Abstract
Monitoring of direct thrombin inhibitors (DTIs) in patients with heparin-induced thrombocytopenia (HIT) is primarily performed using the activated partial thromboplastin time (aPTT). This assay is poorly standardized, reagent dependent, and not DTI specific. We compared aPTT, thrombin time (TT), and prothrombin time (PT) to drug levels obtained by the ecarin chromogenic assay (ECA). We analyzed 495 samples of patients with confirmed or suspected HIT on treatment with either argatroban (n = 37) or lepirudin (n = 80). Mean DTI levels ± standard deviation (SD) were 0.41 ± 0.36 µg/mL for argatroban and 0.20 ± 0.21 µg/mL for lepirudin. Results of aPTT were highly variable: 67 ± 22 seconds for argatroban and 55 ± 20 seconds for lepirudin. Significant correlations (P < .01) were found between ECA-based DTI level and TT (argatroban, r = .820 and lepirudin, r = .830), PT (argatroban, r = −.544), and aPTT (lepirudin, r = .572). However, there was no correlation of aPTT with argatroban or PT with lepirudin concentration. Multiple regression analyses revealed that the TT predicted 54% of argatroban and 42% of lepirudin levels, but no significant impact was seen for PT or aPTT. The aPTT-guided monitoring of DTI therapy leads to a high percentage of patients with inaccurate plasma levels, hence resulting to either undertreatment or overtreatment. Knowledge of baseline values prior to DTI therapy and inclusion of clinical settings are essential for dosing DTIs when using aPTT. However, due to several limitations of aPTT, monitoring according to exact plasma concentrations as obtained by specific tests such as ECA may be more appropriate.
Keywords: monitoring direct thrombin inhibitors, heparin-induced thrombocytopenia, ecarin chromogenic assay
Introduction
Heparin-induced thrombocytopenia (HIT) type II is a serious adverse event of heparin, induced by formation of antibodies against the platelet factor 4/heparin complex. These antibodies, specifically those of the subclass immunoglobulin G, cause thrombocytopenia and can activate platelets via interaction with the Fc-γ-RIIA receptor, with or without thrombosis.1 Patients with HIT have a 30% to 50% risk of thrombosis within the subsequent 28 days.2 Therefore, affected patients require intensive anticoagulation with alternative anticoagulants. Besides danaparoid, the direct thrombin inhibitors (DTIs) argatroban and lepirudin are licensed for anticoagulation in patients with HIT (lepirudin was retrieved from the market in 2012, which did not base on safety considerations). Moreover, desirudin, a hirudin variant, and bivalirudin, a synthetic thrombin inhibitor, have been used for the treatment of patients with HIT. The DTIs inhibit thrombin very efficiently by forming reversible (argatroban) or almost irreversible complexes (hirudin) and have therefore a relatively small therapeutic window. The potential impairment of the regulatory protein C pathway triggered by thrombomodulin-bound thrombin, the inhibition of clot-bound thrombin, or the influence on other activities of the multifunctional enzyme thrombin must be taken into consideration. Today, fondaparinux is also frequently used (“off label”) for anticoagulation in patients with HIT.3
Monitoring is required when using DTIs, preferably with specific assays because there are marked differences in the ability of DTIs to prolongate standard coagulation tests, including prothrombin time (PT), activated partial thromboplastin time (aPTT), or thrombin time (TT). For monitoring DTI therapy, these assays are not well standardized, have only a limited measuring range, do not always display a linear dose–response relationship, and may even show a plateau at higher concentrations (aPTT) influenced by various determinants other than the plasma level of the drug.4 For aPTT, lupus anticoagulant and coagulation factor sensitivity and instruments used for clot detection contribute to variability, an issue that is being well known for heparin monitoring. Moreover, residual heparin in the sample after discontinuing heparin may influence the aPTT, at least for some time. Despite such limitations, aPTT is widely used for monitoring DTIs, and it is also recommended by their manufacturers and the current guidelines.5
Ecarin clotting time (ECT) has been introduced in order to provide a more specific monitoring of DTIs.6 This assay is insensitive to heparin and displays a linear dose–response relationship. The method is widely used, but no standardized commercial version is available. The ECT relies on prothrombin and fibrinogen concentrations of the patient’s sample, which might be disadvantageous in certain clinical settings.7 More recently, superior specificity for monitoring of DTIs has been achieved with the ecarin chromogenic assay (ECA). This assay is independent of any variability of the patient’s fibrinogen and prothrombin. This is accomplished by using an excess of prothrombin in the test and a chromogenic substrate-based kinetic assay instead of clotting end point detection.8 Neither heparin nor therapy with vitamin K antagonists or antiphospholipid antibodies interferes with the ECA. The assay is commercially available, has a CE mark, can be adapted to automated instruments, and can be calibrated for any type of oral or parenteral DTI, providing exact drug levels instead of a clotting time.
The use of DTIs is not only associated with bleeding but also with thromboembolic complications in case of undertreatment. The ALicia study compared argatroban and lepirudin in a randomized controlled trial and found comparable effectiveness of both drugs, with a tendency toward a lower bleeding rate in the argatroban group. Like in most other studies, the DTI was dosed according to the aPTT.9
It was the aim of this retrospective study to determine the specific drug levels of either argatroban or lepirudin with ECA and to examine the correlation with “classical” coagulation tests in patients with HIT, in which the dosage of the DTIs was adjusted according to the aPTT. Of particular interest was the proportion of patients within the therapeutic range.
Methods
Patients
Laboratory routine data in 37 patients on argatroban and 80 patients on lepirudin therapy were collected in this retrospective study between March 2008 and February 2011. A total of 495 (argatroban, n = 189 and lepirudin, n = 306) patient samples were analyzed under clinical and laboratory routine conditions. Median frequency of sample per patient was 2 for both groups (mean value: 5 and 3.8 ranging 1-28 and 1-26 in argatroban patients and in lepirudin patients, respectively). Venous blood samples were drawn in citrate plasma (0.109 mol/L; BD Vacutainer, Heidelberg, Germany), and routine coagulation assays were performed using standard methods (Behring Coagulation System, BCS Analyzer; Siemens, Marburg, Germany). The TT, PT, and aPTT were determined with reagents from Siemens (Pathromtin SL, BC Thrombin and Thromborel S). Normal ranges were 17 to 22 seconds for TT and 26 to 37 seconds for aPTT. The normal range for PT, expressed in percentage normal activity, is >70%. Ecarin chromogenic assay (Diagnostica Stago, Asnieres, France) was adapted to the BCS Analyzer. In the ECA assay, undiluted patient plasma mixed with an excess of purified human prothrombin was subsequently activated with ecarin to generate meizothrombin. Part of the generated meizothrombin was instantaneously inactivated by the DTI of the sample. The residual meizothrombin, which was inversely proportional to the DTI concentration in plasma, was detected with a specific chromogenic substrate by p-nitroaniline release.6 For calibration of the methods, drug-specific standards obtained from Diagnostica Stago were used. Typical examples for hirudin (lepirudin, Refludan; Celgene, International Sàrl, Boudry, Switzerland) and argatroban (Argatra, Mitsubishi Tanabe Pharma, London, UK) are depicted in Figure 1.
Figure 1.

Reference curves for quantitative determination of argatroban (A) and lepirudin (B) concentrations adapted to the BCS Analyzer. Linearity existed up to a concentration of 2.5 µg/mL. The measurement range of the ecarin chromogenic assay (ECA) assay in the study was 0.0 ng/mL to 1.8 µg/mL for argatroban (mean value: 0.41 ± 0.35) and 0 µg/mL to 1.0 ng/mL for lepirudin (mean value: 0.20 ± 0.21). Y-axis: ECA reaction time (seconds).
To examine any effect caused by changes in hemostasis other than DTI therapy, we made a predefined differentiation for samples taken from intensive care units (ICUs) and non-ICU wards. Therefore, a total of 98 (52%) analyses of 20 patients were investigated from ICU in the argatroban group and 229 (75%) of 51 patients in the lepirudin group to minimize selection bias resulted from comorbidity (eg, hepatic dysfunction), comedication (eg, propofol, hemodilution by crystalloids or colloids), and therapeutic interventions, that is, the use of extracorporeal devices or other alteration in hemostasis, for example, extreme high or low level of fibrinogen due to sepsis and/or disseminated intravascular coagulation or elevated aPTT prior to anticoagulation due to a stronger activation of the contact phase, for example, by extracorporeal membrane oxygenation, intra-aortic balloon pump, and so on.
Statistics
As sufficient clinical data, especially related to patient outcome, were not available, results were solely interpreted from the laboratory point of view, correlating each coagulation test with ECA-based DTI level. Statistical significance was defined by P values (P < .01) employing Spearman rank correlation. Statistical descriptive values (calculated arithmetic means and standard deviations [SDs]) are shown in Tables 1 and 2. The statistical analysis was performed by SPSS Statistics 19.0 (SPSS, Chicago, Illinois).
Table 1.
Mean Values and Standard Deviations for DTI Level, Determined by ECT (Argatroban and Lepirudin), aPTT, TT, and PT.
| Argatroban | Lepirudin | |
|---|---|---|
| Samples | ||
| All | n = 189 (100%) | n = 306 (100%) |
| ICU | n = 98 (52%) | n = 229 (75%) |
| Non-ICU | n = 91 (48%) | n = 77 (25%) |
| Patients | ||
| All | n = 37 (100%) | n = 80 |
| ICU | n = 20 (54%)a | n = 51 (64%)a |
| Non-ICU | n = 23 (62%)a | n = 31 (39%)a |
| DTI (determined by ECT) | Mean ± SD (µg/mL) | Mean ± SD (µg/mL) |
| All | 0.41 ± 0.36 | 0.20 ± 0.21 |
| ICU | 0.25 ± 0.21 | 0.19 ± 0.20 |
| Non-ICU | 0.58 ± 0.40 | 0.24 ± 0.23 |
| aPTT | Mean ± SD (seconds) | Mean ± SD (seconds) |
| All | 67 ± 22 | 55 ± 20 |
| ICU | 72 ± 25 | 54 ± 19 |
| Non-ICU | 62 ± 18 | 59 ± 21 |
| TT | Mean ± SD (seconds) | Mean ± SD (seconds) |
| All | 94 ± 45 | 94 ± 60 |
| ICU | 79 ± 41 | 89 ± 60 |
| Non-ICU | 110 ± 43 | 109 ± 56 |
| PT | Mean ± SD (%) | Mean ± SD (%) |
| All | 51 ± 17 | 73 ± 20 |
| ICU | 56 ± 19 | 65 ± 21 |
| Non-ICU | 45 ± 12 | 77 ± 18 |
Abbreviations: aPTT, activated partial thromboplastin time; DTI, direct thrombin inhibitor; ECT, ecarin clotting time; ICU, intensive care unit; PT, prothrombin time; SD, standard deviation; TT, thrombin time.
aAs patients were transferred from ICU to ward (some patients belong to both groups), sum of ICU and non-ICU patients is more than 100%.
Table 2.
Percentage of Patients Within, Below, and Above the Therapeutic Range Defined by DTI Level, Determined by ECT (0.50-1.50 µg/mL) and aPTT (45-85 seconds).
| Within | Below | Above | |||||
|---|---|---|---|---|---|---|---|
| DTI Level,a 0.5-1.5 µg/mL | aPTT, 45-85 seconds | Both DTI Levela and aPTT | DTI Level,a <0.5 µg/mL | aPTT, <45 seconds | DTI Level,a >1.5 µg/mL | aPTT, >85 seconds | |
| Argatroban | |||||||
| All | 57/189 (30%) | 147/189 (77%) | 49/189 (26%) | 132/189 (70%) | 14/189 (7%) | 3/189 (2%) | 28/189 (15%) |
| ICU | 9/98 (9%) | 66/98 (67%) | 7/98 (7%) | 88/98 (90%) | 9/98 (9%) | 0/98 (0%) | 22/98 (22%) |
| Non-ICU | 48/91 (52%) | 80/91 (88%) | 42/91 (46%) | 43/91 (47%) | 5/91 (5%) | 3/91 (3%) | 6/91 (6%) |
| Hirudin | |||||||
| All | 28/306 (9%) | 198/306 (64%) | 25/306 (8%) | 278/306 (91%) | 90/306 (30%) | 0/306 (0%) | 16/302 (5%) |
| ICU | 23/229 (10%) | 151/229 (65%) | 21/229 (9%) | 206/229 (90%) | 70/229 (30%) | 0/229 (0%) | 7/229 (3%) |
| Non-ICU | 5/77 (6%) | 47/72 (65%) | 4/77 (5%) | 72/77 (94%) | 20/72 (28%) | 0/72 (0%) | 9/72 (13%) |
Abbreviations: aPTT, activated partial thromboplastin time; DTI, direct thrombin inhibitor, ECT, ecarin clotting time; ICU, intensive care unit.
aDetermined by ECT.
Ethical Approval
The investigation was approved by the ethics committee of the Heinrich Heine University Medical Center, Dusseldorf, Germany.
Results
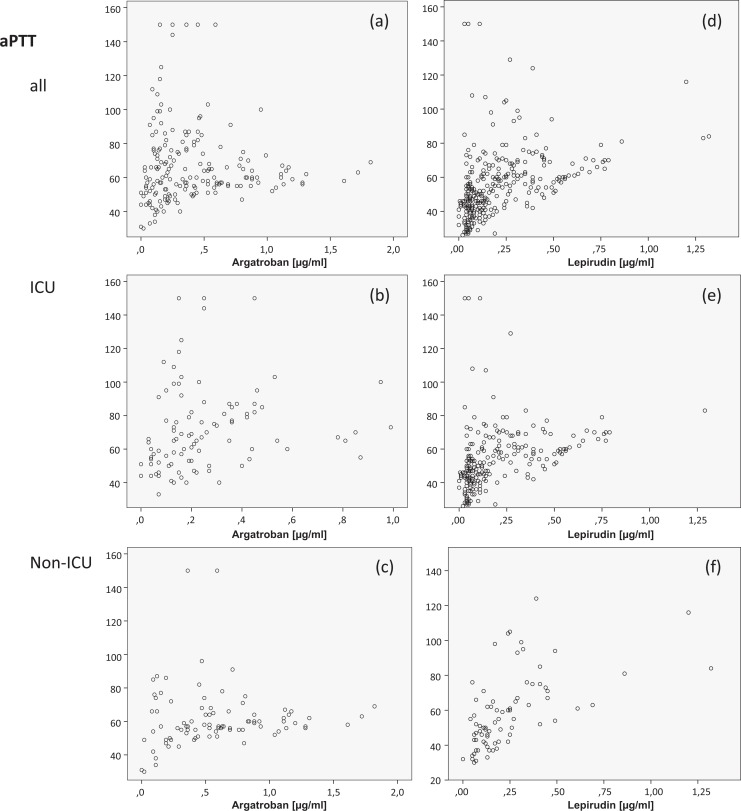
For all assays (PT, aPTT, TT, and ECA-based level of DTI), we observed a wide scatter, as shown in Table 1. In the case of TT, multiple samples showed unclottable results, especially in the lepirudin group. With the ECA, mean DTI levels ± SD were 0.41 ± 0.36 µg/mL for argatroban (range: 0.0-1.8 µg/mL) and 0.20 ± 0.21 µg/mL for lepirudin samples (range: 0.0-1.0 µg/mL) (Table 1). Results of PTT were highly variable. However, except for aPTT in the argatroban group, there were no significant differences comparing ICU patients (mean values: 72 ± 25) and non-ICU patients (mean values: 62 ± 18). The number of patients within (therapeutic DTI level: 0.50-1.5 µg/mL, aPTT between 45 and 85 seconds), below, and above the therapeutic range is summarized in Table 2. Interestingly, when the results of ECA and aPTT were used in combination for analysis, only 26% of argatroban and 8% of lepirudin samples were in the therapeutic range. Only 7% and 9% (argatroban and lepirudin) fulfilled these conditions in samples of ICU and 46% and 4% of non-ICU patients. For both DTIs, a large proportion of patients would be below the therapeutic concentration range, considering the exact plasma concentrations with ECA, despite the “therapeutical” aPTT data.
In contrast to the curvilinear profile of dose–responses of DTIs in vitro (Figure 1), the clotting times (in vivo) determined in our study measured by aPTT and TT only partially correlated with the DTI concentration, and there was a large scatter. Almost identical aPTT values were found for a wide concentration range in numerous samples.
Significant correlations (P < .01) were found between ECA-determined specific DTI levels and TT (r = .820 with argatroban and r = .830 with lepirudin), PT (r = −.544 with argatroban), and aPTT (r = .572 for lepirudin; Figure 2A and D). However, there was no correlation of aPTT (r = .136) with argatroban or PT (r = −.063) with lepirudin. Multiple regression analyses revealed that TT predicted 54% of argatroban and 42% of lepirudin levels, but no significant impact was seen for PT or aPTT.
Figure 2.
Direct thrombin inhibitor (DTI) concentrations assessed by ecarin chromogenic assay (µg/mL) for argatroban in comparison with lepirudin and in relation to activated partial thromboplastin time (aPTT) and thrombin time (TT; seconds) for all patients (A and D), intensive care unit (ICU) patients (B and E), and non-ICU patients (C and F). n = 98 (51%) and n = 91 (48%) samples were obtained from the ICU and non-ICU patients on argatroban n = 78 (25%) and n = 229 (75%) on lepirudin therapy, respectively.
Discussion
Therapeutic Range and Variability of aPTT
The therapeutic range used for thromboprophylaxis and treatment of acute thrombosis is defined by a 1.5- to 3.0-fold prolongation of aPTT for argatroban and a 1.5- to 2.5-fold prolongation of aPTT for lepirudin.10,11 Several aPTT reagents have been evaluated for argatroban sensitivity, and significant influence is considered almost unlikely by the choice of various reagents.12 One study concluded even small interindividual variability of pharmacological parameters and a predictable dose relationship for argatroban. However, this study included patients after percutaneous coronary interventions, obviously a group with a much less complex coagulopathy than patients with HIT.13 In contrast, other studies demonstrated significant differences.14,15 Poor correlation between aPTT and argatroban or lepirudin concentrations was reported.16,17 It was found that the influence of argatroban on coagulation tests was significantly increased by coagulation factor deficiencies.18 In HIT, several changes of the coagulation factors may occur. Thus, dose–response profiles and influence of clotting factors and fibrinogen levels or other variables such as lupus anticoagulants or hemodilution may lead to variability of the aPTT in individual patients. These various effects on aPTT bear the risk of either overdosing or underdosing and potential clinical sequelae for the individual patient.19,20 Several common drugs (among others antibiotics, antidepressants, and antihypertensives) and infections can induce antiphospholipid antibodies, which may contribute to a prolongation of phospholipid-depending tests.21 Furthermore, apart from rare hereditary defects, an acquired alteration of the intrinsic coagulation pathway due to diagnostic and therapeutic interventions and/or acute phase reaction, especially in ICU patients, may influence the aPTT course. But also other deficiencies of clotting factors, especially hepatic coagulopathy, may contribute to a preexisting or acquired prolongation of the aPTT. In addition, fibrin(ogen) split products, which are commonly found in critically ill patients, can influence all clotting assays to a variable extent. At least, switching from heparin to DTI bears the risk of measuring residual heparin and therefore influencing aPTT, thus definition of “baseline aPTT” (ie, the aPTT before starting anticoagulation) might be impossible.
Problems in Practice
In clinical routine practice, baseline aPTT is often not known or not available. Hence, aPTT ratio (present aPTT/baseline aPTT) as the recommended value for monitoring DTI therapy is often absent, and absolute aPTT values are used instead with a range of 45 to 85 seconds. Obviously, the known bleeding risk, in particular in critically ill patients,22 of both DTIs, used in this study, enforces a tendency of unassertive dosing, resulting in a high level of patients in whom the dose is below the recommended plasma concentration for both drugs, though about two-thirds were in the therapeutic range according to the aPTT reagent used in this study.
However, our study exhibited a wide scatter and a poor correlation between present aPTT and ECA-based specifically determined DTI levels under clinical routine conditions. This main finding of our study indicates that absolute aPTT values are not sufficient to estimate the present anticoagulation intensity. Thus, values of the aPTT in the “recommended therapeutic range” could indicate an obviously effective drug level, an overdosing, or more frequently an underdosing. However, in routine practice, DTI-specific tests like ECT or even plasma concentration of these agents are usually not available for each patient. Following current therapeutic dosage recommendations and monitoring with DTI-unspecific coagulation tests, that is aPTT, a wide range of DTI plasma levels can be assumed.
Significance of Prothrombinase-Induced Clotting Time, ECA, and TT
In the study by Calatzis et al, the same issue has been reported for the prothrombinase-induced clotting time assay, a method which is sensitive not only for heparins but also for DTIs and for factor Xa-inhibitors or for antiphospholipid antibodies.20,23 Using a more specific test such as the ECT may help to achieve a more accurate plasma concentration of DTI, as suggested by in vitro studies.24 However, the ECT assay is still biased by variable determinants such as the patients’ fibrinogen and prothrombin levels, fibrinogen degradation products, or other variables that impair fibrin polymerization. The more recent photometric ECA, which was used in this study, is completely independent of such patient-related variables and may therefore provide a better accuracy. The TT showed a linear dose–response profile, indicated by its good correlation with DTI plasma level, but due to its undiluted performance subsequently, no further discrimination between therapeutic and supratherapeutic ranges could be made, as shown in Figure 2. In the case of TT, of course the protocol used in our study is not necessarily representative for other laboratories since thrombin concentration and quality may vary to some extent in different protocols.
Pharmacology and Clinical Outcome
The pharmacological properties of parenteral DTIs used in this study are complex. Hirudin is primarily excreted via the kidneys, while argatroban is excreted primarily in the feces, presumably through biliary secretion, but also to a significant extent via kidneys. Over 20% of argatroban is excreted into the urine.25 Many patients with HIT are elderly individuals, are multimorbid, and have already some degree of organ dysfunction or develop it during their actual illness. In particular, renal insufficiency is a common finding in elderly individuals. The creatinine clearance as a marker of kidney function has many limitations, especially in elderly patients. Iatrogenic complications related to drug toxicity, for example, contrast media-induced nephropathy, may not be detected, or with some delay only. The use of more specific and sensitive early renal injury markers such as neutrophil gelatinase-associated lipocalin26 is yet not being used routinely. Therefore, in particular, patients with renal impairment on treatment with lepirudin or bivalirudin require specific monitoring to avoid toxic levels as a consequence of bioaccumulation. Caution is required when administering argatroban to patients with hepatic dysfunction, but also renal impairment should not be neglected.27 Although DTIs provide protection against thrombosis, bleeding remains a concern.28–30
Economic Burden
Resulting from high drug prices, DTI therapy is cost intensive. In a recent German analysis of multiple HIT cases, the costs for alternative anticoagulants represented the second largest portion of total costs.31 Costs will be significantly higher for those patients who develop bleeding or thrombosis in spite of expensive anticoagulation. Hospitals are interested in inexpensive and routinely employed monitoring methods such as aPTT. However, this involves the risk that an expensive therapy is applied, but the potential clinical success might be limited, if proper plasma level of the drug is not achieved, resulting to an elevated bleeding and/or thromboembolic event. These adverse events may further increase costs. At this point, it should be mentioned that accurate clinical diagnosis for HIT (eg, by using the 4 T score) plays a pivotal role for the decision, whether DTIs are required or not. In critically ill patients, this might be difficult; thus, rapid newer immunoassays should be considered to use to avoid DTI and therefore possible adverse events in patients with not-confirmed HIT.32,33
Limitations of our Study, Perspective, and Conclusion
Of course, our results neither prove that patients with DTI levels outside the therapeutic range have a higher thrombotic or bleeding risk nor that ECT monitored patients on DTI therapy have less adverse events. Currently, we are not aware of any systematic study that has compared in a prospective approach the complication rate of DTI therapy with dosage adjustment with more specific assays such as ECA as compared to the currently used aPTT approach. Such study is highly warranted for parenteral DTIs and may improve therapy with DTIs.
However, we found a high proportion of patients outside the therapeutic range (aPTT) and this is especially true for critically ill patients, which could be confirmed by recently submitted data.34 These data suggest a clinically relevant amount of avoidable adverse advents even in critically ill patients.
To conclude, concurrent determination of ECA and TT, knowledge of baseline values before DTI therapy, and consideration of clinical circumstances (eg, use of extracorporeal devices, antiphospholipid antibodies, other coagulation factor deficiencies) are essential for laboratory evaluation and correct interpretation of the individual anticoagulant effects of DTIs. This should be considered especially for critically ill patients and therefore mentioned in current guidelines, respectively product label information.
Acknowledgement
The authors thank Scharf RE, Buchbinder S, and Morgenrot U, affiliated with Department of Experimental and Clinical Hemostasis, Hemotherapy and Transfusion Medicine, Heinrich Heine University Medical Center, Dusseldorf.
Footnotes
Authors’ Note: Hans-Jürgen Kolde worked as a consultant for Diagnostica Stago, however in a different project, at the time preparing the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Greinacher A, Warkentin TE. Risk of heparin-induced thrombocytopenia in patients receiving thromboprophylaxis. Expert Rev Hematol. 2008;1(1):75–85. [DOI] [PubMed] [Google Scholar]
- 2. Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101(5):502–507. [DOI] [PubMed] [Google Scholar]
- 3. Bhatt VR, Dahal S, Verma V, et al. Fondaparinux for management of heparin-induced thrombocytopenia after cardiovascular intervention: a systematic review. Cardiovasc Hematol Agents Med Chem. 2015;13(2):82–86. [DOI] [PubMed] [Google Scholar]
- 4. Nowak G. Clinical monitoring of hirudin and direct thrombin inhibitors. Semin Thromb Hemost. 2001;27(5):537–541. [DOI] [PubMed] [Google Scholar]
- 5. Watson H, Davidson S, Keeling D; Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159(5):528–540. [DOI] [PubMed] [Google Scholar]
- 6. Nowak G, Bucha E. Quantitative determination of hirudin in blood and body fluids. Semin Thromb Hemost. 1996;22(2):197–202. [DOI] [PubMed] [Google Scholar]
- 7. Pötzsch B, Hund S, Madlener K, Unkrig C, Müller-Berghaus G. Monitoring of recombinant hirudin: assessment of a plasma-based ecarin clotting time assay. Thromb Res. 1997;86(5):373–383. [DOI] [PubMed] [Google Scholar]
- 8. Lange U, Nowak G, Bucha E. Ecarin chromogenic assay—a new method for quantitative determination of direct thrombin inhibitors like hirudin. Pathophysiol Haemost Thromb. 2003. –2004;33(4):184–191. [DOI] [PubMed] [Google Scholar]
- 9. Treschan TA, Schaefer MS, Geib J, et al. Argatroban versus Lepirudin in critically ill patients (ALicia): a randomized controlled trial. Crit Care. 2014;18(5):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Warkentin TE, Greinacher A, Koster A, Lincoff AM; American College of Chest Physicians. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):340S–380S. [DOI] [PubMed] [Google Scholar]
- 11. Gosselin RC, Dager WE, King JH, et al. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and INR values. Am J Clin Pathol. 2004;121(4):593–599. [DOI] [PubMed] [Google Scholar]
- 12. Francis JL, Hursting MJ. Effect of argatroban on the activated partial thromboplastin time: a comparison of 21 commercial reagents. Blood Coagul Fibrinolysis. 2005;16(4):251–257. [DOI] [PubMed] [Google Scholar]
- 13. Akimoto K, Klinkhardt U, Zeiher A, Niethammer M, Harder S. Anticoagulation with argatroban for elective percutaneous coronary intervention: population pharmacokinetics and pharmacokinetic-pharmacodynamic relationship of coagulation parameters. J Clin Pharmacol. 2011;51(6):805–818. [DOI] [PubMed] [Google Scholar]
- 14. Gosselin RC, King JH, Janatpour KA, Dager WE, Larkin EC, Owings JT. Comparing direct thrombin inhibitors using aPTT, ecarin clotting times, and thrombin inhibitor management testing. Ann Pharmacother. 2004;38(9):1383–1388. [DOI] [PubMed] [Google Scholar]
- 15. Siegmund R, Boer K, Poeschel K, Wolf G, Deufel T, Kiehntopf M. Comparison of the ecarin chromogenic assay and different aPTT assays for the measurement of argatroban concentrations in plasma from healthy individuals and from coagulation factor deficient patients. Thromb Res. 2008;123(1):159–165. [DOI] [PubMed] [Google Scholar]
- 16. Lind SE, Boyle ME, Fisher S, Ishimoto J, Trujillo TC, Kiser TH. Comparison of the aPTT with alternative tests for monitoring direct thrombin inhibitors in patient samples. Am J Clin Pathol. 2014;141(5):665–674. [DOI] [PubMed] [Google Scholar]
- 17. Ivandic B, Zorn M. Monitoring of the anticoagulants argatroban and lepirudin: a comparison of laboratory methods. Clin Appl Thromb Hemost. 2011;17(5):549–555. [DOI] [PubMed] [Google Scholar]
- 18. Siegmund R, Boer K, Poeschel K, Wolf G, Deufel T, Kiehntopf M. Influence of direct thrombin inhibitor argatroban on coagulation assays in healthy individuals, patients under oral anticoagulation therapy and patients with liver dysfunction. Blood Coagul Fibrinolysis. 2008;19(4):288–293. [DOI] [PubMed] [Google Scholar]
- 19. Siegmund R, Boer K, Poeschel K, Wolf G, Deufel T, Kiehntopf M. Comparison of the ecarin chromogenic assay and different aPTT assays for the measurement of argatroban concentrations in plasma from healthy individuals and from coagulation factor deficient patients. Thromb Res. 2008;123(1):159–165. [DOI] [PubMed] [Google Scholar]
- 20. Salmela B, Joutsi-Korhonen L, Saarela E, Lassila R. Comparison of monitoring methods for lepirudin: impact of warfarin and lupus anticoagulant. Thromb Res. 2010;125(6):538–544. [DOI] [PubMed] [Google Scholar]
- 21. Dlott JS, Roubey RA. Drug-induced lupus anticoagulants and antiphospholipid antibodies. Curr Rheumatol Rep. 2012;14(1):71–78. [DOI] [PubMed] [Google Scholar]
- 22. Doepker B, Mount KL, Ryder LJ, Gerlach AT, Murphy CV, Philips GS. Bleeding risk factors associated with argatroban therapy in the critically ill. J Thromb Thrombolysis. 2012;34(4):491–498. [DOI] [PubMed] [Google Scholar]
- 23. Calatzis A, Peetz D, Haas S, Spannagl M, Rudin K, Wilmer M. Prothrombinase-induced clotting time assay for determination of the anticoagulant effects of unfractionated and low-molecular-weight heparins, fondaparinux, and thrombin inhibitors. Am J Clin Pathol. 2008;130(3):446–454. [DOI] [PubMed] [Google Scholar]
- 24. Curvers J, van de Kerkhof D, Stroobants AK, van den Dool EJ, Scharnhorst V. Measuring direct thrombin inhibitors with routine and dedicated coagulation assays: which assay is helpful? Am J Clin Pathol. 2012;138(4):551–558. [DOI] [PubMed] [Google Scholar]
- 25. Argatroban [package insert]. Mitsubishi Tanabe Pharma GmbH, Juli 2016. [Google Scholar]
- 26. Shemin D, Dworkin LD. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for early acute kidney injury. Crit Care Clin. 2011;27(2):379–389. [DOI] [PubMed] [Google Scholar]
- 27. Arpino PA, Hallisey RK. Effect of renal function on the pharmacodynamics of argatroban. Ann Pharmacother. 2004;38(1):25–29. [DOI] [PubMed] [Google Scholar]
- 28. Smythe MA, Stephens JL, Koerber JM, Mattson JC. A comparison of lepirudin and argatroban outcomes. Clin Appl Thromb Hemost. 2005;11(4):371–374. [DOI] [PubMed] [Google Scholar]
- 29. Tardy B, Lecompte T, Boehlen F, et al. ; GEHT-HIT Study Group. Predictive factors for thrombosis and major bleeding in an observational study in 181 patients with heparin-induced thrombocytopenia treated with lepirudin. Blood. 2006;108(5):1492–1496. [DOI] [PubMed] [Google Scholar]
- 30. Greinacher A, Warkentin TE. The direct thrombin inhibitor hirudin. Thromb Haemost. 2008;99(5):819–829. [DOI] [PubMed] [Google Scholar]
- 31. Wilke T, Tesch S, Scholz A, Kohlmann T, Greinacher A. The costs of heparin-induced thrombocytopenia: a patient-based cost of illness analysis. J Thromb Haemost. 2009;7(5):766–773. [DOI] [PubMed] [Google Scholar]
- 32. Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia. A systematic review and meta-analysis. Thromb Haemost. 2016;115(5):1044–1055. [DOI] [PubMed] [Google Scholar]
- 33. Nagler M, Bakchoul T. Clinical and laboratory tests for the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost. 2016;116(5):823–834. [DOI] [PubMed] [Google Scholar]
- 34. Beiderlinden M, Werner P, Bahlmann A, et al. Monitoring of argatroban and lepirudin anticoagulation in critically ill patients. 2017. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]