD and LOQ were 16 and 53 ng/mL, respectively, for Biophen DiXaI, 4 and 12 ng/mL for Biophen DiXaI LOW, and 11 and 36 ng/mL for STA LAX.
Interference of the Anti-Xa Activity of LMWH Administered ≥24 Hours Ago With Biophen DiXaI, Biophen DiXaI LOW, and STA LAX
Among patients in Group C, the residual LMWH activity could not be estimated in 4 samples because of insufficient plasma. For the remaining 22 samples, the LMWH activity was below the LOQ of the anti-Xa assay (ie, Biophen Heparin LRT) in 4 samples, and between 0.05 and 0.25 UI/mL in 18 samples. Figure 3 shows the impact of residual LMWH anti-Xa activity on the estimation of rivaroxaban in these 18 samples. The correlation was nonsignificant for Biophen DiXaI (Spearman r: 0.09; 95% CI: −0.41 to 0.55) but was significant for Biophen DiXaI LOW (Spearman r: 0.76; 95% CI: 0.44-0.91) and STA LAX (Spearman r: 0.73; 95% CI: 0.39-0.90).
Figure 3.
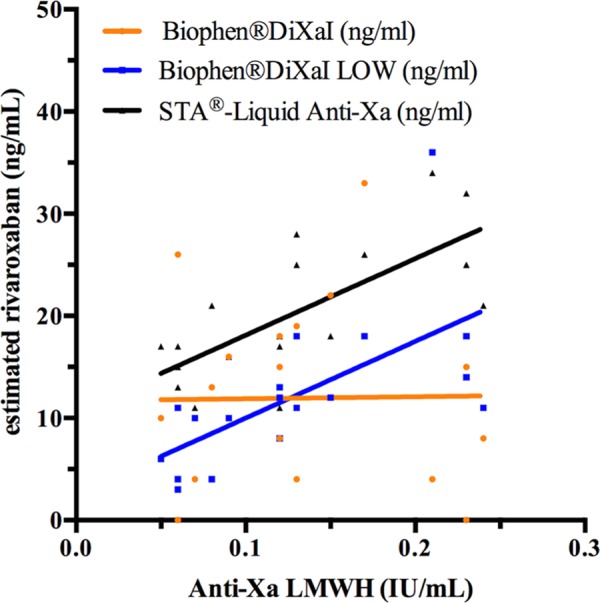
Influence of LMWHs on the estimation of rivaroxaban plasma concentration with the 3 chromogenic anti-Xa assays. Biophen DiXaI LOW and STA LAX are influenced by the presence of LMWH. Up to 0.12 IU/mL, Biophen DiXaI LOW performs better than Biophen DiXaI but above this threshold, the overestimation of the Biophen DiXaI LOW is higher than that of Biophen DiXaI which is not influenced by LMWH at these concentrations. Samples with LMWH concentrations below the LOQ of the heparin assay were not included. LMWH indicates low-molecular-weight heparin; LOQ, limit of quantitation.
Discussion
These results demonstrate the value of the low version of the Biophen DiXaI assay (Biophen DiXaI LOW) for the estimation of low rivaroxaban concentrations. Due to its higher sensitivity compared to the standard procedure, the LOD and LOQ of Biophen DiXaI LOW were roughly 4 times lower than those of the standard procedure, allowing accurate measurements of plasma concentrations <50 ng/mL, which is a limitation of the standard Biophen DiXaI.19 We found that STA LAX was also reliable for the estimation of rivaroxaban plasma concentrations at CTROUGH. However, and importantly, agreement with LC-MS/MS depended on the presence of LMWH. Indeed, the performance of Biophen DiXaI LOW and STA LAX decreased when residual LMWH activity was present, demonstrating that these tests are not specific to the direct factor Xa inhibitors (Table 1; Figures 2 and 3). To our knowledge, this study is the first that enables the assessment of the performance of chromogenic anti-Xa assays with high and low procedures in blood samples containing both residual rivaroxaban and LMWH.
However, some limitations concerning this study need to be discussed. First, the range of rivaroxaban plasma concentration in patients bridged with LMWH (groups B and C) was quite limited (from 0 to 10 ng/mL) and was also below the LOQ of the more sensitive chromogenic anti-Xa assay, the Biophen DiXaI LOW. Samples from patients bridged with LMWH in the 10 to 50 ng/mL rivaroxaban concentration range would have been needed to assess the performance of the specific chromogenic tests in the concentration range for which they are designed. However, due to the design of our study, which followed the initial perioperative management protocol proposed by the French working group on perioperative hemostasis (Groupe d’Intérêt en Hémostase Périopératoire22), patients stopped their rivaroxaban well before the invasive procedure with high bleeding risks, resulting in very low residual rivaroxaban concentrations at the time of the intervention compared to samples recruited at CTROUGH in patients outside the perioperative context (Table 1). Furthermore, the number of samples recruited in group A could only suggest a tendency to a statistical significant difference between Biophen DiXaI and STA LAX. Therefore, these findings need to be confirmed in a larger cohort, preferably within a multicenter study also using coagulometers from different manufacturers as it is recognized that this may affect the sensitivity.23 According to the previous findings of Harenberg et al, a similar study should also be performed with other chromogenic assays available on the market to analyze the interassay variability and reduce it using a mathematical modeling.9 Finally, our conclusions cannot be extrapolated to blood samples of patients receiving prophylactic bridging, where 12-hour discontinuation of LMWH is considered sufficient to undergo an invasive procedure with appropriate hemostatic conditions.
Report of Previous Findings
Mani et al have already published the interest of using low calibrators for the measurement of low rivaroxaban concentrations with 3 other chromogenic anti-FXa assays (COAMATIC Heparin from Chromogenix Instrumentation Laboratory (Bedford, USA), and Technochrom anti-Xa with and without exogenous antithrombin (AT) from Diagnostica Stago, Asnière, France). The LOQ was decreased from 25 to 10 ng/mL, when the low calibrators were used instead of the high calibrators. Therefore, they recommended to rerun plasma samples with the low calibrators when rivaroxaban was estimated <25 ng/mL with the high calibrators. They demonstrated also that anti-Xa assays including exogenous AT were unsuitable for the measurement of rivaroxaban levels as AT falsely elevates rivaroxaban plasma concentrations. This was explained by the ability of AT in excess to complex residual factor Xa. The ex vivo samples came from patients who had received rivaroxaban 10 mg once daily for postoperative prevention of deep venous thromboembolism (DVT) after elective hip or knee replacement, so none had residual heparin.12
Königsbrügge et al have also used high and low commercial standards of rivaroxaban to calibrate the Biophen DiXaI and Biophen Heparin LRT, 2 chromogenic anti-Xa assays which are and are not, respectively, specific to rivaroxaban. For the low procedure of the Biophen DiXaI, they used a different dilution of the plasma samples in the buffer (1:20 instead of 1:8 in our methodology). They demonstrated that the use of the low calibrators improved the LOD, the LOQ, the mean difference, and the standard deviation (in ng/mL) of the 2 anti-Xa assays compared to high calibrators, for rivaroxaban concentrations below 102 ng/mL.13 For the Biophen DiXaI, the LOD and LOQ decreased from 35 and 107 ng/mL to 9 and 28 ng/mL, respectively. For the Biophen LRT assay, the LOD and LOQ decreased from 49 and 149 ng/mL to 2 and 6 ng/mL, respectively. All their samples came from patients treated with rivaroxaban without LMWH bridging. Therefore, we cannot evaluate if the difference in their methodology of the Biophen DiXaI LOW (dilution 1:20) would have less interference with LMWH than our methodology (dilution 1:8). We can only observe an expected decrease in the LOD and LOQ with the dilution 1:8 compared to the dilution 1:20 (ie, 4 and 12 ng/mL compared to 9 and 28 ng/mL, respectively). The dilution 1:8 might be more appropriate in the perioperative setting, as some experts group recommend reaching a rivaroxaban plasma concentration ≤30 ng/mL before an emergency procedure with high bleeding risk.22
Proposal for Sample Management
Low concentrations of rivaroxaban can only be assessed properly with chromogenic anti-Xa assays.1,8,19,24 However, most of these anti-Xa assays have a LOQ around 30 to 50 ng/mL, making them unreliable for assessing concentrations below the LOQ. Measurements of residual rivaroxaban concentrations in the perioperative setting are currently recommended before emergencies that cannot be delayed (eg, thrombolysis in the setting of an acute cerebral thrombosis) or emergencies with high bleeding risks. If a patient is suspected to have an insufficient preoperative interruption of rivaroxaban (eg, a clinical context with suspected supratherapeutic plasma concentration), the measurement of rivaroxaban is arguable, especially if the invasive procedure is at high bleeding risk.25
Without LMWH bridging, the Biophen DiXaI LOW showed a better correlation than Biophen DiXaI with the LC-MS/MS measurement (Table 1). This difference of correlation with LC-MS/MS between STA LAX and Biophen DiXaI tended toward significance. However, one of the advantages of the STALAX is its wide application range as the operator does not have to choose between a low and a normal procedure, simplifying the management of samples for the estimation of residual rivaroxaban concentrations. Therefore, we suggest that Biophen DiXaI LOW and STA LAX may be used for the estimation of low rivaroxaban, for example, when rivaroxaban has been stopped for more than 24 hours.
The Problem of Bridging Therapy
Recent publications demonstrated the increased risk of major bleedings without a decrease of thrombotic events in patients group bridged with heparins during the perioperative interruption of oral anticoagulants.3,26–28 The most recent updated guidelines recommend to bridge only patients who are at high risk of thromboembolic events, especially as those were underrepresented in clinical trials analyzing the benefit or harm of bridging therapies (eg, the BRIDGE clinical trial).29–31
With the exception of the Biophen DiXaI and its specific buffer, LMWH anti-Xa activity leads to an overestimation of the rivaroxaban concentration.15 We expected that the low procedure of the Biophen DiXaI would also have this specificity as it is directly derived from the Biophen DiXaI assay. Unfortunately, this study reveals that in the absence of any rivaroxaban effect, increasing concentrations of residual LMWH overestimated rivaroxaban plasma concentrations measured by Biophen DiXaI LOW (Figures 2 and 3). From our data, above 0.12 IU/mL of LMWH, there is no benefit from the low procedure of Biophen DiXaI (Figure 3). These findings may be explained by the fact that, in order to improve the sensitivity, the LOD and the LOQ, the sample is less diluted in the high ionic strength buffer that is less apt to inhibit the catalytic action of LMWHs. The overestimation of rivaroxaban by STA LAX was predictable as it is not specific for rivaroxaban, however, it had no advantage compared to Biophen DiXaI even in samples with 0.05 IU/mL of LMWH. Therefore, both Biophen DiXaI LOW and STA LAX are not suitable for the measurement or the exclusion of residual rivaroxaban concentrations in bridged patients. The standard Biophen DiXaI was, as expected, not influenced by the presence of LMWH, even at therapeutic LMWH concentrations (data not shown), but its lack of precision at low levels of rivaroxaban precludes its use for the accurate estimation of residual rivaroxaban in this concentration range.
Some manufacturers have proposed the use of a global anti-Xa plasma activity calibrated with heparin. However, these assays are affected by a high interassay variability and the absence of correlation between anti-Xa concentration and heparin anti-Xa IU/mL, except for the low anti-Xa concentrations.32,33 In case of a patient initially on rivaroxaban and bridged with LMWH, the result will reflect the activity of residual rivaroxaban and LMWH. These assays are thus suitable to exclude presence of anti-Xa activity but should not be used to quantify direct anti-Xa activity or to predict the bleeding risk of the patients receiving LMWH after rivaroxaban suspension.
Perspectives
This study represents real-life conditions, without routine access to LC-MS/MS. Thus, a chromogenic anti-Xa assay that is not sensitive to heparin and is more accurate than Biophen DiXaI for the low concentrations of rivaroxaban should be developed. Our findings should help to improve the performance of these dedicated tests in the presence of heparins. These results suggest that both STA LAX and Biophen DiXaI LOW could be tested with a high ionic strength buffer which, in the case of Biophen DiXaI LOW, should be 6 times more concentrated than the current buffer to recover the specificity of Biophen DiXaI. Second, as suggested by Asmis et al, the use of a heparin-neutralizing agent, such as polybrene or heparinase, could be tested to evaluate whether it improves the specificity to rivaroxaban.10 Meanwhile, clinicians need to inform their laboratory about the timing and dose of the last LMWH and rivaroxaban administration, so that the most reliable test can be used depending on the patient’s specific situation and the clinical question.
Conclusion
This study confirms previous data and recommendations about the use of low methodologies to estimate low rivaroxaban concentrations. However, the main point is that it highlights important limitations of the chromogenic tests currently available for the management of patients bridged with LMWHs. Although the low procedures may be preferred for the estimation of rivaroxaban concentrations below 50 ng/mL, these methods are sensitive to LMWHs and may be influenced even 24 hours after of the last LMWH administration. Currently, clinicians and laboratories are ill-equipped to assess bridged patients when a reliable estimate of the residual rivaroxaban concentration is required. Even if bridging therapies with heparins will decrease in the near future, there will still be patients at high thromboembolic risks who will benefit from it. Therefore, the development of a chromogenic anti-Xa assay accurate at low rivaroxaban concentrations and insensitive to heparins is required to optimize their perioperative management.
Acknowledgments
The authors thank Justine Baudar, Maïté Guldenpfennig, Françoise Biot, Celia Devroye, Christelle Vancrayenest, and Charlotte Tordoir for their technical support and Professor Edith Collard for her logistic support. The authors also thank Dr Elizabeth Wager for language editing and Benoît Bihin for reviewing the statistic section of our manuscript.
Authors’ Note: S. Lessire, J. Douxfils, and F. Mullier concepted and designed the study. S. Lessire, J. Douxfils, L. Pochet, A-S. Dincq, A-S. Larock, M. Gourdin, J-M. Dogné, B. Chatelain, and F. Mullier participated in the analysis and the interpretation of the data. S. Lessire, J. Douxfils and F. Mullier have written the manuscript. L. Pochet wrote the methodology of the liquid chromatography coupled with tandem mass spectrometry. A-S. Dincq, A-S. Larock, M. Gourdin, J-M. Dogné, and B. Chatelain revised the intellectual content of the manuscript. All authors approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sarah Lessire received a research grant from the Fondation Mont-Godinne to complete her thesis.
References
- 1. Douxfils J, Mani H, Minet V, et al. Non-VKA oral anticoagulants: accurate measurement of plasma drug concentrations. BioMed Res Int. 2015;2015:345138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129(18):1850–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beyer-Westendorf J, Gelbricht V, Forster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35(28):1888–1896. [DOI] [PubMed] [Google Scholar]
- 4. Godier A, Martin AC, Leblanc I, et al. Peri-procedural management of dabigatran and rivaroxaban: Duration of anticoagulant discontinuation and drug concentrations. Thromb Res. 2015;136(4):763–768. [DOI] [PubMed] [Google Scholar]
- 5. Kepplinger J, Prakapenia A, Barlinn K, et al. Standardized use of novel oral anticoagulants plasma level thresholds in a new thrombolysis decision making protocol. J Thromb Thrombolysis. 2016; 41(2):293–300. [DOI] [PubMed] [Google Scholar]
- 6. Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64(11):1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Samama MM, Contant G, Spiro TE, et al. ; Rivaroxaban Anti-Factor Xa Chromogenic Assay Field Trial Laboratories. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107(2):379–387. [DOI] [PubMed] [Google Scholar]
- 8. Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogne JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130(6):956–966. [DOI] [PubMed] [Google Scholar]
- 9. Harenberg J, Kramer R, Giese C, Marx S, Weiss C, Wehling M. Determination of rivaroxaban by different factor Xa specific chromogenic substrate assays: reduction of interassay variability. J Thromb Thrombolysis. 2011;32(3):267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Asmis LM, Alberio L, Angelillo-Scherrer A, et al. Rivaroxaban: quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res. 2012;129(4):492–498. [DOI] [PubMed] [Google Scholar]
- 11. Douxfils J, Lessire S, Dincq AS, et al. Estimation of dabigatran plasma concentrations in the perioperative setting. An ex vivo study using dedicated coagulation assays. Thromb Haemost. 2015;113(4):862–869. [DOI] [PubMed] [Google Scholar]
- 12. Mani H, Rohde G, Stratmann G, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost. 2012;108(1):191–198. [DOI] [PubMed] [Google Scholar]
- 13. Konigsbrugge O, Quehenberger P, Belik S, et al. Anti-coagulation assessment with prothrombin time and anti-Xa assays in real-world patients on treatment with rivaroxaban. Ann Hematol. 2015;94(9):1463–1471. [DOI] [PubMed] [Google Scholar]
- 14. Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti-Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol. 2016;38(5):505–513. [DOI] [PubMed] [Google Scholar]
- 15. Samama MM, Amiral J, Guinet C, Perzborn E, Depasse F. An optimised, rapid chromogenic assay, specific for measuring direct factor Xa inhibitors (rivaroxaban) in plasma. Thromb Haemost. 2010;104(5):1078–1079. [DOI] [PubMed] [Google Scholar]
- 16. Narouze S, Benzon HT, Provenzano DA, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications: guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2015;40(3):182–212. [DOI] [PubMed] [Google Scholar]
- 17. Douketis JD, Woods K, Foster GA, Crowther MA. Bridging anticoagulation with low-molecular-weight heparin after interruption of warfarin therapy is associated with a residual anticoagulant effect prior to surgery. Thromb Haemost. 2005;94(3):528–531. [DOI] [PubMed] [Google Scholar]
- 18. O’Donnell MJ, Kearon C, Johnson J, et al. Brief communication: Preoperative anticoagulant activity after bridging low-molecular-weight heparin for temporary interruption of warfarin. Ann Intern Med. 2007;146(3):184–187. [DOI] [PubMed] [Google Scholar]
- 19. Douxfils J, Tamigniau A, Chatelain B, et al. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110(4):723–731. [DOI] [PubMed] [Google Scholar]
- 20. Rohde G. Determination of rivaroxaban—a novel, oral, direct Factor Xa inhibitor—in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872(1-2):43–50. [DOI] [PubMed] [Google Scholar]
- 21. Administration FaD. FDA Guidelines for Industry for Bioanalytical Method Validation. 2001.
- 22. Pernod G, Albaladejo P, Godier A, et al. ; Working Group on Perioperative Haemostasis. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP)—March 2013. Arch Cardiovasc Dis. 2013;106(6-7):382–393. [DOI] [PubMed] [Google Scholar]
- 23. Van Blerk M, Bailleul E, Chatelain B, et al. Influence of dabigatran and rivaroxaban on routine coagulation assays. A nationwide Belgian survey. Thromb Haemost. 2015;113(1):154–164. [DOI] [PubMed] [Google Scholar]
- 24. Cuker A. Laboratory measurement of the non-vitamin K antagonist oral anticoagulants: selecting the optimal assay based on drug, assay availability, and clinical indication. J Thromb Thrombolysis. 2015. [DOI] [PubMed] [Google Scholar]
- 25. Godier A, Gouin-Thibault I, Rosencher N, Albaladejo P; Groupe d’Interet en Hemostase P. Management of direct oral anticoagulants for invasive procedures [In French]. J Mal Vasc. 2015;40(3):173–181. [DOI] [PubMed] [Google Scholar]
- 26. Douketis JD, Healey JS, Brueckmann M, et al. Perioperative bridging anticoagulation during dabigatran or warfarin interruption among patients who had an elective surgery or procedure. Substudy of the RE-LY trial. Thromb Haemost. 2015;113(3):625–632. [DOI] [PubMed] [Google Scholar]
- 27. Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC. Periprocedural heparin bridging in patients receiving vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation. 2012;126(13):1630–1639. [DOI] [PubMed] [Google Scholar]
- 28. Steinberg BA, Peterson ED, Kim S, et al. ; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients. Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circulation. 2015;131(5):488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Douketis JD, Spyropoulos AC, Kaatz S, et al. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation; BRIDGE Investigators. New Engl J Med. 2015;373(9):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douketis JD, Spyropoulos AC, Spencer FA, et al. ; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2): e326S–e350S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mar PL, Familtsev D, Ezekowitz MD, Lakkireddy D, Gopinathannair R. Periprocedural management of anticoagulation in patients taking novel oral anticoagulants: review of the literature and recommendations for specific populations and procedures. Int J Cardiol. 2016;202:578–585. [DOI] [PubMed] [Google Scholar]
- 32. Adcock DM, Gosselin R. Direct Oral Anticoagulants (DOACs) in the Laboratory: 2015 Review. Thromb Res. 2015;136(1):7–12. [DOI] [PubMed] [Google Scholar]
- 33. Gosselin RC, Francart SJ, Hawes EM, Moll S, Dager WE, Adcock DM. Heparin-Calibrated Chromogenic Anti-Xa Activity Measurements in Patients Receiving Rivaroxaban: Can This Test Be Used to Quantify Drug Level? Ann Pharmacother. 2015;49(7):777–783. [DOI] [PubMed] [Google Scholar]