Abstract
Weight-based, nurse-driven heparin nomograms are reported in the medical literature to improve the time it takes to reach a minimum threshold for anticoagulation without compromising patient safety in specific indications or patient populations. This is the first report in the literature of an institution-wide protocol implementation and evaluation of effectiveness with simultaneous transition to an electronic health record. The purpose of implementing this practice change at our institution was to standardize practice, improve time to reach therapeutic anticoagulation, and improve patient safety. We conducted a retrospective analysis utilizing a pre/postimplementation design to compare outcomes. The primary end point evaluated was the time to reach minimum threshold value for therapeutic anticoagulation. Additionally, we assessed the percentage of patients who reached minimum threshold therapeutic anticoagulation within 24 hours, the percentage of patients with a critically supratherapeutic activated partial thromboplastin time (aPTT) value (≥120 seconds) during therapy, and a description of heparin titration for the first 4 aPTT results with nomogram use. Overall time to therapeutic anticoagulation decreased from a mean 18.7 to 11.7 hours (hazard ratio [HR] 1.59; 95% confidence interval 1.22-2.08; P < .0005). Percentage of patients receiving therapeutic anticoagulation within 24 hours increased from 74.4 to 88.5 (odds ratio [OR 2.97, P = .002) and the percentage of patients with an aPTT ≥120 seconds remained constant at 49.9 versus 46.8 (OR 0.92, P = .73). This practice change reduced time to therapeutic anticoagulation without an increase in the proportion of patients with a critically supratherapeutic aPTT at our institution.
Keywords: heparin, nomogram, anticoagulation, titration
Background
Unfractionated heparin (UFH) is a parenterally administered anticoagulant commonly utilized in the acutely ill medical or surgical inpatient population. UFH is often preferred in this setting for its quick onset of action and reversibility in the setting of bleeding or need for invasive procedures. A standard treatment option for both treatment of active thromboembolic processes and prophylaxis against such, UFH is utilized at our institution for acute venous thromboembolism (VTE), acute coronary syndrome (ACS), and stroke prevention in atrial fibrillation, among other indications. Between institutions, however, there is no standardized method for titrating UFH. Nurse-driven protocols have been utilized to titrate UFH to achieve therapeutic anticoagulation with the first successful report in 1993.1 Since then, others have published their experiences with this titration scheme in various patient populations.2,3 With the recent rapid growth of electronic health record (EHR) use and expected continued rise, institutions will be tasked with incorporating such programs into their practice and documentation.4 Recently, our institution adopted a new EHR, and a major practice change was implemented transitioning from a nonstandardized, provider-driven practice to an institution-wide, nurse-driven, weight-based nomogram for continuous infusion UFH administrations.
Unfractionated heparin is typically monitored for safety and efficacy via the activated partial thromboplastin time (aPTT).5,6 The aPTT is performed by adding surface activator and partial thromboplastin to a plasma sample and measuring the clotting time.7 The test is limited in that clotting times are variable and subject to different reagents and can be prolonged due to factor deficiencies and the presence of inhibitors. Measuring the anti-Xa activity is an alternative test that is standardized to a therapeutic range of 0.3 to 0.7 IU/mL but does not reflect the full anticoagulant effects of UFH.8 While each test has its limitations, data have shown that targeting an aPTT goal value of at least 1.5 times the baseline value with UFH significantly reduces recurrence of VTE during treatment.9 Reaching a threshold lower limit of the goal aPTT range within 24 hours reduces the frequency of VTE recurrence in patients treated with UFH.10 Achieving therapeutic aPTT values within 12 to 24 hours when treating ACS is associated with improved outcomes including fewer reinfarctions.11 Supratherapeutic aPTT values resulting from high UFH doses are associated with major bleeding.12 Due to its narrow therapeutic range, UFH must be titrated to maintain efficacy while preventing harm associated with bleeding. The short half-life of UFH facilitates the titratability of the drug.5
Traditionally, UFH dosing for VTE and ACS included a bolus and infusion of a fixed rate of heparin. For example, some references have recommended an initial intravenous bolus of 5000 units followed by an infusion of 24 000 to 32 000 units per 24 hours although practices varied widely.5,13 Adjusting the UFH dose based on weight and utilizing a standard nomogram for dose adjustment decreases the time it takes to reach therapeutic anticoagulation and restores therapeutic aPTT from subtherapeutic aPTT values more rapidly, which is extrapolated to result in better patient outcomes.1,14,15 Nomograms can be utilized by nurses to titrate heparin resulting in similar or faster times to reach therapeutic anticoagulation compared to a more traditional provider-driven approach.2,16 Pharmacist involvement and the use of technology in heparin monitoring have also been shown to improve the time it takes to reach therapeutic goals.17–19 At our institution, all of these strategies are utilized to improve our time to reach therapeutic anticoagulation and monitor for patient safety. Here we describe our experience with the implementation of our nomograms and comparison with our previous traditional provider-driven approach.
The impetus behind implementing this change at our institution was to standardize practice, improve time to reach therapeutic anticoagulation, and improve patient safety. The purpose of this article is to describe our practice change to a weight-based, nurse-driven approach for UFH management on a full institutional scale in the setting of simultaneous transition to a new EHR and our evaluation of whether the goals for this implementation have been met.
Program Description
The setting of this practice change is institution-wide at the Brigham and Women’s Hospital (BWH), a 793-bed tertiary academic medical center and teaching affiliate of Harvard Medical School, both located in Boston, Massachusetts. The aPTT assay using a silica activator (PTT Automate; Diagnostica Stago Inc, Parsippany, New Jersey) run on the STA-R Evolution (Diagnostica Stago Inc) is utilized for adult patients in whom therapeutic anticoagulation targeting either a goal aPTT of 50 to 70 seconds or 60 to 80 seconds with bolus dosing was felt to be appropriate by the patient’s providers. At BWH, UFH is managed and monitored through collaborative multidisciplinary teams, including providers, pharmacists, and nurses. In order to standardize practice for UFH administration, institution-wide use of 2 noncustomizable UFH nomograms targeting specific aPTT ranges has been adopted (Supplementary Appendix 1). These nomograms are approved for use in patients with an acute pulmonary embolism (PE) or deep venous thrombosis (DVT) targeting an aPTT range of 60 to 80 seconds, patients with acute ACS targeting an aPTT range of 50 to 70 seconds, and patients with a target range of 50 to 70 seconds or 60 to 80 seconds outside the previously mentioned indications where the risk to benefit analysis is deemed appropriate. For patients whose clinical indications are not appropriate for either of the nomograms, a custom weight-based infusion may be utilized with provider-guided titration. An “as-needed” aPTT order for laboratory draws is built into the UFH order set for patients ordered for the nomogram, allowing the nurse to obtain an appropriately timed aPTT per protocol based on the time of the last titration. Appropriate timing specified in the protocol is 6 hours after any heparin dose adjustment, and every 6 hours until 2 consecutive aPTT results are within the therapeutic goal range, at which point aPTT draws are extended out to every 12 hours. After the aPTT value results, the nurse titrates the UFH dose per the nomogram. Clinicians have the option of utilizing an initial UFH bolus when starting therapy (80 units per kg or 60 units per kg for the 60- to 80-second goal and 50- to 70-second goal nomograms, respectively). Bolus doses used as part of the titration nomogram for subtherapeutic aPTT values are noncustomizable and cannot be removed from the order set. Unit-based clinical pharmacists and unit-based nurse educators monitor UFH daily through use of the EHR, daily reports, and a computerized clinical surveillance system.20
Clinical content for the nomograms was developed through a collaborative effort between physicians, pharmacists, nurses, and informatics professionals from all Partners HealthCare institutions, including BWH and Partners eCare. Implementation of the standard UFH nomograms took place with the rollout of a new EHR. To prepare for this change, significant resources were dedicated to multidisciplinary staff education with a major focus on nursing for 6 months leading up to the transition and continued thereafter. Informatics teams and EHR builders worked to ensure intuitive user interfaces. Additional interventions included a mandatory online learning module created for nursing, updated smart infusion pump libraries, and unit-based nursing education. On the day of implementation, safety checklists were completed by teams of nurses and pharmacists to insure the correct conversion was made for each patient. Following implementation, members of the conversion team reviewed the records of patients on UFH continuous infusions daily to ensure the nomogram was utilized correctly and to provide one-on-one education to nursing staff on the new protocol. The review of practice continues daily with new staff or in areas where the nomogram is less frequently ordered.
Methods
In order to evaluate the nomogram implementation, we utilized a pre- versus post-design to compare the elapsed time to reach a minimum threshold anticoagulation defined as the lower limit of the therapeutic goal range (aPTT value of 50 or 60 seconds depending on nomogram selection). This was a retrospective evaluation comparing internal quality assurance data (two 3-month periods from September through November 2013 and 2014) to a group of patients receiving therapeutic anticoagulation with our heparin nomograms during a predefined time period (September 2015). This study period was chosen to allow for a 3-month wash-in period after nomogram implementation to account for staff acclimation. Secondary end points included the percentage of patients receiving therapeutic anticoagulation within 24 hours of UFH and the percentage of patients with an aPTT value ≥120 seconds while receiving UFH. Additionally, we included a descriptive evaluation of the average first 4 aPTT values while patients were receiving nomogram-titrated UFH.
Patients were eligible for inclusion if they were receiving a continuous infusion of UFH during the study time periods. Patients were excluded if they received UFH for less than 24 hours, if UFH was initiated at an outside hospital, if nonnomogram provider-driven UFH titration was used (post-nomogram), or if UFH was used without a specified aPTT goal (pre-nomogram).
Comparing our pre- and post-implementation groups, we performed a power analysis demonstrating 93% power to detect a 30% change in our primary end point with an α of .05. For time-to-event data, the log-rank test was used to determine a hazard ratio using the Kaplan-Meier method to develop a survival curve. Chi-square test was used for categorical and student t test for continuous parametric data analysis. Descriptive statistics were utilized to describe the heparin titration during the first 4 aPTT values in the postnomogram population.
Results
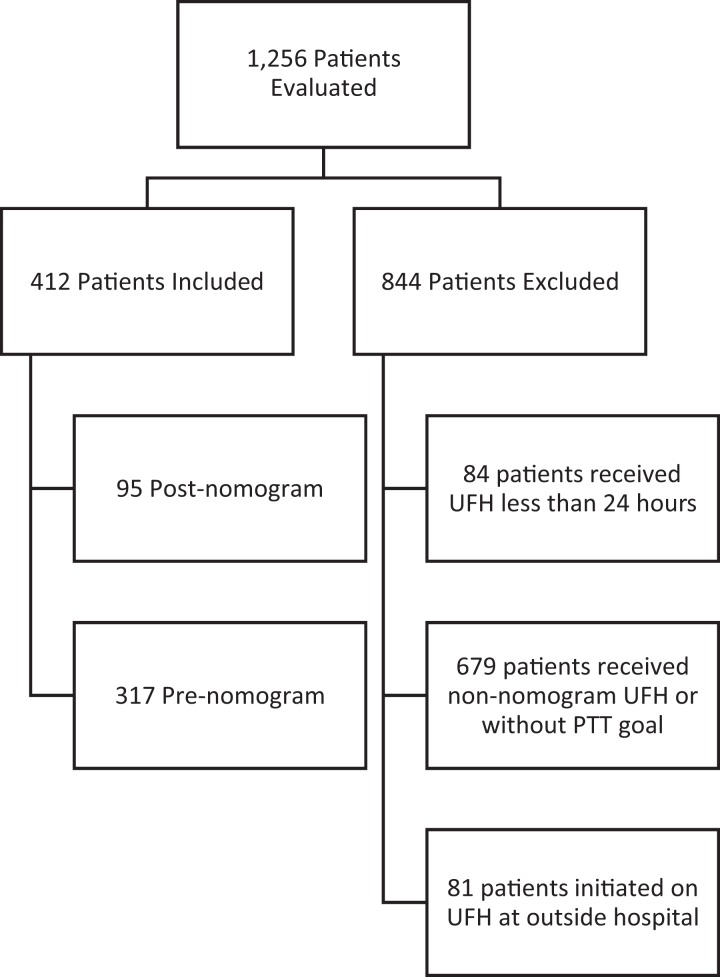
A total of 1255 patient records were evaluated for inclusion (Figure 1). Of these, 844 were excluded and 411 were included (317 patients in the pre-nomogram group and 95 patients in the post-nomogram group). The disparity in numbers is explained by the wide time period chosen for the pre-nomogram group to have a comparator group reflective of multiple periods of practice. Baseline characteristics and indications for therapeutic anticoagulation with heparin are listed in Table 1. Venous thromboembolism was more common among the post-nomogram group, while stroke prevention in atrial fibrillation was more common in the pre-nomogram population. The pre-nomogram population had a higher proportion of surgical patients including cardiac and neurosurgery in which providers felt bolus dosing associated with each nomogram would not be appropriate and therefore opted to use the customized provider-driven infusion rather than the nomogram, resulting in underrepresentation of these populations.
Figure 1.
Patient inclusion flowchart.
Table 1.
Baseline Characteristics.a
| Pre-nomogram (N = 317) | Post-nomogram (N = 95) | P Value | |
|---|---|---|---|
| Male | 197 (62.1) | 56 (58.9) | .574 |
| Age, years | 66.4 ± 28.5 | 66.9 ± 14.3 | .869 |
| Weight, (kg | 79.7 ± 20.6 | 85.8 ± 26.5 | .019 |
| Ethnicity | |||
| White | 264 (83.3) | 78 (82.1) | .785 |
| Black | 31 (9.8) | 6 (6.3) | .296 |
| Other | 22 (7.0) | 11 (11.6) | .149 |
| 50-70 s Nomogram Goal | N/A | 34 (35.8) | - |
| 60-80 s Nomogram Goal | N/A | 61 (64.2) | - |
| Indication | |||
| VTE | 70 (22.1) | 36 (37.9) | .002 |
| Atrial Fibrillation | 120 (37.9) | 24 (25.3) | .024 |
| ACS | 38 (12.0) | 22 (23.2) | .007 |
| Cancer-related thrombosis | 32 (10.1) | 4 (4.2) | .101 |
| Stroke | 9 (2.8) | 1 (1.1) | .413 |
| Other | 48 (15.1) | 8 (8.4) | .095 |
Abbreviations: ACS, acute coronary syndrome; N/A, not applicable; SD, standard deviation; VTE, venous thromboembolism.
aData presented as mean ± SD or number (%)
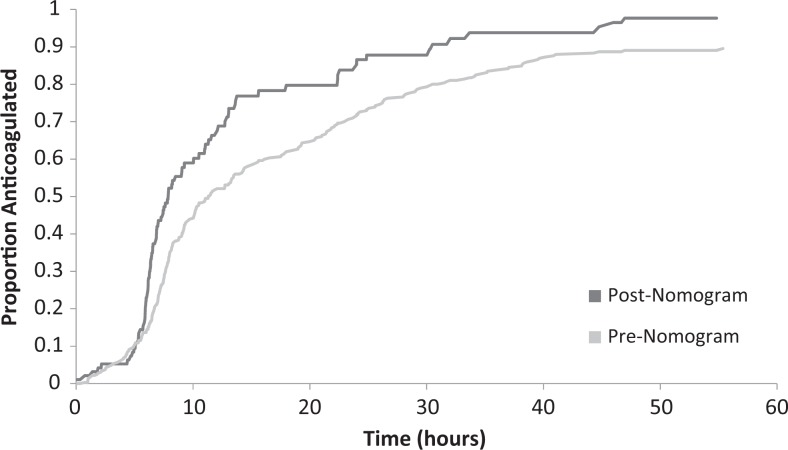
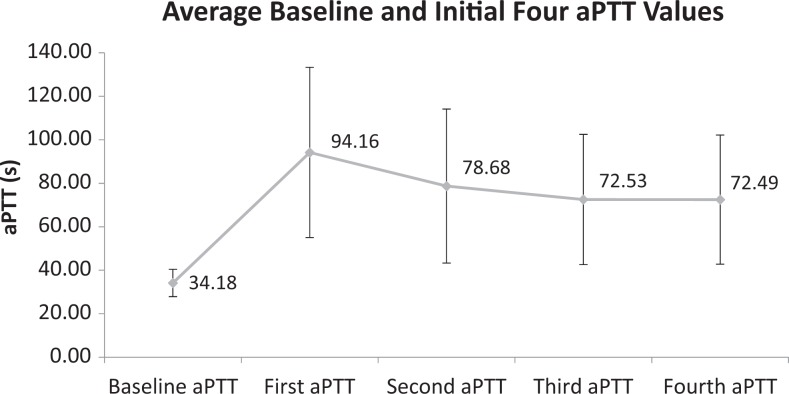
Primary and secondary outcomes can be seen in Table 2. Comparison of the pre-nomogram and post-nomogram groups revealed an improvement in the time to reach threshold therapeutic anticoagulation with a decrease in average time from 18.7 to 11.7 hours (hazard ratio: 1.59; 95% confidence interval 1.22-2.08; P < .0005; Figure 2). A statistically significant increase in the percentage of patients therapeutically anticoagulated at 24 hours from 74.4% to 88.5% was observed in the postnomogram group (P < .0005). No difference was observed in the percentage of patients with an aPTT value ≥120 seconds while receiving UFH with 49.2% in the prenomogram group and 46.8% in the postnomogram group (P = .73). The average baseline aPTT value was 34.18 ± 6.27 seconds in the postnomogram group. To evaluate the success of titration, the first 4 correctly drawn aPTT values (representing 6, 12, 18, and 24 hours [±1 hour]) after UFH initiation were collected in the post-nomogram group with the titration seen in Figure 3. On average, the first aPTT drawn after UFH initiation was slightly supratherapeutic; aPTT then came down into range by the second draw. Supplementary Appendix 2 demonstrates the time required for the UFH nomogram titration to bring a patient into therapeutic goal range with 69% of patients having achieved therapeutic anticoagulation within the goal aPTT range by the fourth aPTT draw. Overall nomogram compliance at our institution was 84.6%, and a more thorough description will be published separately. No major or fatal bleeding events were noted for patients anticoagulated on UFH during the nomogram period.
Table 2.
Program Evaluation Outcomes.a
| Prenomogram (N = 317) | Postnomogram (N = 95) | Hazard Ratio/Odds Ratio | P Value | |
|---|---|---|---|---|
| Primary end point | ||||
| Time to therapeutic (hours) | 18.7 ± 18.6 | 11.7 ± 11.9 | 1.59 | <.0005 |
| Secondary end points | ||||
| Percentage therapeutic at 24 hours | 236 (74.4) | 84 (88.5) | 2.97 | .002 |
| Percentage of patients with PTT ≥120 secondsb | 156 (49.3) | 45 (46.8) | 0.92 | .73 |
aData presented as mean ± SD or as number (%).
bExcluding improperly drawn PTT after initial heparin bolus.
Figure 2.
Kaplan-Meier curve for proportion of patients reaching therapeutic anticoagulation.
Figure 3.
Heparin titration utilizing the Partners eCare Heparin Nomograms used at Brigham and Women’s Hospital (BWH; Data presented as mean value with error bars representing the standard deviation).
Discussion
Heparin has been identified as a high-risk medication by the Joint Commission, and many steps have been taken at our institution to improve patient safety when using this drug, including the use of smart infusion pumps.3,21 The use of computerized surveillance for heparin monitoring by pharmacists has also proven effective.20 As many health systems continue to adopt EHRs, challenges and opportunities arise with management of high-risk infusions such as UFH. With the continued goal of improving patient safety, we adopted a weight-based, nurse-driven strategy for therapeutic anticoagulation. The outcomes in our study are measured as laboratory values serving as surrogate markers for safety and efficacy. Supra- and subtherapeutic aPTT values have been associated with bleeding and thrombosis, respectively, justifying this decision.10,12
From our experience, 2 salient points arise in that a weight-based, nurse-driven UFH nomogram can be successfully and safely implemented on a full institutional scale, and it can be accomplished during the simultaneous adoption of a new enterprise-wide EHR. Numerous reports of weight-based, nurse-driven UFH nomograms exist in the literature, but to our knowledge, this is the first report of an institution-wide adoption occurring with the simultaneous implementation of an enterprise-wide EHR.2,16 We hope to set the standard for other institutions to implement their own institution-specific nomograms with the confidence that it can be successfully accomplished on a full hospital scale. Through a collaborative effort between medical, nursing, and pharmacy departments, the Partners eCare nomograms have accomplished the specified goal of decreasing the time to reach therapeutic anticoagulation while incurring no increase in the number of supratherapeutic aPTT values.
Utilizing a pre- versus poststudy design allowed us to directly compare practice strategies evaluating whether the goal of improving practice through standardization had been accomplished. Our pre-nomogram patient population consisted of patients whose records were identified through internal quality assurance data. Furthermore, we chose to allow for a 3-month wash-in period after nomogram and EHR implementation to allow for staff acclimation to the new practice approach. Despite a great deal of preparation, this was a challenging transition that required ongoing education by nursing informatics and pharmacist teams. We believe the wash-in period was appropriate to account for these difficulties, but there is a continued need for ongoing education and intervention more than 1 year out from implementation.
Raschke and colleagues reported on their success with a weight-based UFH titration nomogram in 1993, setting the standard against which others would be compared.1 In their protocol, nurses were instructed to obtain aPTT laboratory values at prespecified times and adjust UFH infusion rates based on these results. Similarly, our protocol also allows nurses to obtain aPTT laboratory values at appropriate times and adjust infusion doses. Both studies had similar outcomes reaching a faster time to therapeutic anticoagulation with a greater percentage of patients anticoagulated within 24 hours. Additionally, assessing how well the nomogram brings patients into therapeutic goal range, Raschke et al demonstrated an average time to reach an aPTT value within range was 14.1 hours with use of the nomogram, which would approximate either the second or third aPTT value drawn. Similarly, in our population, 23.2% of patients were in range by the first aPTT laboratory value, 35.8% by the second aPTT value, and 53.7% by the third.
Nurse-titrated nomograms have also been reported by Williams and Brown to show equally fast times to therapeutic anticoagulation in critically ill populations.2,16 Our study included both critically ill and noncritically ill patients without differentiating due to the challenge of accounting for transitioning care between patient care areas. While practices may have differed between these populations, our study inclusion makes the results generalizable institution wide. Additionally, we felt it prudent to exclude patients initiated on UFH at an outside institution, as this may have confounded our results due to differing practice approaches. Many previous studies set the standard of utilizing the percentage of patients receiving therapeutic anticoagulation at 24 hours as an end point; we therefore excluded patients receiving therapy for less than this time period.1,22
In analyzing their experience with a nonweight-based heparin titration nomogram, Hull and colleagues describe supratherapeutic aPTT values that persisted for 24 hours or more in 24% of their patients. However, they did not find these patients to be at higher risk of bleeding complications than those who did not have supratherapeutic aPTTs.23 This in combination with evidence that shows increased VTE with subtherapeutic aPTTs may suggest that it is better to be initially supratherapeutic than subtherapeutic.24 In our study, the average first aPTT value was 94.16 seconds, somewhat above the therapeutic goal ranges. Only 7.3% of patients had supratherapeutic aPTT values that persisted for at least 24 hours, a dramatic decrease from the rate seen in the analysis by Hull.23 Overall, 17.9% and 58.9% of patients had subtherapeutic and supratherapeutic first aPTT values, respectively. Interestingly, the average weight of our postgroup was higher than the pregroup. This may have resulted in the postgroup receiving higher UFH doses, although the pregroup was not utilizing weight-based dosing as a standard. There were no instances of major bleeding as defined by the Society for Thrombosis and Hemostasis in our nomogram group despite many patients having supratherapeutic aPTT values.
Our study is limited by its retrospective nature, potential inaccuracy of documentation, and inability to account for provider-driven noncompliance with the nomogram. We attempted to control for this by excluding patients whose titration nomograms had been altered in the EHR, considering them to be nonnomogram UFH use. However, changes made at the bedside that were not documented could not be accounted for. While our study had adequate power to detect a difference in the primary end point, the uneven pre- and postimplementation group numbers represent a confounding factor for analysis. Using a pre- versus postdesign was also a limitation in that our control group was not a true comparator and could be subject to chronological bias and patient differences. Differences in our baseline populations were observed regarding indications for UFH reflecting higher rates of VTE and ACS as well as a lower rate of stroke prevention in atrial fibrillation in the nomogram group. Use of a lower aPTT goal for certain indications as determined by the ordering provider could also lead to not reaching therapeutic anticoagulation as fast. Additionally, we did not differentiate between critically and noncritically ill patients, potentially confounding the data. Although we set out to standardize practice, we did not achieve full adoption as evidenced by numerous services opting to use the provider-driven custom infusion approach to therapy.
Ultimately, implementation of a weight-based, nurse-driven nomogram resulted in faster times to reach therapeutic anticoagulation, without resulting in more patients with critically high aPTT values. These results suggest that institution-wide UFH nomograms can be implemented simultaneously with a new EHR when there is a dedicated multidisciplinary team to lead the implementation, significant education, and close monitoring of practice during the implementation. All of these factors are critical to the success of the practice and will help ensure improved patient care and safety.
Acknowledgments
The authors would like to acknowledge Partners eCare for their work in implementing the order sets for the heparin nomogram at Brigham and Women’s Hospital.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jean Connors: reports consulting for Bristol Meyer Squibb and serving on Scientific Advisory Boards for Bristol Meyer Squibb and Boehringer Ingleheim.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplementary material is available for this article online.
References
- 1. Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119(9):874–881. [DOI] [PubMed] [Google Scholar]
- 2. Brown G, Dodek P. An evaluation of empiric vs. nomogram-based dosing of heparin in an intensive care unit. Crit Care Med. 1997;25(9):1534–1538. [DOI] [PubMed] [Google Scholar]
- 3. Shalansky KF, FitzGerald JM, Sunderji R, et al. Comparison of a weight-based heparin nomogram with traditional heparin dosing to achieve therapeutic anticoagulation. Pharmacotherapy. 1996;16(6):1076–1084. [PubMed] [Google Scholar]
- 4. Abraham S. Technological trends in health care: electronic health record. Health Care Manag (Frederick). 2010;29(4):318–323. [DOI] [PubMed] [Google Scholar]
- 5. Hirsh J, Dalen JE, Deykin D, Poller L. Heparin: mechanism of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest. 1992;102(suppl 4):337S–351S. [DOI] [PubMed] [Google Scholar]
- 6. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(suppl 1):64S–94S. [DOI] [PubMed] [Google Scholar]
- 7. Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112(4):e53–e60. [DOI] [PubMed] [Google Scholar]
- 8. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013;139(4):450–456. [DOI] [PubMed] [Google Scholar]
- 9. Basu D, Gallus A, Hirsh J, Cade J. A prospective study of the value of monitoring heparin treatment with the activated partial thromboplastin time. N Engl J Med. 1972;287(7):324–327. [DOI] [PubMed] [Google Scholar]
- 10. Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. Relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157(22):2562–2568. [PubMed] [Google Scholar]
- 11. Granger CB, Hirsch J, Califf RM, et al. Activated partial thromboplastin time and outcome after thrombolytic therapy for acute myocardial infarction: results from the GUSTO-I trial. Circulation. 1996;93(5):870–878. [DOI] [PubMed] [Google Scholar]
- 12. Morabia A. Heparin doses and major bleedings. Lancet. 1986;1(8492):1278–1279. [DOI] [PubMed] [Google Scholar]
- 13. Reilly BM, Raschke R, Srinivas S, Nieman T. Intravenous heparin dosing: patterns and variations in internists’ practices. J Gen Intern Med. 1993;8(10):536–542. [DOI] [PubMed] [Google Scholar]
- 14. Cruickshank MK, Levine MN, Hirsh J, Roberts R, Siguenza M. A standard heparin nomogram for the management of heparin therapy. Arch Intern Med. 1991;151(2):333–337. [PubMed] [Google Scholar]
- 15. Jaff MR, Olin JW, Piedmonte M, Pirzada C, Young JR. Heparin administration via nomogram versus a standard approach in venous and arterial thromboembolic disease. Vasc Med. 1996;1(2):97–101. [DOI] [PubMed] [Google Scholar]
- 16. Williams TD, Sullivan K, Lacey C, Adoryan S, Watts B. Nurse-driven intravenous heparin protocol: quality improvement initiative. AACN Adv Crit Care. 2010;21(2):152–161. [DOI] [PubMed] [Google Scholar]
- 17. Rivey MP, Peterson JP. Pharmacy-managed, weight-based heparin protocol. Am J Hosp Pharm. 1993;50(2):279–284. [PubMed] [Google Scholar]
- 18. Oyen LJ, Nishimura RA, Ou NN, Armon JJ, Zhou M. Effectiveness of a computerized system for intravenous heparin administration: using information technology to improve patient care and patient safety. Am Heart Hosp J. 2005;3(2):75–81. [DOI] [PubMed] [Google Scholar]
- 19. Saya FG, Coleman LT, Martinoff JT. Pharmacist-directed heparin therapy using a standard dosing and monitoring protocol. Am J Hosp Pharm. 1985;42(9):1965–1969. [PubMed] [Google Scholar]
- 20. Hohlfelder B, Stashek C, Anger KE, Szumita PM. Utilization of a pharmacy clinical surveillance system for pharmacist alerting and communication at a tertiary academic medical center. J Med Syst. 2016;40(1):24. [DOI] [PubMed] [Google Scholar]
- 21. Wilson K, Sullivan M. Preventing medication errors with smart infusion technology. Am J Health Syst Pharm. 2004;61(2):177–183. [DOI] [PubMed] [Google Scholar]
- 22. Rothschild JM, Keohane CA, Cook EF, et al. A controlled trial of smart infusion pumps to improve medication safety in critically ill patients. Crit Care Med. 2005;33(3):533–540. [DOI] [PubMed] [Google Scholar]
- 23. Hull RD, Raskob GE, Rosenbloom D, et al. Optimal therapeutic level of heparin therapy in patients with venous thrombosis. Arch Intern Med. 1992;152(8):1589–1595. [PubMed] [Google Scholar]
- 24. Hull RD, Raskob GE, Hirsh J, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315(18):1109–1114. [DOI] [PubMed] [Google Scholar]