Abstract
Little is known about aging in von Willebrand disease (VWD). It is uncertain whether VWD patients experience an age-related increase in von Willebrand factor (VWF) levels, and if so, it is unknown whether normalization of VWF levels with aging ameliorates bleeding risk. We aimed to determine the association of age with VWF levels and bleeding risk in patients with type 1 VWD. This is a retrospective chart review of patients with type 1 VWD presenting to the Hemophilia Clinic of Western Pennsylvania for regularly scheduled clinic visits. Data collected included VWF antigen level and condensed molecular and clinical markers for the diagnosis and management of Type 1 (MCMDM-1) VWD bleeding assessment tool (BAT) bleeding score based on bleeding symptoms during the previous 3 years. Thirty-nine patients participated in the study, and 32 were female. The average age of participants was 41.8 ± 18.0 years. The mean VWF antigen level was 0.83 ± 0.37 IU/mL, and the mean bleeding score was 2.51 ± 2.90. The bleeding score was inversely associated with age, β = −0.080 (SE = 0.023), P < .01. There was a nonsignificant association between VWF antigen levels and age. To our knowledge, this is the first report showing an association between aging and decreased bleeding symptoms in patients with type 1 VWD. Determining whether or not bleeding risk is reduced in older patients with type 1 VWD is essential for optimal clinical management. Moreover, VWF concentrate is costly, and unwarranted use represents a significant waste of health-care dollars. These findings warrant further investigation.
Keywords: von Willebrand disease, von Willebrand factor, aging, bleeding score
Introduction
Little is known about the effect of aging on von Willebrand factor (VWF) levels and bleeding in von Willebrand disease (VWD). While it is has been well established that VWF levels increase with age among healthy adults, it is uncertain whether this phenomenon is observed in VWD, and if so, if it is restricted to a certain subset of patients.1 Limited data suggest an age-related increase in VWF levels is observed in patients with type 1, but not type 2, VWD.2,3 Furthermore, if normalization of VWF levels does occur in patients with type 1 VWD, it is unknown if this results in amelioration of bleeding phenotype. This issue has been addressed in a few studies, which suggest that, despite increasing VWF levels, bleeding symptoms do not lessen with age; however, several matters related to study design may have confounded the results, including inclusion criteria and the methods used to administer bleeding assessment tools (BATs).3–6
Determining whether or not bleeding risk is reduced in elderly patients with type 1 VWD is essential for providing proper medical care to affected patients. Aging patients often require invasive procedures, and patients with VWD require periprocedural prophylactic therapy with 1-Desamino-8d-Arginine Vasopressin (DDAVP) or VWF concentrate to ensure appropriate hemostasis. Administering DDAVP or VWF concentrate to older patients with type 1 VWD having normalized VWF levels may be harmful, placing patients at risk of thrombosis, electrolyte disturbance, and other adverse effects. Additionally, such therapy is costly and represents a considerable waste of health-care dollars if its use is not warranted. For these reasons, investigation into the effect of aging on VWF levels and bleeding risk in patients with VWD is sorely needed.
Methods
We performed a retrospective chart review of consecutive patients, aged 18 or older, with a new or historical diagnosis of type 1 VWD presenting to the Hemophilia Clinic of Western Pennsylvania (HCWP) for regularly scheduled clinic visits between February 2016 and January 2017. During this time, we began obtaining current VWF levels and bleeding scores as standard of care on all patients with VWD presenting to clinic at HCWP. All diagnoses of type 1 VWD were made at HCWP by a hematologist with expertise in disorders of hemostasis and based on clinical history and a VWF antigen level and/or VWF ristocetin cofactor activity <0.50 IU/dL. If the VWF antigen level and VWF ristocetin cofactor activity were ≥0.50 IU/dL in the setting of exogenous estrogen use, but the clinical history was highly suspicious, type 1 VWD was still diagnosed. Exclusion criteria included concomitant hereditary bleeding disorder and pregnancy. Data collected include demographic information: age, sex, race, and ethnicity; medical information: exogenous estrogen use and menopause status; laboratory results: VWF antigen level (via BCS-XP Coagulation Analyzer utilizing the STA-Liatest VWF kit), VWF ristocetin cofactor activity (via BCS-XP Coagulation Analyzer utilizing the BC von Willebrand Reagent), and factor VIII activity (via BCS-XP Coagulation Analyzer utilizing Pathromtin SL and factor VIII deficient plasma); and the condensed MCMDM-1 VWD BAT bleeding score based on bleeding symptoms during the previous 3 years.7 The prophylactic use of DDAVP and/or VWF concentrate prior to invasive procedures was not included in the determination of the bleeding score.
Descriptive statistics consisted of mean, frequency, range, and standard deviation for continuous variables and frequency and percentages for categorical variables. Simple linear regression was calculated to predict VWF antigen level and bleeding score based on age. Multiple linear regression analysis was conducted to assess the influence of age on VWF antigen level and bleeding score after adjustment for sex, exogenous estrogen use, and menopause status.
Results
The study consisted of 39 patients with type 1 VWD presenting to HCWP for regularly scheduled clinic visits between February 2016 and January 2017 (Table 1). Seven patients were newly diagnosed with type 1 VWD at the time of presentation, and the remaining 32 patients had an established diagnosis. The mean age of participants was 41.8 ± 18.0 years. Eighteen patients were 45 years of age or older, including 7 patients aged 60 or older. In all, 82% of patients were female, including 89% of patients aged 45 years or older. The mean VWF antigen level was 0.83 ± 0.37 IU/mL, and the mean VWF ristocetin cofactor activity was 0.59 ± 0.34 IU/mL. The mean bleeding score was 2.51 ± 2.90. Of female patients, 34% reported the use of estrogen-containing hormonal therapy, and 41% were postmenopausal.
Table 1.
Baseline Characteristics.
| N | 43 |
| Age, years ± SD, range | 41.8 ± 18.0 (19, 85) |
| Female sex, % | 82 |
| Race | |
| Caucasian, % | 85 |
| African American, % | 15 |
| VWF antigen level, IU/mL ± SD, range | 0.83 ± 0.37 (0.10, 2.20) |
| VWF ristocetin cofactor activity, IU/mL ± SD, range | 0.59 ± 0.34 (0.10, 1.60) |
| FVIII activity, IU/mL ± SD, range | 0.91 ± 0.37 (0.10, 2.91) |
| Bleeding score ± SD, range | 2.51 ± 2.90 (0, 11) |
| Hormone use, % | 34 |
| Postmenopausal, % | 41 |
Abbreviations: FVIII, factor VIII; SD, standard deviation; vWF, von Willebrand factor.
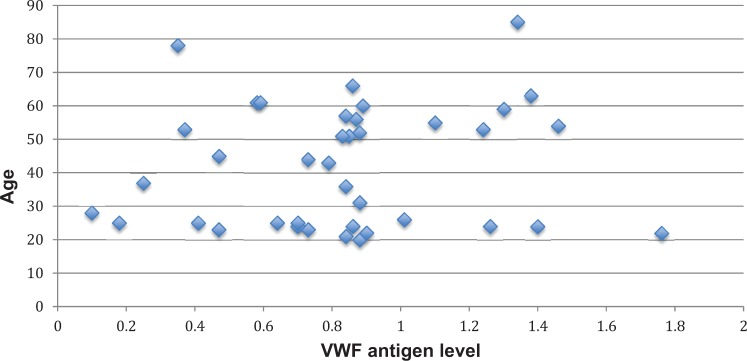
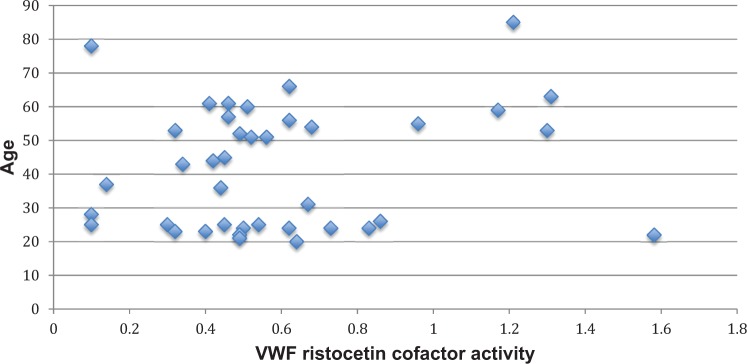
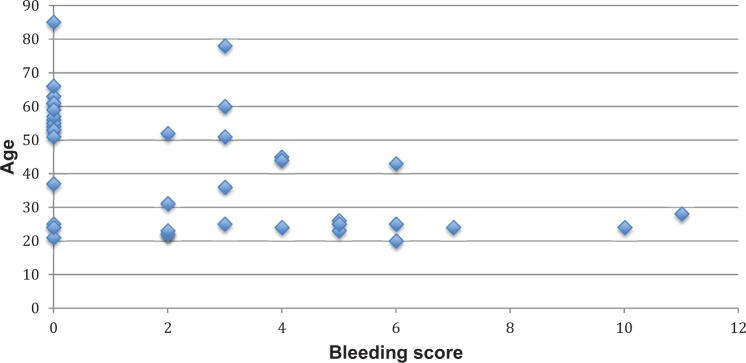
A simple linear regression was calculated to predict bleeding score based on age. The bleeding score was inversely associated with age, with a β = −0.080 (standard error [SE] = 0.023) per year of age, P < .01. This association was no longer significant after adjusting for sex, exogenous estrogen use, and menopause with a β = −0.015 (SE = 0.034), P = .66. There was a nonsignificant association between VWF antigen level and age, β = −0.002 (SE = 0.003) for each year of age, P = .45, which was unaffected following adjustment for sex, hormonal therapy, and menopause, β = 0.001 (SE = 0.006), P = .84. Similarly, there was a nonsignificant association between VWF ristocetin cofactor activity and age, with a β = .003 (SE = 0.003) per year of age, P = .29, which was not affected by adjustment for sex, estrogen use, and menopause, β = 0.002 (SE = 0.005), P = .71. Figures 1 to 3 depict the association of age with VWF antigen levels, ristocetin cofactor activity, and bleeding score.
Figure 1.
The association between age and von Willebrand factor (VWF) antigen levels.
Figure 2.
The association between age and von Willebrand factor (VWF) ristocetin cofactor activity.
Figure 3.
The association between age and condensed MCMDM-1 VWD bleeding assessment tool (BAT) bleeding score.
Discussion
To our knowledge, this is the first study showing an association between aging and decreased bleeding symptoms in patients with type 1 VWD. We were able to demonstrate aging is associated with a decrease in bleeding score using the condensed MCMDM-1 VWD BAT. We found no association between aging and VWF antigen levels, which may, in part, be related to sample size. Our study is unique in the fact that we modified the BAT to determine the bleeding score based on recent, rather than lifetime, bleeding symptoms.
Our findings are contradictory to prior studies that have investigated this issue and suggested that, despite increasing VWF levels, bleeding symptoms do not diminish with aging in patients with type 1 VWD; however, several matters related to study design might explain these differences. One issue is the method of BAT administration.3–6 The BATs allow a detailed and objective bleeding history to discriminate between patients with and without VWD and gauge the severity of VWD. Previous studies determined the bleeding score based on lifetime bleeding symptoms; however, this may not accurately reflect current bleeding risk. For example, a patient may have had a significant number of bleeding symptoms in early adulthood corresponding to a high bleeding score; however, even if bleeding manifestations lessen, or completely resolve, with aging, the bleeding score will remain unchanged. This prevents a proper assessment of the patient’s current bleeding phenotype.
In addition to the method of BAT administration, inclusion criteria may explain the dissimilar findings between our study and prior studies. In the Willebrand in the Netherlands (WiN) study, patients with types 2 and 3 VWD accounted for nearly half of enrolled participants, and enrollment was restricted to patients with more severe VWD (VWF antigen level and/or ristocetin cofactor activity less than 0.30 IU/mL).3 An age-related increase in VWF levels is not expected in type 3 VWD due to a complete lack of VWF production. Similarly, an increase in VWF levels might not occur in severe type 1 and type 2 VWD, as demonstrated by Sanders et al in elderly patients with type 2 VWD, and may be partially explained by the presence of mutations in the VWF gene.3 Pathogenic VWF gene mutations have been identified in most cases of type 2 VWD, and several large population studies have identified VWF mutations in approximately 65% of type 1 VWD cases, which occur with greater frequency in more severe disease.8–11 So, an age-related increase in VWF levels may not be seen in patients with VWF mutations, and VWF mutations are most common in severe type 1 and types 2 and 3 VWD; therefore, it is likely that VWF mutations affected a sizable portion of participants in the WiN study and may explain the lack of improvement in bleeding symptoms with aging. Of note, while there was a statistically significant increase in VWF antigen levels among patients older than 65 years of age, 0.30 and 0.38 IU/mL, P = .033, VWF antigen levels remained less than normal.3
There are several limitations to our study that need to be addressed. First, we would like to point out that while we found an inverse association between aging and bleeding symptoms, correlation does not equal causation. A prospective longitudinal study is necessary to discern whether or not aging results in amelioration of bleeding symptoms in patients with type 1 VWD. Second, VWF antigen levels were higher than expected. An estrogen-related increase in VWF levels is seen with hormonal therapy, and 34% of female patients were receiving estrogen-containing hormonal therapy, which may partially explain the higher than expected VWF antigen levels. However, even after adjusting for estrogen use, there was no association between aging and VWF antigen levels. VWF antigen is an acute-phase reactant, and inflammatory states, such as autoimmune diseases and infections, neither of which were assessed, are other potential explanations for these findings. Third, the sample size was small and may have prevented us from detecting an association between aging and increased VWF levels, which has been reported in previous studies.2,3
Finally, BATs have several drawbacks and may not truly reflect the underlying bleeding phenotype. We used the condensed MCMDM-1 VWD BAT to evaluate bleeding symptoms over the previous 3 years; however, it has not been validated for this specific application. Nevertheless, this method of BAT administration and bleeding score calculation is necessary to determine if bleeding risk declines with aging. Whether or not 3 years provides enough time for the occurrence of hemostatic challenges, such as surgery, dental extraction, or trauma, is another area of concern; however, based on clinical experience, this represents a reasonable period for the incidence of such events. Moreover, greater periods of time, such as 10 years, may be subject to recall bias. Another potential limitation of BATs relates to the use of menorrhagia and postpartum hemorrhage in determining the bleeding score. In our study, the inverse association between aging and bleed score was no longer seen after adjusting for menopause. This may be explained by the fact that menopause serves as a surrogate maker for age. Alternatively, the decrease in bleeding score associated with aging may be due to differences in BAT scoring according to menopause status rather than a true improvement in bleeding symptoms. Finally, the prophylactic use of DDAVP and/or VWF concentrate, usually prior to invasive procedures to prevent bleeding symptoms that may occur in the absence of such treatment, represents another limitation of BATs and source of potential bias; however, this effect should be equal across all groups except newly diagnosed patients, of which there were only 7 in our study.
Our study has several strengths, too. Most importantly, we modified the BAT to determine the bleeding score based on recent bleeding symptoms over the previous 3 years to provide a more accurate assessment of current bleeding phenotype. This has been an important limitation in prior studies. Also, we restricted participation to individuals with type 1 VWD, which may represent the subset of patients most likely to experience an age-related decrease in bleeding symptoms. Finally, to our knowledge, this is the first study primarily designed to investigate whether or not an association exists between age and bleeding symptoms in VWD.
In conclusion, we were able to demonstrate aging is associated with a reduction in bleeding symptoms but did not find an association between aging and increased VWF levels. These findings warrant further investigation to provide more conclusive answers. Therefore, we are planning a multicenter, cross-sectional study to investigate the association of aging with VWF levels and bleeding risk in patients with type 1 VWD.
Footnotes
Authors’ Note: CDS and MVR designed and completed the research, performed the statistical analysis, analyzed the data, formulated the conclusions, and wrote the paper.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Coppola R, Mari D, Lattuada A, Franceschi C. von Willebrand factor in Italian centenarians. Haematologica. 2003;88(1):39–43. [PubMed] [Google Scholar]
- 2. Rydz N, Grabell J, Lillicrap D, James PD. Changes in von Willebrand factor level and von Willebrand activity with age in type 1 von Willebrand disease. Haemophilia. 2015;21(5):636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders YV, Giezenaar MA, Laros-van Gorkom BA, et al. von Willebrand disease and aging: an evolving phenotype. J Thromb Haemost. 2014;12(7):1066–1075. [DOI] [PubMed] [Google Scholar]
- 4. Tosetto A, Rodeghiero F, Castaman G, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost. 2006;4(4):766–773. [DOI] [PubMed] [Google Scholar]
- 5. De Wee EM, Sanders YV, Mauser-Bunschoten EP, et al. Determinants of bleeding phenotype in adult patients with moderate or severe von Willebrand disease. Thromb Haemost. 2012;108(4):683–692. [DOI] [PubMed] [Google Scholar]
- 6. Flood VH, Christopherson PA, Gill JC, et al. Clinical and laboratory variability in a cohort of patients diagnosed with type 1 VWD in the United States. Blood. 2016;127(20):2481–2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowman M, Mundell G, Grabell J, et al. Generation and validation of the Condensed MCMDM-1VWD Bleeding Questionnaire for von Willebrand disease. J Thromb Haemost. 2008;6(12):2062–2066. [DOI] [PubMed] [Google Scholar]
- 8. Lillicrap D. von Willebrand disease: advances in pathogenetic understanding, diagnosis, and therapy. Blood. 2013;122(23):3735–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. James PD, Notley C, Hegadorn C, et al. The mutational spectrum of type 1 von Willebrand disease: results from a Canadian cohort study. Blood. 2007;109(1):145–154. [DOI] [PubMed] [Google Scholar]
- 10. Goodeve A, Eikenboom J, Castaman G, et al. Phenotype and genotype of a cohort of families historically diagnosed with type 1 von Willebrand disease in the European study, molecular and clinical markers for the diagnosis and management of type 1 von Willebrand disease. Blood. 2007;109(1):112–121. [DOI] [PubMed] [Google Scholar]
- 11. Gill JC, Endres-Brooks J, Bauer PJ, Marks WJ, Montgomery RR. The effect of ABO blood group on the diagnosis of von Willebrand disease. Blood. 1987;69(6):1691–1695. [PubMed] [Google Scholar]