Abstract
In patients with venous thromboembolism (VTE), male sex has been associated with an increased risk of occult cancer. The influence of sex on clinical characteristics, treatment, cancer sites, and outcome has not been thoroughly investigated yet. We used the Registro Informatizado Enfermedad TromboEmbólica registry to compare the clinical characteristics, treatment strategies, cancer sites, and clinical outcomes in patients with VTE having occult cancer, according to sex. As of June 2014, 5864 patients were recruited, of whom 444 (7.6%; 95% confidence interval: 6.8-8.2) had occult cancer. Of these, 246 (55%) were men. Median time elapsed from VTE to occult cancer was 4 months (interquartile range: 2-8.4), with no sex differences. Women were older, weighed less, and were less likely to have chronic lung disease than men. The most common cancer sites were the lung (n = 63), prostate (n = 42), and colorectal (n = 29) in men and colorectal (n = 38), breast (n = 23), uterine (n = 18), hematologic (n = 17), or pancreas (n = 15) in women. Men were more likely to have lung cancer than women (2.18% vs 0.30%; P < .01) and less likely to have pancreatic cancer (0.17% vs 0.5%; P = .03). Interestingly, breast cancer was more likely found in women aged ≥50 years than in those aged <50 years (0.97% vs 0.14%; P = .03). This study highlights the existence of sex differences in patients with VTE having occult cancer. One in every 2 men had lung, prostate, or colorectal cancer. In women, there is a heterogeneity of cancer sites, increasing risk of breast cancer in those aged >50 years.
Keywords: neoplasm, venous thromboembolism, pulmonary embolism, deep vein thrombosis, sex
Introduction
In a recent study by our group, we built a prognostic model to identify which patients with venous thromboembolism (VTE) were at an increased risk of occult cancer.1 On multivariable analysis, male sex, age >70 years, chronic lung disease, anemia, and raised platelet count at baseline were significantly associated with an increased risk of occult cancer, while prior VTE and recent surgery were associated with a lower risk. Then, we built a prognostic score assigning points to each variable according to β coefficient. Among patients scoring ≤2 points, the prevalence of occult cancer was 5.8% (241 of 4150 patients). Among those scoring ≥3 points, the prevalence was 12% (203 of 1713 patients). However, the influence of sex on clinical characteristics, treatment strategies, cancer site, and clinical outcomes was not reported.
The Registro Informatizado de Enfermedad TromboEmbólica (RIETE) registry is an ongoing, multicenter, international observational registry of consecutive patients with objectively confirmed acute VTE. Data from this registry have been used to evaluate outcomes after acute VTE, such as the frequency of recurrent VTE, bleeding and mortality, and risk factors for such outcomes.2–5 The aim of the current study was to compare the clinical characteristics, treatment strategies, cancer sites, and clinical outcomes in patients with VTE having occult cancer, according to sex.
Material and Methods
Inclusion Criteria
Consecutive patients with symptomatic, acute deep vein thrombosis (DVT) or pulmonary embolism (PE), confirmed by objective tests, were enrolled in RIETE. Patients were excluded if they were currently participating in a therapeutic clinical trial with a blinded therapy. All patients (or their relatives) provided written or oral consent for participation in the registry, in accordance with local ethics committee.
Data were recorded on to a computer-based case report form at each participating hospital and submitted to a centralized coordinating center through a secure website. The coordinating center assigned patients with a unique identification number to maintain patient confidentiality and was responsible for all data management. Data quality was regularly monitored electronically, including checks to detect inconsistencies or errors, which were resolved by contacting the local coordinators. Data quality was also monitored by periodic visits to participating hospitals by contract research organizations that compared medical records with the submitted data.
Study Design
We performed a post hoc analysis from a case–control study within a cohort of patients with VTE included in the RIETE registry.1 Patients with no cancer at the moment of the VTE event were followed up for at least 2 years or until cancer was diagnosed. We defined occult cancer as a cancer diagnosed beyond the first 30 days after VTE. For diagnosing cancer, tissue biopsy was always required. We analyzed and compared the clinical characteristics, treatment strategies, cancer sites, and clinical outcomes in patients with VTE having occult cancer, according to sex.
Baseline Variables
Patients enrolled in the RIETE registry had data collected from around the time of VTE diagnosis that included but were not limited to age; sex; weight; presence of coexisting conditions such as chronic heart or lung disease; recent (<30 days before VTE) major bleeding; presence of risk factors for VTE, including recent immobility (defined as nonsurgical patients assigned to bed rest with bathroom privileges for >4 days in the 2 months before VTE diagnosis); surgery (defined as those who had undergone major surgery in the 2 months before VTE); extent of the venous thrombosis (distal thrombosis was thrombosis confined to the infrapopliteal veins); clinical signs and symptoms on admission; and laboratory results at baseline that included hemoglobin levels, platelet count, and serum creatinine at baseline. Anemia was defined as hemoglobin levels <13 g/dL for men and <12 g/dL for women.
Treatment and Follow-Up
Patients were managed according to the current clinical practice of each participating hospital (ie, there was no standardization of treatment). The type, dose, and duration of anticoagulant therapy were recorded. During each visit, any signs or symptoms suggesting cancer, symptomatic VTE, or major bleeding were noted. In patients with suspected malignancy, the attending doctors decided what diagnostic tests to be performed.
Statistical Analysis
Quantitative variables are expressed as mean (standard deviation), and the qualitative variables are expressed as percentages. For confidence intervals of 95% (95% CI), we used the Clopper-Pearson exact method. We used the Student t test (or Mann-Whitney U test when appropriate) and the χ2 test (or Fisher exact test when appropriate) to compare continuous or categorical variables. We analyzed the time to occult cancer diagnosis using the Kaplan-Meier method (Mantel-Cox log-rank test). Receiver–operator characteristic curve analyses (C-statistic) were generated. We also estimated the clinical usefulness and net benefit of the new predictive models using decision curve analysis, as described by Vickers et al.6 For the statistical analysis, we used the IBM SPSS Statistics program (version 19; SPSS Inc, Chicago, Illinois), and a 2-sided P < .05 was considered to be statistically significant.
Results
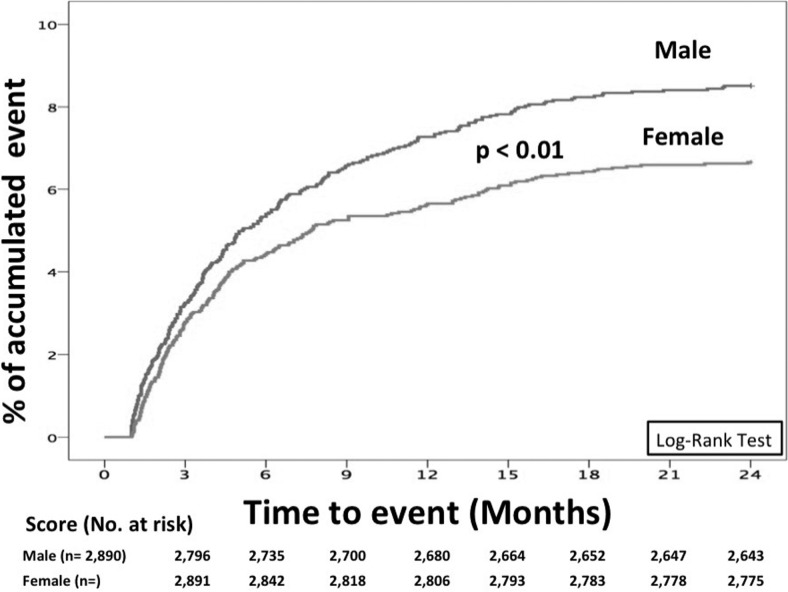
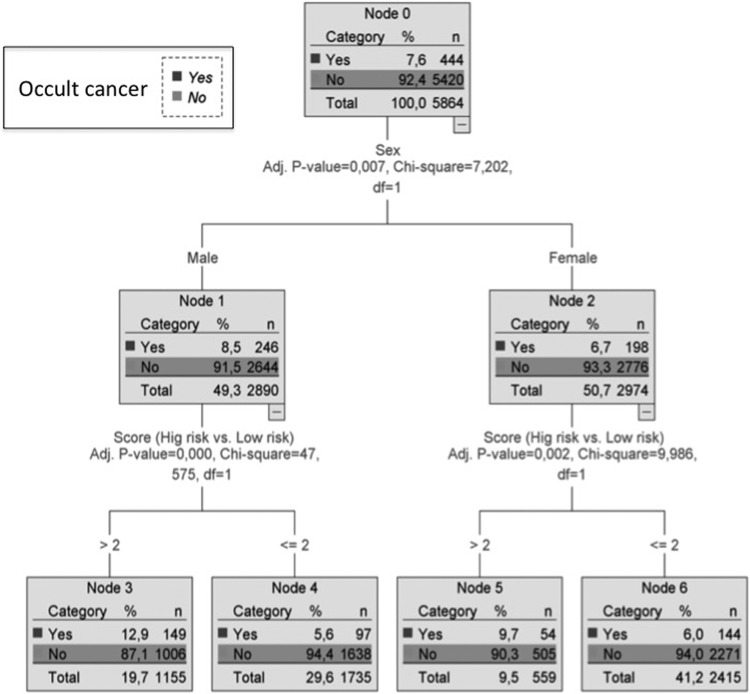
As of June 2014, 5864 patients were followed up for at least 2 years or until they were diagnosed with occult cancer. Of these, 444 (7.6%; 95% CI: 6.90-8.28) were diagnosed with cancer beyond the first 30 days of VTE (occult cancer): 246 (55%) were men and 198 women. Median time elapsed from VTE to occult cancer was 4 months (interquartile range [IQR]: 2-8.4 months), with no differences between men and women (4.2, IQR: 2-8.4 months vs 3.9, IQR: 2-7.7 months, respectively; P = .76). However, some differences were observed in time to cancer diagnosis using log-rank test (P < .001; Figure 1). At the moment of occult cancer diagnosis, 41% were metastatic, with nonsignificant differences according to sex (males 40% vs females 43%; P = .6). Clinical characteristics, attending sex, are in Table 1. In Figure 2, we show a decision tree according to sex and score. There were no sex differences in risk factors for VTE (Table 2), and 2 in every 3 patients were on therapy with vitamin K antagonists when the cancer was diagnosed, with no sex differences either.
Figure 1.
Cumulative rates of occult cancer according to sex. Time-to-event data.
Table 1.
Clinical Characteristics, Laboratory Findings, and Outcomes of Patients With Occult Cancer, Attending Sex.
| Variable | Men | Women | P |
|---|---|---|---|
| Patients, n | 246 | 198 | |
| Clinical characteristics | |||
| Age, years, mean (SD) | 68.9 (12.0) | 73.0 (13.7) | <.001 |
| Weight, kg, mean (SD) | 77.1 (13.3) | 70.0 (13.1) | <.001 |
| Height, cm, mean (SD) | 169 (7) | 157 (7) | <.001 |
| Comorbid diseases | |||
| Chronic heart failure, n (%) | 13 (5.3) | 17 (8.6) | .168 |
| Chronic lung disease, n (%) | 55 (22.4) | 19 (9.6) | <.001 |
| Recent major bleeding, n (%) | 4 (1.6) | 5 (2.5) | .504 |
| Signs and symptoms | |||
| Cough, n (%) | 31 (12.9) | 20 (10.3) | .411 |
| Hemoptysis, n (%) | 15 (6.2) | 2 (1) | .005 |
| Dyspnea, n (%) | 90 (37.3) | 101 (51.5) | .003 |
| Syncope, n (%) | 15 (6.2) | 13 (6.7) | .84 |
| Chest pain, n (%) | 59 (24.5) | 51 (26.3) | .666 |
| Lower limb pain, n (%) | 147 (60) | 98 (49.7) | .031 |
| Swelling, n (%) | 155 (63.5) | 106 (53.8) | .039 |
| Side DVT | .573 | ||
| Right, n (%) | 91 (47.9) | 55 (42) | |
| Left, n (%) | 88 (46.3) | 67 (51.1) | |
| Bilateral, n (%) | 11 (5.8) | 9 (6.9) | |
| Proximal DVT, n (%) | 149 (82.3) | 108 (87.8) | .194 |
| Laboratory findings, n (%) | |||
| Hemoglobin levels (g/dL) | 13.5 (2.0) | 12.3 (1.9) | <.001 |
| Leukocyte count (×1000/mm3), mean (SD) | 9.6 (3.1) | 9.5 (3.7) | .26 |
| Platelet count (×1000/mm3), mean (SD) | 242 (103) | 261 (94) | .004 |
| Creatinine levels, mg/dL, mean (SD) | 1.32 (0.73) | 1.16 (0.59) | .013 |
| Outcomes, n (%) | |||
| Recurrences | 35 (14.2%) | 28 (14.1%) | .98 |
| Major bleeding | 16 (6.5%) | 17 (8.6%) | .39 |
| Death | 64 (26%) | 68 (34.3%) | .056 |
Abbreviations: DVT, deep vein thrombosis; SD, standard deviation.
Figure 2.
Decision tree. Score as predictor of occult cancer, according to sex. Patient is classified according to sex and as low or high risk according to score (≥3: high risk and ≤2: low risk).
Table 2.
Occult Cancer in Patients, Attending to Sex and Risk Factors for VTE.
| Variable | Total | Male | Female | P |
|---|---|---|---|---|
| Patients, n | 5864 | 2890 | 2974 | |
| Risk factors for VTE, n (%) | ||||
| Recent surgery (n = 592) | 28 (4.7) | 12 (4.9) | 16 (4.6) | .87 |
| Recent immobility ≥4 days (n = 1184) | 90 (7.6) | 46 (8.3) | 44 (7) | .37 |
| Hormonal therapy (n = 332) | 8 (2.4) | 1 (20) | 7 (2.1) | .27 |
| Recent travel (n = 150) | 8 (5.3) | 6 (6) | 2 (4) | .89 |
| Pregnancy/puerperium (n = 112) | 0 | - | 0 | |
| None of the above (unprovoked; n = 3666) | 312 (8.5) | 181 (9) | 131 (7.9) | .26 |
| Previous VTE (n = 1098) | 62 (5.6) | 34 (5.8) | 28 (5.4) | .78 |
Abbreviation: VTE, venous thromboembolism.
Data comparing accuracy of the score according to sex are shown in Table 3, where we can observe better results in male, with better sensitivity, odds ratio, positive predictive value, a C-statistic 0.64 (95% CI: 0.6-0.68), and a number needed to screen of 14 (95% CI: 11-20). In women, C-statistic was 0.61 (95% CI: 0.57-0.65) and the number needed to screen was 28 (95% CI: 16-94). Net benefit in males and females was 1.9% and 0.6%, respectively. There were no differences in Cox proportional models. A decision curve model was built to show the net benefit of screening based on the prediction model (Supplemental Figure).
Table 3.
Accuracy for High- Versus Low-Risk Categories to Predict Occult Cancer, According to Sex.
| Male | Female | |||
|---|---|---|---|---|
| Variable | (246/2644) | (198/2974) | ||
| Occult Cancer/Patients | 95% CI | 95% CI | ||
| C-statistic | 0.64 | 0.60-0.68 | 0.61 | 0.57-0.65 |
| Sensitivity | 60.6% | 54.3%-66.5% | 27.3% | 21.5%-33.9% |
| Specificity | 62.0% | 60.1%-63.8% | 81.8% | 80.3%-83.2% |
| Positive predictive value | 12.9% | 11.1%-15.0% | 9.7% | 7.5%-12.4% |
| Negative predictive value | 94.4% | 93.2%-95.4% | 94.0% | 93.0%-94.9% |
| Odds ratio diagnosis | 2.50 | 1.91-3.27 | 1.69 | 1.22-2.34 |
| Positive likelihood ratio | 1.59 | 1.42-1.78 | 1.50 | 1.18-1.91 |
| Negative likelihood ratio | 0.64 | 0.54-0.75 | 0.89 | 0.81-0.98 |
| Pretest probability (prevalence) | 8.5% | 7.5%-9.6% | 6.7% | 5.8%-7.6% |
| Posttest probability high risk (Bayes) | 12.9% | 11.1%-14.9% | 9.7% | 7.5%-12.5% |
| Number needed to screen | 14 | 11-20 | 28 | 16-94 |
Abbreviation: CI, confidence interval.
The most common cancer sites in men were the lung (n = 63), prostate (n = 42), and colorectal (n = 29), accounting for 54% of all cancers in men. Among women, the most common sites were colorectal (n = 38), breast (n = 23), uterine (n = 18), hematologic (n = 17), and pancreas (n = 15), accounting for 56% of all cancers. When comparing cancer sites according to sex, men were more likely to have lung cancer than women (2.18%, 95% CI: 1.68-2.78 vs 0.30%, 95% CI: 0.14-0.57; P < .01) and less likely to have pancreatic cancer (0.17%, 95% CI: 0.06-0.4 vs 0.5%, 95% CI: 0.28-0.83; P = .03), as shown in Figure 3. Interestingly, breast cancer was more likely found in women aged ≥50 years than in those aged <50 (0.97% vs 0.14%, respectively, P = .03), as shown in Figure 4.
Figure 3.
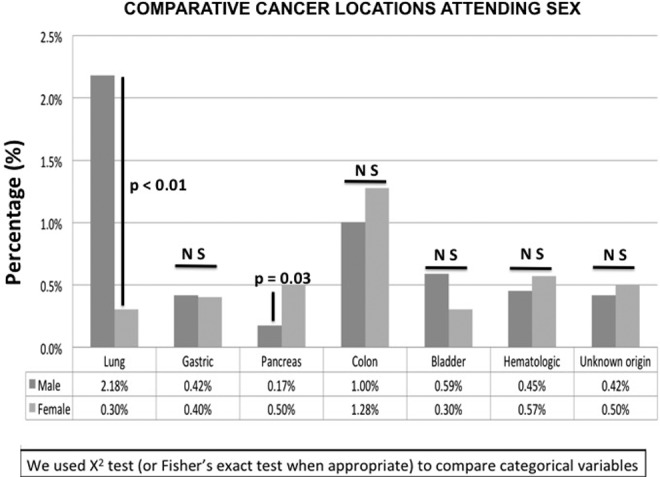
Comparative cancer sites according to sex.
Figure 4.
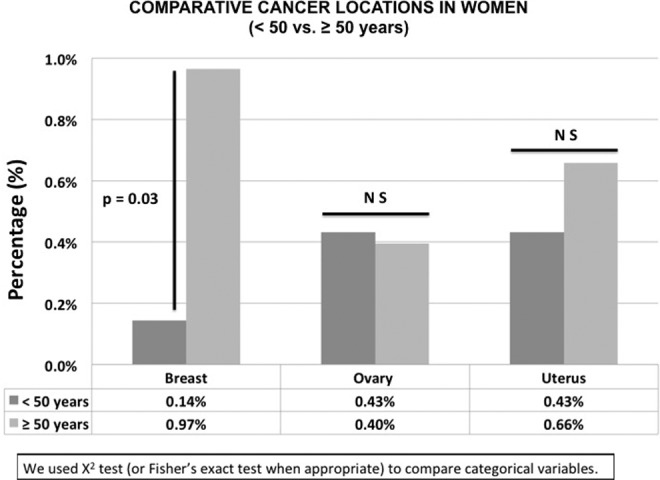
Comparative cancer sites in women by age (<50 vs ≥50 years).
In 2890 men, during the study period, there were 500 VTE recurrences (17.3%), with nonsignificant differences between patients with occult cancer versus no occult cancer (14.2% vs 17.6%, P = .18), and 220 bleed (7.6%). Major bleeding was significantly more common in patients with occult cancer than in those without occult cancer (14.6% vs 7%, P < .001). In 2974 women, there were 398 VTE recurrences (13.4%), with nonsignificant differences between occult cancer versus no occult cancer (14.1% vs 13.3%, P = .75), and 253 bleed (8.5%). Again, major bleeding was more common in women with occult cancer than in those without occult cancer (17.7% vs 7.9%, P < .001).
In patients with occult cancer, during follow-up, 132 patients (30%, 95% CI: 25-34) died. The leading causes of death were disseminated cancer (67%), bleeding (3.7%), and objectively confirmed PE (2.7%). In all, 64 men (26%) and 68 women (34%) died during follow-up (P = .056). There were 63 recurrences of VTE and 33 major bleeding, with no sex differences (Table 1).
Discussion
Our study is the largest published cohort of patients with acute VTE and occult cancer and provides interesting data on the clinical profile of at-risk patients. Men had an increased risk of occult cancer, particularly in the lung, prostate, or colorectal. Hence, men scoring ≥3 points should probably undergo a chest computed tomography (CT) scan (the most frequently used test to confirm PE diagnosis), a rectal examination, and prostate specific antigen (PSA) test. Among women, the risk of cancer was slightly lower, and the most common sites included the colon, breast, uterus, pancreas, and hematologic. Thus, the diagnostic workup for cancer in women scoring ≥3 points is more complicated. We found an increased risk of breast cancer in females older than 50 years. Several factors increased the risk of breast cancer, including early menarche, late childbearing, fewer pregnancies, or use of menopausal hormone therapy. The incidence of breast cancer has a distinctive age-specific curve, with a rapid rate of increase before the menopause (ages 40-50) and slows down after that, probably owing to diminishing levels of circulating estrogens.7,8 In many ways, the incidence of occult breast cancer in females with VTE was higher than expected, particularly in women aged >50 years.
The risk of lung cancer was much higher in men than in women. This difference might be attributed to the higher prevalence of chronic lung disease in men and the likely influence of tobacco smoking on both diseases. Unfortunately, smoking habit was not routinely gathered in the RIETE registry. We also found a nonsignificantly higher mortality rate in women (34% vs 26%; P = .056) and there were no differences in the rate of major bleeding or VTE recurrences.
The results in trials emphasize that performing an extensive occult cancer screening strategy does not seem to be beneficial. The Screening for Occult Malignant disease in Idiopathic venous Thromboembolism (SOMIT) trial randomized patients with a first episode of unprovoked VTE and negative limited occult cancer screening to either no further testing or additional investigations.9 Approximately 10% of patients in the control group were diagnosed with cancer over the 2-year follow-up period. The Trousseau’s study was a prospective cohort study assessing the added value of performing mammography in women and thoracic and abdominal CT in all patients presenting with unprovoked VTE.10 Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism (SOME) Occult cancer was diagnosed at enrollment in 2.4% and 3.5% of 630 patients receiving limited screening alone or in combination with CT, respectively. The Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism (SOME) trial failed to demonstrate, in patients with a first unprovoked VTE, any additional benefit of extensive occult cancer screening.11 In this multicenter study, 854 patients were randomized to either a limited screening strategy alone or in combination with comprehensive CT. At 1 year, 33 patients (3.9%, 95% CI: 2.8-5.4) were diagnosed with cancer in the interval between randomization and follow-up, with no significant differences between the 2 groups (P = .28). A French randomized controlled trial randomized 494 patients to a limited occult cancer screening alone or in combination with fludeoxyglucose positron emission tomography (FDG-PET)/CT.12 There was no significant difference in the primary outcome measure, with 2% and 5.6% of patients being diagnosed with occult cancer in the limited screening and limited screening + FDG-PET groups, respectively. Moreover, there were no differences in overall or cancer-related mortality. In a registry-based cohort study based on the entire Danish population of 5.4 million people, the risk of cancer after a diagnosis of superficial venous thrombosis in the legs, DVT, and PE was determined.13 They found that patients with a diagnosis of superficial venous thrombosis had a clearly higher occurrence of cancer than expected, particularly during the first year after diagnosis, with a standardized incidence ratio of 2.46 (95% CI: 2.10-2.86). For all these findings, it’s important to identify a high-risk population in which screening could obtain benefit.
One limitation of this study is that we do not have information about complete cancer staging (tumor/node/metastasis [TNM] classification) or diagnostic tools used to diagnose occult cancer, although our findings let us identify high-risk population, first step in the screening process.14 Our study has several strengths. All patients selected from RIETE were followed up for at least 2 years or until occult cancer was diagnosed, thus avoiding selection bias, and the number of involved patients was high (N = 5864). The proportion of patients with occult cancer in our series (7.6%) was consistent with that found in similar studies.11,15,16 Considering high-risk population, we obtained a number needed to screen of 14 for males and 28 for females, which should be considered relevant because other diseases like breast cancer screening showed number needed to screen of 133 to 588.17 Also, the increased rate of breast cancer in women aged >50 years should be noted (0.97%, 95% CI: 0.6-1.46 vs 0.14%, 95% CI: 0.003-0.8; P = .03).
According to our data, we may conclude that we should have a high suspicion of lung cancer in men scoring ≥3 points (particularly if they also have chronic lung disease), as well as prostate cancer and colorectal cancer. By contrast, women have more variety of cancer sites, though clinicians should be alert for breast and colorectal cancer in those aged ≥50 years. Anyway, these results should be externally validated and the prognostic score should be carefully managed.
Conclusions
This study emphasizes the existence of sex differences in patients with VTE who develop occult cancer. In men, there are more lung cancers and fewer pancreatic cancers than in women. In men, 2 cancer sites accounted for more than half the cancers. In women, there is a heterogeneity of cancer sites, with an increased risk of breast cancer in those aged >50 years. Women with occult cancer had a nonsignificantly higher mortality (P = .056).
Supplemental Material
Supplementary_Figure for Sex Differences in Patients With Occult Cancer After Venous Thromboembolism by Luis Jara-Palomares, Remedios Otero, David Jiménez, Juan Manuel Praena-Fernández, Agustina Rivas, Carme Font, Philip S. Wells, Raquel López-Reyes, José González-Martínez and Manuel Monreal, in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors express our gratitude to Sanofi Spain for supporting this registry with an unrestricted educational grant. We also express our gratitude to Bayer Pharma AG for supporting this registry. Bayer Pharma AG’s support was limited to the part of RIETE outside Spain, which accounts for a 23.52% of the total patients included in the RIETE registry. The authors also thank the RIETE registry Coordinating Center, S & H Medical Science Service, for their quality control data, logistic, and administrative support.
Appendix A
Coordinator of the RIETE registry: Manuel Monreal. RIETE Steering Committee Members: Hervé Decousus, Paolo Prandoni and Benjamin Brenner. RIETE National Coordinators: Raquel Barba (Spain), Pierpaolo Di Micco (Italy), Laurent Bertoletti (France), Inna Tzoran (Israel), Abilio Reis (Portugal), Marijan Bosevski (R. Macedonia), Henri Bounameaux (Switzerland), Radovan Malý (Czech Republic), Philip Wells (Canada), and Peter Verhamme (Belgium). RIETE Registry Coordinating Center: S & H Medical Science Service. Members of the RIETE Group SPAIN: Adarraga MD, Aibar MA, Alfonso M, Arcelus JI, Ballaz A, Barba R, Barrón M, Barrón-Andrés B, Bascuñana J, Blanco-Molina A, Cañas I, Chic N, del Pozo R, del Toro J, Díaz-Pedroche MC, Díaz-Peromingo JA, Falgá C, Fernández-Aracil C, Fernández-Capitán C, Fidalgo MA, Font C, Font L, Gallego P, García I, García MA, García-Bragado F, García-Ródenas M, Gavín O, Gómez C, Gómez V, González J, Grau E, Grimón A, Guijarro R, Guirado L, Gutiérrez J, Hernández-Comes G, Hernández-Blasco L, Jara-Palomares L, Jaras MJ, Jiménez D, Jiménez J, Joya MD, Llamas P, Lobo JL, López P, López-Jiménez L, López-Reyes R, López-Sáez JB, Lorente MA, Lorenzo A, Lumbierres M, Marchena PJ, Martín-Martos F, Mellado M, Monreal M, Nieto JA, Nieto S, Núñez A, Núñez MJ, Otalora S, Otero R, Ovejero A, Pedrajas JM, Pérez G, Pérez-Ductor C, Peris ML, Pons I, Porras JA, Reig O, Riera-Mestre A, Riesco D, Rivas A, Rodríguez M, Rodríguez-Dávila MA, Rosa V, Ruiz-Artacho P, Ruiz-Giménez N, Sahuquillo JC, Sala-Sainz MC, Sampériz A, Sánchez-Martínez R, Sanz O, Soler S, Sopeña B, Suriñach JM, Tolosa C, Torres MI, Trujillo-Santos J, Uresandi F, Usandizaga E, Valero B, Valle R, Vela J, Velez-Mendizábal E, Vidal G, Vila M, Villalobos A, Xifre B; Belgium: Vanassche T, Verhamme P; Brazil: Yoo HHB; Canada: Wells P; Czech Republic: Hirmerova J, Malý R; Ecuador: Salgado E; France: Bertoletti L, Bura-Riviere A, Falvo N, Farge-Bancel D, Hij A, Mahé I, Moustafa F; Israel: Braester A, Brenner B, Tzoran I; Italy: Antonucci G, Barillari G, Bilora F, Bortoluzzi C, Brandolin B, Bucherini E, Candeloro G, Cattabiani C, Ciammaichella M, Dentali F, Di Micco P, Duce R, Giorgi-Pierfranceschi M, Grandone E, Imbalzano E, Lessiani G, Maida R, Mastroiacovo D, Pace F, Parisi R, Pellegrinet M, Pesavento R, Pinelli M, Poggio R, Prandoni P, Quintavalla R, Rocci A, Tiraferri E, Tonello D, Tufano A, Visonà A; Latvia: Gibietis V, Skride A, Vitola B; Republic of Macedonia: Bosevski M, Zdraveska M; Switzerland: Bounameaux H, Mazzolai L; United States: Caprini JA.
Authors’ Note: LJP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. LJP, MM, ROC, and DJ contributed to study concept and design. All authors contributed to acquisition, analysis, or interpretation of data, drafted the manuscript, and critically revised the manuscript for important intellectual content. JMPF and LJP performed statistical analysis. LJP, ROC, DJ, CF, JMPF RLR, PW, JGM, and MM supervised the study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article:
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Jara-Palomares L, Otero R, Jimenez D, et al. ; RIETE Investigators. Development of a risk prediction score for occult cancer in patients with VTE. Chest. 2017;151(3):564–571. [DOI] [PubMed] [Google Scholar]
- 2. Monreal M, Falgá C, Valdés M, et al. ; RIETE Investigators. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost. 2006;4(9):1950–1956. [DOI] [PubMed] [Google Scholar]
- 3. Tzoran I, Brenner B, Sakharov G, et al. ; RIETE Investigators. Clinical outcome in patients with venous thromboembolism receiving concomitant anticoagulant and antiplatelet therapy. Eur J Intern Med. 2014;25(9):821–825. [DOI] [PubMed] [Google Scholar]
- 4. Spiezia L, Campello E, Trujillo-Santos J, et al. ; RIETE Investigators. The impact of disseminated intravascular coagulation on the outcome of cancer patients with venous thromboembolism. Blood Coagul Fibrinolysis. 2015;26(6):709–711. [DOI] [PubMed] [Google Scholar]
- 5. Farge D, Trujillo-Santos J, Debourdeau P, et al. ; RIETE Investigators. Fatal events in cancer patients receiving anticoagulant therapy for venous thromboembolism. Medicine (Baltimore.) 2015;94(32):e1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. [DOI] [PubMed] [Google Scholar]
- 8. Bray F, McCarron P, Parkin DM. The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res. 2004;6(6):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Piccioli A, Lensing AWA, Prins MH, et al. Extensive screening for occult malignant disease in idiopathic venous thromboembolism: a prospective randomized clinical trial. J Thromb Haemost. 2004;2(6):884–889. [DOI] [PubMed] [Google Scholar]
- 10. van Doormaal FF, Terpstra W, van der Griend R, et al. Is extensive screening for cancer in idiopathic venous thromboembolism warranted? J Thromb Haemost. 2011;9(1):79–84. [DOI] [PubMed] [Google Scholar]
- 11. Carrier M, Lazo-Langner A, Shivakumar S, et al. ; SOME Investigators. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med. 2015;373(8):697–704. [DOI] [PubMed] [Google Scholar]
- 12. Robin P, Le Roux PY, Planquette B, et al. ; MVTEP Study Group. Limited screening with versus without (18)F-fluorodeoxyglucose PET/CT for occult malignancy in unprovoked venous thromboembolism: an open-label randomised controlled trial. Lancet Oncol. 2016;17(2):193–199. [DOI] [PubMed] [Google Scholar]
- 13. Sørensen HT, Sværke C, Farkas DK, et al. Superficial and deep venous thrombosis, pulmonary embolism and subsequent risk of cancer. Eur J Cancer. 2012;48(4):586–593. [DOI] [PubMed] [Google Scholar]
- 14. Armstrong K, Kim JJ, Halm EA, Ballard RM, Schnall MD. Using lessons from breast, cervical, and colorectal cancer screening to inform the development of lung cancer screening programs. Cancer. 2016;122(9):1338–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kearon C, Akl EA, Comerota AJ, et al. American College of Chest Physicians. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun LM, Chung WS, Lin CL, Liang JA, Kao CH. Unprovoked venous thromboembolism and subsequent cancer risk: a population-based cohort study. J Thromb Haemost. 2016;14(3):495–503. [DOI] [PubMed] [Google Scholar]
- 17. Hendrick RE, Helvie MA, Hardesty LA. Implications of CISNET modeling on number needed to screen and mortality reduction with digital mammography in women 40–49 years old. AJR Am J Roentgenol. 2014;203(6):1379–1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figure for Sex Differences in Patients With Occult Cancer After Venous Thromboembolism by Luis Jara-Palomares, Remedios Otero, David Jiménez, Juan Manuel Praena-Fernández, Agustina Rivas, Carme Font, Philip S. Wells, Raquel López-Reyes, José González-Martínez and Manuel Monreal, in Clinical and Applied Thrombosis/Hemostasis