Abstract
Two large randomized controlled trials examined the efficacy and safety of rivaroxaban for the treatment of venous thromboembolism (VTE). The aim of this epidemiological study was to analyze a cohort of Italian patients affected by VTE who were treated with rivaroxaban in clinical practice. The data were collected by physicians using an online electronic questionnaire. The study was performed during a 6-month period from January to June 2014. We analyzed the clinical characteristics, risk factors for VTE, comorbidities, diagnostic techniques, and treatments in the whole population and in the subgroups with deep vein thrombosis (DVT) only, pulmonary embolism (PE) only, and DVT+PE. Overall, 75.9% of patients were affected by DVT; 20% of patients had DVT and PE; and 4.8% of patients had only PE. Approximately 90% of patients were symptomatic upon diagnosis, and 46.3% of patients required hospitalization. More than half of the patients switched to rivaroxaban after receiving another anticoagulant therapy. The main reasons for changing treatment included difficulties in managing vitamin K antagonists, patient choice, and prothrombin time-international normalized ratio (PT-INR) instability. The switch to rivaroxaban occurred after a mean of 1.8 PT-INR measurements. Clinical characteristic were overall similar to those of patients enrolled in prior clinical trials evaluating the safety and efficacy of rivaroxaban.
Keywords: anticoagulants, pulmonary embolism, rivaroxaban, venous thromboembolism, deep vein thrombosis, epidemiology
Introduction
Venous thromboembolism (VTE) includes 2 clinical manifestations: deep vein thrombosis (DVT) and pulmonary embolism (PE). The VTE causes morbidity and mortality and is the third most common cause of cardiovascular disease.1 According to a recent population cohort study in the United Kingdom, the incidence rate of VTE was 1.3 first VTE events per 1000 person-years. Additionally, the recurrence rate of VTE was 11.1 per 100 person-years during the first 6 months following the first VTE event.2
The standard of care for VTE includes an initial treatment with heparin followed by vitamin K antagonists (VKAs).3–5 This treatment poses several problems associated with frequent laboratory monitoring, food and drug interactions, and dosage changes.6 However, new oral anticoagulants (NOACs) do not require more frequent routine monitoring and can be administered at a fixed dose. These drugs are available and are approved in many countries for the treatment and prevention of recurrent VTE.7–12 The NOACs offer the same efficacy and have a decreased risk of bleeding compared to warfarin.13 The drug rivaroxaban is a direct, oral reversible factor Xa inhibitor that allows treatment without an initial parenteral therapy with heparin.14–16
The efficacy and safety of rivaroxaban for the treatment of VTE was studied in the EINSTEIN program, which included 2 large randomized controlled trials (EINSTEIN-DVT and EINSTEIN-PE).11,12 The EINSTEIN-DVT trial consisted of 2 parts. The first part (Acute DVT Study) of the study was an open-label randomized study of patients with acute and symptomatic DVT. The second part (Continued Treatment Study) of the study was a double-blind randomized study of patients with confirmed-symptomatic DVT or PE who completed 6 to 12 months of anticoagulant therapy.12 The Acute DVT Study compared rivaroxaban treatment (15 mg twice daily for 3 weeks, followed by 20 mg once daily) with a standard therapy consisting of enoxaparin and a VKA. In the Continued Treatment Study, rivaroxaban treatment (20 mg once daily) was compared to a placebo. These 2 studies revealed that rivaroxaban is as effective as the standard therapy for the treatment of acute DVT. Additionally, rivaroxaban has superior efficacy and has an acceptable risk of bleeding in patients with preventing recurrences.12
Similar results (noninferiority to standard therapy) were obtained in the EINSTEIN-PE study. This study was a randomized open-label trial comparing rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily) with standard therapy (enoxaparin followed by warfarin or acenocoumarol) for 3, 6, or 12 months in patients with acute symptomatic PE with or without DVT.11 In 2013, a pooled analysis of EINSTEIN-DVT and EINSTEIN-PE data was conducted and found similar results for the safety and efficacy of rivaroxaban. Similar results were also found for the following key patient subgroups: fragile patients, patients with cancer, patients presenting with large clots, and patients with a history of recurrent VTE.17 Moreover, a “real-life” Spanish (RLS) study evaluated a cohort of patients with DVT and/or PE who were treated with rivaroxaban, and the results were similar to the EINSTEIN studies.18
The aim of this epidemiological analysis was to evaluate a real-life Italian population to determine the characteristics (risk factors of VTE, diagnostic techniques, comorbidities, and previous treatments; Tables 1 and 2, Figure 1) of patients with VTE receiving rivaroxaban.
Table 1.
Clinical Characteristics of Patients Diagnosed With VTE.a
| Whole Population | DVT Only | PE Only | DVT+PE | P | |
|---|---|---|---|---|---|
| n | 458 | 348 | 22 | 88 | |
| Age, years | 62.5 (13.7) | 61.7 (16) | 63.7 (5.5) | 65.0 (17.1) | .205 |
| Male gender, n (%) | 239 (52.2%) | 190 (54.6%) | 8 (36.4%) | 41 (46.6%) | .127 |
| Female gender, n (%) | 219 (47.8%) | 158 (45.4%) | 14 (63.6%) | 47 (53.4%) | |
| BMI, kg/m2 | .382 | ||||
| <25 | 173 (37.7%) | 135 (38.8%) | 4 (18.2%) | 34 (38.6%) | |
| 25 to 30 | 189 (41.4%) | 143 (41.1%) | 11 (50.0%) | 35 (39.8%) | |
| >30 | 96 (20.9%) | 70 (20.1%) | 7 (31.8%) | 19 (21.6%) | |
| SBP, mm Hg | 132.6 (17.6) | 132.2 (15.6) | 134.3 (19.3) | 127.8 (21.5) | .071 |
| DBP, mm Hg | 80.3 (9.8) | 81.0 (10.3) | 77.3 (10.2) | 78.1 (9.5) | .022 |
| Heart rate, beats/min | 78.3 (9.7) | 76.2 (8.4) | 83.7 (10.5) | 84.2 (11.7) | <.001 |
| CrCl, mL/min | 85.7 (36.8) | 85.8 (36.4) | 92.3 (43.4) | 84.5 (36.8) | .519 |
| VTE type | |||||
| Proximal DVT | 56.9% | ||||
| Distal DVT | 18.9% | ||||
| DVT+PE | 20% | ||||
| PE | 4.8% |
Abbreviations: VTE, venous thromboembolism; DVT, deep vein thrombosis; PE, pulmonary embolism; DVT+PE, patients presenting both deep vein thrombosis and pulmonary embolism; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CrCl, creatinine clearance calculated with the Cockcroft-Gault formula19.
aValues are expressed as means (SDs) or as % of patients.
Table 2.
Associated Comorbidities and Possible Risk Factors Identified in the Whole Population and in Subgroups.a
| Whole Population | DVT Only | PE Only | DVT+PE | P c | P d | P e | |
|---|---|---|---|---|---|---|---|
| Comorbidities | |||||||
| n | 458 | 348 | 22 | 88 | |||
| Hypertension | 220 (48.0%) | 167 (48.0%) | 9 (40.9%) | 44 (50.0%) | .747 | ||
| Varicose vein | 133 (29.0%) | 107 (30.7%) | 3 (13.6%) | 23 (26.1%) | .184 | ||
| Diabetes mellitus | 90 (19.7%) | 66 (18.9%) | 4 (18.2%) | 20 (22.7%) | .719 | ||
| COPD | 73 (15.9%) | 46 (13.2%) | 3 (13.6%) | 24 (27.3%) | .005 | .955 | .001 |
| Congestive heart failure | 42 (9.2%) | 23 (6.6%) | 1 (4.5%) | 18 (20.5%) | <.001 | .703 | <.001 |
| Atrial fibrillation | 36 (7.9%) | 25 (7.2%) | 1 (4.5%) | 10 (11.4%) | .360 | ||
| Peripheral artery disease | 36 (7.9%) | 30 (8.6%) | 2 (9.1%) | 4 (4.5%) | .436 | ||
| Previous acute coronary syndrome | 33 (7.2%) | 22 (6.3%) | 0 (0.0%) | 11 (12.5%) | .055 | ||
| Previous stroke or TIA | 26 (5.7%) | 18 (5.2%) | 2 (9.1%) | 6 (6.8%) | .651 | ||
| Chronic inflammatory disease | 23 (5.0%) | 19 (5.5%) | 0 (0.0%) | 4 (4.5%) | .510 | ||
| Risk factors | |||||||
| Previous VTE | 140 (30.6%) | 115 (33.0%) | 2 (9.1%) | 23 (26.1%) | .037 | .019 | .213 |
| Hospitalizationb | 90 (19.7%) | 57 (16.4%) | 7 (31.8%) | 26 (29.5%) | .007 | .063 | .005 |
| Prolonged immobilization | 85 (18.6%) | 66 (19.0%) | 2 (9.1%) | 17 (19.3%) | .502 | ||
| Known thrombophilia | 68 (14.8%) | 52 (14.9%) | 1 (4.5%) | 15 (17.0%) | .335 | ||
| Postthrombotic syndrome | 60 (13.1%) | 52 (14.9%) | 2 (9.1%) | 6 (6.8%) | .111 | ||
| Recent trauma/fracture | 35 (7.6%) | 24 (6.9%) | 3 (13.6%) | 8 (9.1%) | .437 | ||
| Recent major surgeryb | 35 (7.6%) | 22 (6.3%) | 2 (9.1%) | 11 (12.5%) | .145 | ||
| Use of oral contraceptives | 24 (5.2%) | 18 (5.2%) | 1 (4.5%) | 5 (5.7%) | .971 | ||
| Long-term air travel | 9 (2.0%) | 9 (2.6%) | 0 (0.0%) | 0 (0.0%) | .234 | ||
| Recent pregnancy | 5 (1.1%) | 4 (1.1%) | 0 (0.0%) | 1 (1.1%) | .880 | ||
| Presence of central venous catheter | 3 (0.7%) | 3 (0.9%) | 0 (0.0%) | 0 (0.0%) | .620 | ||
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; DVT+PE, patients presenting both DVT and PE; COPD, chronic obstructive pulmonary disease; TIA, transient ischemic attack; VTE, venous thromboembolism.
aValues are expressed as % of patients.
bDuring the 3 months before diagnosis.
cχ2 test comparing 3 groups.
dDVT only versus PE only.
eDVT only versus PE+DVT.
Figure 1.
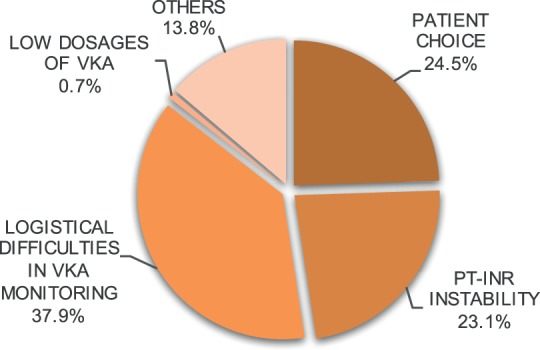
Reasons for switching therapy from VKA or heparin to rivaroxaban. PT-INR indicates prothrombin time-international normalized ratio; VKA, vitamin K antagonist.
Materials and Methods
We performed an epidemiological survey of a cohort of Italian patients. An electronic questionnaire, including demographic data (sex, age, and body mass index) and patient medical history, was prepared. Physicians, affiliated with a hospital or university and with experience in prescribing rivaroxaban, were asked to complete the questionnaire to collect clinical data on adult patients with suspected VTE (DVT and/or PE) who were prescribed rivaroxaban during the defined period. The data collected included risk factors for VTE, diagnostic techniques, comorbidities, and previous treatments. The survey was performed during a period of 6 months from January to June 2014. The information collected by the physicians was not monitored or controlled and was sent to the central statistical center automatically (as aggregate data).
Statistical Analyses
The central statistical center performed a descriptive analysis on the aggregate data. Relative frequencies, expressed as percentages, of qualitative variables and means and standard deviations of quantitative variables were analyzed. All variables were analyzed for the whole population and for the following patient subgroups: patients diagnosed with DVT only (DVT only), patients with PE only (PE only), and patients presenting both DVT and PE (DVT+PE). Chi-Square test, or Fisher exact test when necessary, was used to compare baseline characteristics and risk factors between the 3 groups. Post hoc comparisons were made between the DTV-only group and the others 2 groups, in case of statistical significance. No adjustment for multiple comparisons was applied. Comparisons of study characteristics with others studies were made by χ2 test. One-way analysis of variance was used to compare baseline quantitative factors between the 3 groups. A P value <.05 was considered statistically significant.
Results
Clinical Characteristics
A total of 38 physicians (of 47 who were contacted) agreed to participate and answered the questionnaire. The physicians participating in this survey had the following backgrounds: internal medicine (45.5%), coagulation clinic physicians (37.5%), cardiology (12.0%), and hematology (3.1%). Physicians collected data on a total of 458 patients. The clinical patient characteristics (upon diagnosis) are listed in Table 1.
The thromboembolic events were localized within the right lower leg (12.9% distal part and 41% proximal part) in 53.9% of patients, left lower leg (12.9% distal part and 34.3% proximal part) in 47.2% of patients, and in the pulmonary vessels in 24% of patients.
At the time of diagnosis, 89.3% of patients were symptomatic. The following primary symptoms were reported: local edema (61.4% of patients), leg pain (60.7%), warm sensation (16.6%), dyspnea (16.4%), erythema (13.3%), thoracic pain (6.9%), syncope (2.2%), hemoptysis (0.6%), and other symptoms (3.3%).
In total, 75.3% of patients had experienced symptoms prior to diagnosis: 73.8% in the DVT group, 59.1% in the PE group, and 85.2% in the DVT+PE group. Additionally, 37.7% of DVT cases and 49.1% of PE/DVT+PE cases reported having symptoms for less than 5 days. Furthermore, 21.7% of DVT cases and 19% of PE/DVT+PE cases reported experiencing symptoms 5 to 10 days before their diagnosis. Among these patients, 4.6% of patients with DVT and 2.7% of patients with PE/DVT+PE reported experiencing symptoms for 11 to 15 days before diagnosis, and 4% of patients with DVT and 1.8% of patients with PE/DVT+PE reported experiencing symtoms 16 to 30 days before diagnosis. Finally, 5.5% of patients with DVT and 6.4% of patients with PE/DVT+PE reported experiencing symptoms for more than 30 days.
Risk Factors and Comorbidities
All previous or concomitant pathological conditions and risk factors associated with VTE and reported in the studied population upon diagnosis are represented in Table 2. In total, 11.8% of patients had a known cancer. The majority of patients with cancer (73.7%) were in complete remission, and 26.3% had active cancer. Overall, 1.3% of patients received chemotherapy during the analysis period, and 0.4% had undergone prior chemotherapy.
In total, 14.8% of patients had known thrombophilia (14.9% of patients with DVT, 17% of patients with DVT+PE, and 4.5% of patients with PE). Within patients with thrombophilia, we observed heterozygous mutation for factor V Leiden in 47.1% of cases, G20210A mutation in prothrombin in 19.1%, protein S deficiency in 10.3%, protein C deficiency in 3% of cases, antithrombin III deficiency in 2.9%, and other defects in 17.6% of cases. Overall, 3.5% of patients had experienced a previous bleeding event, 1.75% had experienced intracranial bleeding, and 1.75% had experienced bleeding in other sites.
Diagnosis and Treatment
The diagnosis of VTE was confirmed in the majority of the patients in a specialty department (47.6%) or emergency unit (39.7%). The following diagnostic techniques were used: compressive ultrasound or Doppler ultrasound (in 94.1% of patients and 99% in DVT cases), d-dimer test (32.1%), contrast tomography (18.3%, 90.9% in PE), Wells score20 (4.8%), pulmonary angiography (3.5%), and other methods (1.1%).
Hospitalizations occurred in 46.3% of patients due to VTE. Among these patients, 24.6% were hospitalized for less than 5 days, 46% were hospitalized for 5 to 10 days, and 28.9% were hospitalized for more than 10 days. Analyzing the subgroups, we found that only 33% of patients with DVT only were hospitalized, while 77.3% of patients with PE only and 90.9% of patients with DVT+PE were hospitalized due to VTE.
Before starting the treatment with rivaroxaban, most patients (52.4%) had used an anticoagulant for a mean of 28.1 ± 86.6 days; 27.1% had received low-molecular-weight heparin; 17.9% had received VKA; 1.7% had received unfractionated heparin; and 5.7% had received fondaparinux. The prothrombin time-international normalized ratio (PT-INR) was evaluated before switching to rivaroxaban in patients taking VKA. An average of 1.8 ± 2.2 PT-INR measurements were conducted before changing drugs and the mean PT-INR value before switching was 2.02 ± 0.46. The mean number of days that elapsed between the last PT-INR measurement, and the first administration of rivaroxaban was 3.37 ± 7.8. The major reasons for switching to rivaroxaban therapy are depicted in Figure 1.
The dosages of rivaroxaban prescribed in this survey included the following: 15 mg twice daily for 21 days followed by 20 mg daily (54.4% of patients), 15 mg twice daily for 21 days followed by 15 mg daily (6.5%), 15 mg daily (3.7%), and 20 mg daily (35.4%). The planned length of rivaroxaban treatment was 3 months (in 15.5% of patients), 6 months (28.4%), 12 months (20.3%), or over 12 months (6.9%). In the remaining cases, the length of treatment was undefined.
Some patients treated with rivaroxaban were also receiving other concomitant treatments: 21% were treated with antiplatelet agents (15.4% received aspirin and 4.8% clopidogrel), 8% were treated with nonsteroidal anti-inflammatory drugs, and 4.8% were treated with steroidal drugs.
Discussion
This epidemiological survey of real-life Italian patients diagnosed with VTE and treated with rivaroxaban revealed interesting differences and similarities with respect to the EINSTEIN clinical trials.
The Italian patients had a similar gender distribution (P = .181) and percentage of patients who were recently immobilized, compared to patients enrolled in the EINSTEIN trials. Conversely, the mean age was higher in our patients (62 years vs 57 years in the EINSTEIN-pooled analysis, P < .001).17 In this study, there was also a higher rate of patients with DVT only (75.9% vs 41.7%, P < .001) and a lower rate of patients with PE only (4.8% vs 58.3%, P < .001). There was also a lower rate of patients with recent trauma or surgery (9.2% vs 18.1%, P < .001) compared to the populations in the EINSTEIN-pooled analysis. However, the subgroups had similar characteristics among patients in our study and patients of the EINSTEIN-DVT and EINSTEIN-PE trials.11,12 The primary baseline characteristics in our survey that were also reported in the EINSTEIN-PE trial (gender, immobilization, recent surgery, active cancer, previous VTE, and hospitalization) were similar in both populations. Additionally, when comparing the DVT subgroup between our Italian cohort and the EINSTEIN-DVT trial, the main difference was the percentage of patients with a recent surgery or trauma (6.9% in our DVT population vs 19.5% in the EINSTEIN-DVT population, P < .001).
The DVT rates in our population (75.9%) were higher than in the RLS study (64.1%, P = .013) and in the EINSTEIN-pooled rates (41.7%, P < .001). Furthermore, the percentage of patients affected by PE in the EINSTEIN-pooled (58.3%) trial was higher than the percentage observed in our survey (4.8%). This result may be due to bias resulting from the physicians’ backgrounds (our survey included internal medicine and coagulation clinic physicians). Also, physicians may have preferred rivaroxaban in particular for those patients who did not need hospitalization. The VTE treatment with rivaroxaban does not require an initial therapy with heparin14 and may therefore help lower hospitalization rates in less severe patients.
When analyzing the medical history, it is noteworthy that in our survey the percentages of patients with concomitant diseases, such as diabetes mellitus (19.7%) and hypertension (48.0%), were higher compared to the RLS study (10.7%, P = .032 and 30.1%, P = .001 respectively). However, the percentages of patients with atrial fibrillation and of those with chronic obstructive pulmonary disease were not significantly different between studies (7.9% vs 10.7%, P = .351 and 15.9% vs 5.8%, P = .08, respectively). These results may have been biased by the physicians involved in our survey; the physicians may have preferred administering rivaroxaban to patients affected by VTE and concomitant diseases due to the easier management and lower associated bleeding risk.13,17,21
The most represented possible risk factors for VTE identified in our population included prolonged immobilization, previous hospitalization, previous VTE, and postthrombotic syndrome.22 In our analysis of risk factors, the percentage of patients undergoing recent surgery or trauma was lower in our survey compared to the EINSTEIN-pooled study. However, the immobilization and active cancer rates were similar. The rates of previous VTE were higher in our analysis (30.6% vs 19.1%). These results may be due to the slightly older patients in our study compared to the patients involved in the EINSTEIN study. Age is a reported risk factor for VTE recurrence.23,24
The prevalence of previous bleeding in our study was lower compared to the RLS study (3.4% vs 8%, respectively).18 The most common complication of anticoagulant treatment is bleeding.25 In the RLS study, 71% of patients switched to rivaroxaban treatment after a previous treatment with heparin or VKA. In this analysis, 52.4% of patients were receiving anticoagulants before changing their treatment to rivaroxaban. This difference could have affected the incidence of previous bleeding events.
The majority of patients were symptomatic (89.3%) upon presentation. A higher percentage of patients with DVT (73.8%) were already symptomatic before their diagnosis compared to patients with PE (59.1%). This result is compatible with the finding that PE symptoms are frequently sudden and may require immediate medical assistance.26
In our study, 52.4% patients were in therapy with an anticoagulant (28.8% received heparin, 17.9% VKA, and 5.7% fondaparinux) before switching to rivaroxaban. Conversion from low-molecular-weight heparin to rivaroxaban requires administering the oral anticoagulant when the next heparin dose is due.14 However, conversion from warfarin requires discontinuing the therapy and initiating rivaroxaban on the next day when the PT-INR value is 2.5 or less.14,27 Interestingly, the mean number of PT-INR measurements needed before changing treatment was only 1.8.
The main reasons reported for switching to rivaroxaban included difficulties in VKA management, patient choice, and PT-INR instability. The VTE therapy and prophylaxis have suboptimal compliance in patients long term.28,29 In a recent study, more than 70% of VTE patients with a high risk of recurrence did not comply with warfarin therapy, and more than 50% of patients discontinued warfarin therapy within 1 year.29 These results may be related to difficulties associated with VKA treatment management. Thus, the use of NOACs may overcome some of the limitations associated with conventional VKA treatments and heparin.
Rivaroxaban is an NOAC and can be an alternative to VKA and heparin. Rivaroxaban can be administered orally at a fixed dose and requires limited monitoring. Additionally, rivaroxaban has limited drug interactions, and an initial treatment with heparin is not required.14 The safety and efficacy of this drug have been evaluated in fragile patients, patients with cancer, patients presenting with large clots, and patients with a history of recurrent VTE.17 In this study, more than 30% of patients had a previous VTE. Furthermore, the drug can be administered to patients with chronic kidney disease (creatinine clearance > 15 mL/min). Recent studies confirm the hypothesis that NOACs, such as rivaroxaban, can positively affect patient compliance.18,30 In a study on patients with atrial fibrillation, patient compliance and adherence to therapy were higher in patients taking rivaroxaban compared to warfarin. The rate of therapy discontinuation at 6 months was 18.5% in patients taking rivaroxaban and 31.7% in patients taking warfarin.30
In conclusion, our survey results support the current literature data and confirm the overall similarity of real-life VTE patients, with patients enrolled in the rivaroxaban clinical trials. Therefore, physicians can expect their patients to achieve the same positive outcomes reported in the trials. The limitations of our survey are due to the use of aggregate data and the absence of information monitoring and control. There may also be reporting bias. The main strength of this real-life survey is that the patients studied represent the actual population that will receive rivaroxaban in clinical practice.
Acknowledgments
The authors would like to thank Bayer for providing unrestricted funding for editorial support in the preparation of this manuscript. Ercules Comunicazioni provided the editorial support.
Authors’ Note: eXperience VTE Italian Group: Maria Amitrano (Napoli), Fulvio Barbieri (Romano di Lombardia, BG), Maria Cristina Bertoncelli (Vercelli), Eugenio Bucherini (Faenza), Umberto Carini (Grosseto), Franco Carmassi (Pisa), Maurizio Cassol (Roma), Attilio Castellaneta (Roma), Sergio Castellani (Firenze), Roberto Catalini (Macerata), Alberto Cogo (Vicenza), Egidio De Gaudenzi (Domodossola), Biagio De Simone (Napoli), Luigi Di Pino (Catania), Michelangelo Di Salvo (Catania), Gabriele Giordano (Acerra, NA), Antonio Lillo (Taranto), Annamaria Margarita (Napoli), Daniela Mastroiacovo (Avezzano, AQ), Antonino Mazzone (Legnano, MI), Pierluigi Edgard Mollo (Frosinone), Fernando Parente (Lecce), Rocco Paternò (Potenza), Fulvio Pomero (Cuneo), Vincenzo Prisco (Mercato San Severino, SA), Pietro Luigi Pujatti (Arzignano, VI), Stefano Radicchia (Perugia), Doda Renzetti (Bari), Carlo Sabbà (Bari), Rita Carlotta Santoro (Catanzaro), Mauro Scanferlato (San Donà di Piave, VE), Piera Sivera (Torino), Piero Sotgiu (Pompei, NA), Eros Tiraferri (Rimini), Maurizio Tonizzo (San Vito al Tagliamento, PN), Antonietta Vanini (Cona, FE), Antonio Versace (Messina), Marco Zaramella (Latisana, UD).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Lindblad B, Sternby NH, Bergqvist D. Incidence of venous thromboembolism verified by necropsy over 30 years. BMJ. 1991;302(6778):709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112(2):255–263. [DOI] [PubMed] [Google Scholar]
- 3. Prandoni P, Lensing AW, Büller HR, et al. Comparison of subcutaneous low-molecular-weight heparin with intravenous standard heparin in proximal deep-vein thrombosis. Lancet. 1992;339(8791):441–445. [DOI] [PubMed] [Google Scholar]
- 4. Columbus Investigators, Büller HR, Gent M, et al. Low-molecular-weight heparin in the treatment of patients with venous thromboembolism. The Columbus Investigators. N Engl J Med. 1997;337(10):657–662. [DOI] [PubMed] [Google Scholar]
- 5. Guyatt GH, Akl EA, Crowther M, et al. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):7S–47S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2014;141(suppl 2):e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geldhof V, Vandenbriele C, Verhamme P, Vanassche T. Venous thromboembolism in the elderly: efficacy and safety of non-VKA oral anticoagulants. Thromb J. 2014;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devabhakthuni S, Yoon CH, Pincus KJ. Review of the target-specific oral anticoagulants in development for the treatment and prevention of venous thromboembolism. J Pharm Pract. 2016;29(4):392–405. [DOI] [PubMed] [Google Scholar]
- 9. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361(24):2342–2352. [DOI] [PubMed] [Google Scholar]
- 10. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369(9):799–808. [DOI] [PubMed] [Google Scholar]
- 11. Büller HR, Prins MH, Lensin AWA, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366(14):1287–1297. [DOI] [PubMed] [Google Scholar]
- 12. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499–2510. [DOI] [PubMed] [Google Scholar]
- 13. Bacchus F, Schulman S. Clinical experience with the new oral anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol. 2015;35(3):513–519. [DOI] [PubMed] [Google Scholar]
- 14. European Medicines Agency. Xarelto Summary of Product Characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed May 20, 2016.
- 15. Turpie AGG. Advances in oral anticoagulation treatment: the safety and efficacy of rivaroxaban in the prevention and treatment of thromboembolism. Ther Adv Hematol. 2012;3(5):309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tripodi A, Palareti G. New anticoagulant drugs for treatment of venous thromboembolism and stroke prevention in atrial fibrillation. J Intern Med. 2012;271(6):554–565. [DOI] [PubMed] [Google Scholar]
- 17. Prins MH, Lensing AW, Bauersachs R, et al. Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;1(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jara-Palomares L, Sanchez-Oro-Gomez R, Elias-Hernandez T, et al. Rivaroxaban for the treatment of venous thromboembolism. A ‘real-life’ perspective in 103 patients. Thromb Res. 2014;134(3):617–621. [DOI] [PubMed] [Google Scholar]
- 19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]
- 20. Geersing GJ, Zuithoff NPA, Kearon C, et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ. 2014;348:g1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeh CH, Hogg K, Weitz JI. Overview of the new oral anticoagulants: opportunities and challenges. Arterioscler Thromb Vasc Biol. 2015;35(5):1056–1065. [DOI] [PubMed] [Google Scholar]
- 22. Konstantinides S, Torbicki A, Agnelli G, et al. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) * Endorsed by the European Respiratory Society (ERS). Eur Heart J. 2014;35(43):3033–3369.25173341 [Google Scholar]
- 23. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199–205. [DOI] [PubMed] [Google Scholar]
- 24. Streiff MB. Predicting the risk of recurrent venous thromboembolism (VTE). J Thromb Thrombolysis. 2015;39(3):353–366. [DOI] [PubMed] [Google Scholar]
- 25. Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Lancet. 1996;348(9025):423–428. [DOI] [PubMed] [Google Scholar]
- 26. Bajaj N, Bozarth AL, Guillot J, et al. Clinical features in patients with pulmonary embolism at a community hospital: analysis of 4 years of data. J Thromb Thrombolysis. 2014;37(3):287–292. [DOI] [PubMed] [Google Scholar]
- 27. Tran H, Joseph J, Young L, et al. New oral anticoagulants: a practical guide on prescription, laboratory testing and peri-procedural/bleeding management. Intern Med J. 2014;44(6):525–536. [DOI] [PubMed] [Google Scholar]
- 28. Kahn SR, Springmann V, Schulman S, et al. Management and adherence to VTE treatment guidelines in a national prospective cohort study in the Canadian outpatient setting. The Recovery Study. Thromb Haemost 2012;108(3):493–498. [DOI] [PubMed] [Google Scholar]
- 29. Chen SY, Wu N, Gulseth M, et al. One-year adherence to warfarin treatment for venous thromboembolism in high-risk patients and its association with long-term risk of recurrent events. J Manag Care Pharm. 2013:19(4):291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laliberté F, Cloutier M, Nelson WW, et al. Real-world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30(7):1317–1325. [DOI] [PubMed] [Google Scholar]


