Abstract
The purpose of this study was to compare the performance of anti-factor Xa concentration versus activated partial thromboplastin time (aPTT) monitoring with multiple indication-specific heparin nomograms. This was a prospective, nonrandomized study with historical control at a large academic medical center. A total of 201 patients who received intravenous heparin in the cardiology units were included. The prospective cohort included patients (n = 101) with anti-factor Xa (anti-Xa) monitoring, and the historical control group included patients (n = 100) who had aPTT monitoring. Patients in the prospective group had both anti-Xa and aPTT samples drawn, but anti-Xa was used for dosing adjustment. The anti-Xa cohort achieved a significantly faster time to therapeutic range (P < .01) and required fewer dose adjustments per 24-hour period compared to the aPTT control (P = .01). Results were consistent across heparin nomograms. The overall discordance rate between the 2 tests was 49%. No significant differences in clinical outcomes were observed. In summary, anti-Xa monitoring improved the time to therapeutic anticoagulation and led to fewer dose adjustments compared to the aPTT with multiple indication-based heparin nomograms.
Keywords: anti-factor Xa, unfractionated heparin, anticoagulation
Despite numerous advances in anticoagulation therapy in recent years, intravenous (IV) unfractionated heparin (UFH) remains a cornerstone of inpatient anticoagulation management. It offers practical advantages in the acute care setting, such as a rapid onset, short half-life, and reversibility.1 The pharmacokinetic advantages coupled with its inexpensive cost make it well suited for inpatient use. However, its inconsistent pharmacodynamic response requires routine monitoring, commonly via the activated partial thromboplastin time (aPTT), and frequent dose adjustment to maintain a therapeutic effect.1 Because aPTT is an indirect value subject to its own limitations and variations in measurement, the College of American Pathologists has recommended that therapeutic aPTT reference ranges be defined according to a direct measurement of heparin activity such as anti-factor Xa (anti-Xa) concentration.2,3 Although the use of aPTT to monitor heparin persists, it has been reported to be discordant from anti-Xa values in 46% to 60% of instances that may result in thromboembolic or bleeding complications.4–6 Furthermore, many biologic factors can affect concordance rates, including abnormal factor II and factor VIII concentrations, liver dysfunction, and hypercoagulable states, such that direct measurements of anticoagulant effect with anti-Xa may be more desirable.3,5 Switching from aPTT-based heparin monitoring to anti-Xa-based heparin monitoring has been shown to decrease the number of laboratory tests required and to shorten the time needed to achieve a therapeutic response.7–9 However, comparative studies among a broad population of patients who received different indication-based IV UFH nomograms are lacking. Therefore, the purpose of this study was to evaluate the performance and outcomes associated with anti-Xa versus aPTT monitoring among patients who received different IV UFH nomograms.
Methods
This was a prospective nonrandomized study of anti-Xa monitoring with aPTT-based historical control. This project received approval from the University of Pittsburgh Medical Center (UPMC) Quality Improvement Board. A convenience sample of all patients who received UFH as a continuous IV infusion on the cardiology units of UPMC Presbyterian Hospital, a 757-bed academic tertiary care center, between March 30, 2015, and June 30, 2015, were included and served as the prospective arm. During this time period, all patients were monitored at the same time with both anti-Xa concentrations and aPTTs as part of a pilot evaluation to determine the feasibility of institution-wide conversion to anti-Xa only monitoring. However, dose adjustments and monitoring were based only on anti-Xa for the prospective cohort. The retrospective control group consisted of a convenience sample of all medical and surgical patients treated with IV UFH on the same cardiology units during the time period immediately prior to initiation of the anti-Xa nomogram implementation on March 30, 2015. Patients in this group had IV UFH monitored and titrated using the aPTT. During the aPTT monitoring period, the aPTT assays were performed by STA-PTT A using the STA-R Max, a fully automated coagulation instrument (Parsippany, New Jersey). The STA-Liquid Anti-Xa Hybrid Assay was used for the quantitative determination of the plasma levels of UFH by measurement of anti-Xa activity in a competitive system using a synthetic chromogenic substrate.
Exclusion criteria for both study arms were use of IV UFH for less than 24 hours, treatment interruption for more than 10 hours, and ≥25% deviation from documented compliance with dose adjustment and/or monitoring with the heparin nomogram. All UFH adjustments during the prospective group were made according to anti-Xa concentrations using institutional nomograms, whereas aPTT nomograms were used for the historical control group. Nomograms for both anti-Xa and aPTT were designed for indication-specific uses with different dose intensities and therapeutic anticoagulation targets (Table 1). However, nomogram selection was at the discretion of the provider. All doses and rates were calculated based on total body weight with specified dose caps. Dosing adjustments for each nomogram were the same for the aPTT and anti-Xa monitoring groups (Table 2). The frequency of monitoring for both aPTT and anti-Xa nomograms was the same: 6 hours after initiation with subsequent rate adjustments until therapeutic anticoagulation was attained. Our institution defined therapeutic anticoagulation as 2 consecutive laboratory test values within the therapeutic range. Once therapeutic anticoagulation was achieved, monitoring frequency occurred once daily.
Table 1.
Heparin Nomograms Based on aPTT and Anti-Xa Concentrations.
| Nomogram | Initial Bolus Dose (Maximum) | Initial Infusion Dose (Maximum) | Goal aPTT (Seconds) | Goal Anti-Xa Concentration (U/mL) |
|---|---|---|---|---|
| DVT/PE | 80 U/kg (10 000 U) | 18 U/kg/h (1600 U/h) | 68-106 | 0.3-0.7 |
| UA/NSTEMI | 60 U/kg (4000 U) | 12 U/kg/h (1000 U/h) | 68-96 | 0.3-0.6 |
| Afib/Post-Op | 60 U/kg (10 000 U) | 10 U/kg/h (1600 U/h) | 68-82 | 0.3-0.45 |
| Stroke/EP/VAD/high-risk bleed | NA | 8 U/kg/h (1600 U/h) | 59-72 | 0.25-0.35 |
Abbreviations: Afib, atrial fibrillation; aPTT, activated partial thromboplastin time; DVT/PE, deep vein thrombosis/pulmonary embolism; EP, electrophysiology; Post-Op, postoperative; UA/NSTEMI, unstable angina/non-ST-segment elevation myocardial infarction; VAD, ventricular assist device.
Table 2.
Heparin Nomogram Dosing Adjustments Based on aPTT and Anti-Xa Concentrations.
| Nomograma | aPTT (seconds) or Anti-Xa (U/mL) Result and Bolus/Rate Changeb | ||||||
|---|---|---|---|---|---|---|---|
| DVT/PE | ≤48 or ≤0.20; 80 U/kg bolus and ↑ previous infusion by 4 U/kg/h | 49-67 or 0.21-0.29; 40 U/kg bolus and ↑ previous infusion by 2 U/kg/h | 68-106 or 0.3-0.7; no change | 107-115 or 0.71-0.80 and ↓ infusion by 2 U/kg/h | 116-125 or 0.81-0.99; hold infusion for 1 hour, then ↓ infusion by 3 U/kg/h | > 125 or ≥ 1.0; hold infusion for 2 hours, then ↓ infusion by 4 U/kg/h | NA |
| UA/NSTEMI | ≤48 or ≤0.20; 60 U/kg bolus and ↑ previous infusion by 4 U/kg/h | 49-67 or 0.21-0.29; 30 unit/kg bolus (not to exceed 2000 U) and ↑ previous infusion by 2 U/kg/h | 68-96 or 0.3-0.6; no change | 97-115 or 0.61-0.80; ↓ infusion by 2 U/kg/h | 116-125 or 0.81-0.99; hold infusion for 1 hour, then ↓ infusion by 3 U/kg/h | >125 or ≥1.0; h Hold infusion for 2 hours, then ↓ infusion by 4 U/kg/h | NA |
| Afib/Post-Op | ≤48 or ≤0.20; 60 U/kg bolus and ↑ previous infusion by 3 U/kg/h | 49-67 or 0.21-0.29; 40 unit/kg bolus and ↑ previous infusion by 2 U/kg/h | 68-82 or 0.3-0.45; no change | 83-96 or 0.46-0.60; hold infusion for 1 hour, then ↓ infusion by 1 U/kg/h | 97-106 or 0.61-0.70; hold infusion for 1 hour, then ↓ infusion by 2 U/kg/h | 107-125 or 0.71-0.99; hold infusion for 1 hour, then ↓ infusion by 4 U/kg/h | >125 or ≥1.0; hold infusion for 2 hours, then ↓ infusion by 6 U/kg/h |
| Stroke/EP/VAD/high-risk bleed | ≤48 or ≤0.20; ↑ previous infusion by 2 U/kg/h | 49-58 or 0.21-0.24; ↑ previous infusion by 1 U/kg/h | 59-72 or 0.25-0.35; no change | 73-82 or 0.36-0.45; ↓ infusion by 1 U/kg/h | 83-106 or 0.46-0.69; hold infusion for 1 hour, then ↓ infusion by 2 U/kg/h | >106 or ≥0.70; stop infusion and call physician | N/A |
Abbreviations: Afib, atrial fibrillation; ; aPTT, activated partial thromboplastin time; DVT/PE, deep vein thrombosis/pulmonary embolism; EP, electrophysiology; Post-Op, post-operative; UA/NSTEMI, unstable angina/non-ST-segment elevation myocardial infarction; VAD, ventricular assist device.
aDVT/PE, UA/NSTEMI, and Afib/Post-Op nomograms may be ordered with or without a bolus. No bolus nomograms omit the use of an initial bolus and additional boluses based on a subtherapeutic aPTT or anti-Xa concentration. Information shown here is for bolus dosing with these nomograms. Bolus dosing of intravenous unfractionated heparin for DVT/PE and Afib/Post-Op nomogram not to exceed 10 000 U; UA/NSTEMI nomogram not to exceed 4000 U (except where otherwise noted). Note that stroke/EP/VAD/high-risk bleed nomogram does not include a bolus at initiation or with adjustments based on aPTT or anti-Xa concentration.
bRepeat STAT aPTT or anti-Xa 6 hours after rate change; goal is 2 consecutive therapeutic results at which point laboratory parameter is checked daily.
Data collection included patient demographics, indication for UFH, laboratory results (anti-Xa concentration, aPTT), dosing adjustments, complications (thromboembolism and bleeding), and time to therapeutic response. Thromboembolism was defined as any new embolic stroke, myocardial infarction, or venous thrombosis. Bleeding was evaluated based on the Thrombolysis in Myocardial Infarction noncoronary artery bypass grafting definition.7 Data collection for both cohorts was based on chart review; however, evaluation of the prospective cohort occurred concurrent to patient treatment, which contrasted with retrospective review only in the control arm. The primary end point was time to therapeutic anticoagulation (time to therapeutic range). Secondary end points were the number of dose adjustments per 24 hours, discordance between anti-Xa and aPTT, and length of stay (LOS). Clinical outcomes that occurred during the inpatient visit while on UFH therapy were also evaluated. The primary clinical outcome of interest was the incidence of venous thromboembolism (VTE), whereas bleeding and mortality were secondary outcomes of interest. Discordance was defined as the presence of a therapeutic anti-Xa associated with any subtherapeutic or supratherapeutic aPTT result.
Patient characteristics were represented as mean ± standard deviation or median (range) for continuous variables and percentages for categorical variables. Paired samples were used from each patient for comparison of aPTT and anti-Xa concentration. We tested for differences between cohorts to ensure that patients were balanced on potential confounders. Differences between aPTT controls and anti-Xa patients were tested using Wilcoxon rank sum tests in continuous variables, as appropriate; categorical variables were tested using Fisher exact test. Subgroup analyses were performed by heparin nomogram used and by presence or absence of a starting bolus. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
A total of 101 patients were monitored using anti-Xa concentration and compared to 100 historical control patients monitored with the aPTT. Patient characteristics and treatment nomogram used are shown in Table 3. There were no significant differences between the historical control and the anti-Xa cohort in age, race, body mass index, renal dysfunction, concurrent medications, treatment nomogram, or the proportion that received a bolus of heparin. Table 4 provides a summary of the primary and secondary end points of the study. The anti-Xa cohort achieved a significantly faster time to therapeutic range (Figure 1). The anti-Xa group was therapeutic at a median (range) time of 16 hours (0.8-69.3 hours) compared to the aPTT group at 24 hours (2.5-118.8 hours). The anti-Xa group also required fewer dose adjustments per 24-hour period compared to the aPTT control. The results were consistent when stratified based on the presence or absence of a UFH bolus and by UFH nomogram used. Figure 2 shows the level of agreement between the aPTT and the anti-Xa concentration. The overall discordance rate between the 2 tests was 49%. The aPTT was therapeutic 35% of the time that the anti-Xa was therapeutic. There were no between-group differences in LOS and adverse events between groups, including VTE and bleeding.
Table 3.
Comparison of Baseline Patient Characteristics in aPTT Controls Versus Anti-Xa Cohort.a
| Clinical Characteristic | aPTT | Anti-Xa | P Value |
|---|---|---|---|
| Number of patients | 100 | 101 | |
| Age, years | 64.4 ± 14.2 | 65.5 ± 13.6 | .59 |
| Race | .43 | ||
| White | 89 (89) | 94 (93.1) | |
| Black | 7 (7) | 4 (4) | |
| Asian | 0 (0) | 1 (1) | |
| Unknown | 4 (4) | 2 (2) | |
| Height, cm | 171.8 ± 11.3 | 170.5 ± 10.1 | .40 |
| Weight, kg | 89.7 ± 27.1 | 87.3 ± 21.3 | .49 |
| BMI, kg/m2 | 30.3 ± 8.3 | 30.1 ± 7.3 | .89 |
| Kidney dysfunction, GFR < 60 mL/min | 44 (44) | 43 (42.6) | .88 |
| Concurrent medications | |||
| ASA | 80 (80) | 71 (70.3) | .14 |
| P2Y12 inhibitor | 32 (32) | 31 (30.7) | .88 |
| GP IIb/IIIa inhibitor | 1 (1) | 1 (1) | 1.00 |
| Warfarin | 46 (46) | 41 (40.6) | .47 |
| Selective Xa inhibitor | 0 (0) | 1 (1) | 1.00 |
| DTI | 0 (0) | 1 (1) | 1.00 |
| Heparin nomogram | .70 | ||
| Afib/Post-Op | 40 (40) | 47 (46.5) | |
| UA/NSTEMI | 36 (36) | 29 (28.7) | |
| DVT/PE | 22 (22) | 23 (22.8) | |
| Stroke/EP/VAD/high-risk bleed | 2 (2) | 2 (2) | |
| Bolus | 39 (39) | 47 (46.5) | .31 |
Abbreviations: Afib, atrial fibrillation; aPTT, activated partial thromboplastin time; ASA, aspirin; BMI, body mass index; DVT/PE, deep vein thrombosis/pulmonary embolism; DTI, direct thrombin inhibitor; EP, electrophysiology; GFR, glomerular filtration rate; GP, glycoprotein; Post-Op, postoperative; UA/NSTEMI, unstable angina/non-ST-segment elevation myocardial infarction; VAD, ventricular assist device.
aContinuous data are represented as mean (standard deviation) or median (range). Categorical data are expressed as n (%).
Table 4.
Primary Results for aPTT Controls Versus Anti-Xa Cohort.a
| Study Endpoints | aPTT | Anti-Xa | P Value |
|---|---|---|---|
| Time to therapeutic range (hours) | 24 (2.5-118.8) | 16 (0.8-69.3) | < .01 |
| Total time on heparin (hours) | 66.5 (14.5-370) | 61.5 (13-427) | .84 |
| Number of tests performed | 7 (1-34) | 6 (2-36) | .53 |
| Number of tests performed per 24 hours on heparin | 2.7 (1.3-6.6) | 2.7 (1-5.5) | .81 |
| Number of adjustments required | 4 (0-24) | 3 (0-16) | .06 |
| Number of adjustments required per 24 hours on heparin | 1.5 (0-5.3) | 1.2 (0-3.7) | .01 |
| Length of stay (days) | 6.5 (1.6-37.9) | 7.5 (1-43) | .46 |
| VTE | 1 (1) | 1 (1) | 1.00 |
| Bleeding | 4 (4) | 12 (11.9) | .07 |
| Mortality | 4 (4) | 0 (0) | .06 |
Abbreviation: aPTT, activated partial thromboplastin time; VTE, venous thromboembolism.
aData reported as medians (range).
Figure 1.
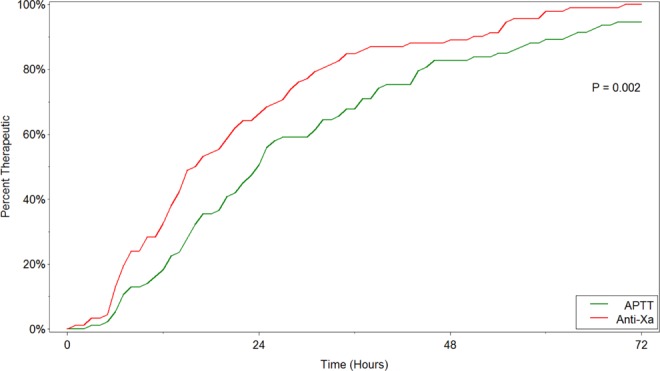
Time to therapeutic range. aPTT indicates activated partial thromboplastin time; anti-Xa, anti-factor Xa concentration.
Figure 2.
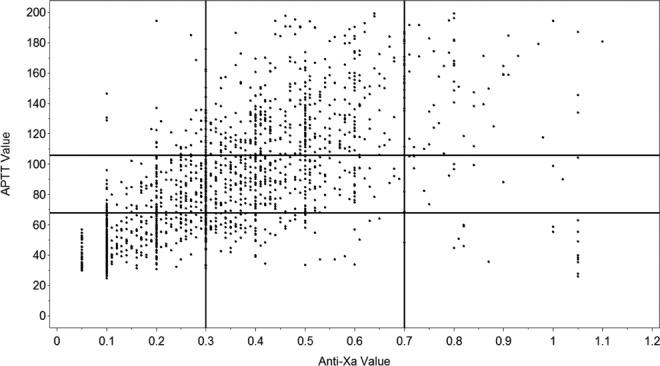
Agreement between activated partial thromboplastin time (aPTT) and anti-Xa concentrations. The bottom left corner represents pairings in the “subtherapeutic” range for aPTT and anti-Xa. The top left corner represents “supratherapeutic” for aPTT and “subtherapeutic” for anti-Xa.
Discussion
This study demonstrates that patients receiving continuous IV infusions of UFH using anti-Xa nomograms reached therapeutic targets faster than those monitored with aPTT nomograms. Our data showed that patients monitored by the anti-Xa assay reached the therapeutic range approximately 8 hours faster than patients monitored with the aPTT. These findings are in agreement with prior studies which demonstrated significant differences in achieving therapeutic ranges more rapidly with anti-Xa compared to aPTT monitoring.8–10 The clinical importance of these results is underscored by an increased risk of recurrent VTE if therapeutic anticoagulation is not achieved by 24 hours.11
To our knowledge, this is the second largest of only 4 previous studies which directly compared aPTT and anti-Xa nomograms for monitoring UFH.8–10,12 Rosborough evaluated 268 patients who received a single treatment protocol based on deep vein thrombosis (DVT) treatment and showed that despite a modest increase in cost, patients in the anti-Xa group required fewer monitoring tests and dose changes compared to the aPTT.8 Fruge and Lee studied 141 patients who received a single protocol for all indications, although dosing differed slightly between the aPTT and anti-Xa nomograms.10 The investigators demonstrated fewer doses changes and a faster time to therapeutic effect (6 hours, but not 24 hours) with anti-Xa monitoring. The study by Miller et al included 119 patients who received 1 of 3 different nomograms (thrombosis, cardiology, and acute coronary syndromes).12 The thrombosis and cardiology nomograms had different initial IV bolus and infusion doses; however, the goal anti-Xa range was the same. The focus of this study was on nomogram adherence, with the primary results showing improved laboratory monitoring at the appropriate time and correct dose adjustments with anti-Xa monitoring. Finally, the study by Guervil et al evaluated 100 patients who were treated with a single protocol for VTE.9 Patients in the anti-Xa arm of this study had significantly greater time in therapeutic range, fewer monitoring tests, and fewer dose changes per 24 hours.
Our study also demonstrated that patients monitored with anti-Xa concentrations required fewer adjustments per 24 hours on UFH than those monitored with the aPTT. We found the need for 1 less IV UFH infusion dose adjustment per 3.3 patient-days on treatment. This is one of several factors that must be considered when weighing costs of aPTT and anti-Xa monitoring, notably nursing labor, phlebotomy, and laboratory expenses. Vandiver and Vondracek showed that anti-Xa monitoring cost per patient per day was US$13.30 compared to US$13.97 with aPTT monitoring.3 The economic advantage was a result of decreased dose adjustments and laboratory testing, which translated to decreased phlebotomy and nursing labor costs while on anti-Xa protocols. This benefit was seen despite a high anti-Xa reagent cost at their institution.3
The discordance between the aPTT and anti-Xa in our study was 49%. A notable pattern was that the aPTT was therapeutic only 35% of the time that the anti-Xa was also therapeutic. This finding is consistent with previous reports, including a study by Price et al that showed a significant association between patients who had laboratory discordance (at least 2 consecutive high aPTT to anti-Xa values) and higher risks of major bleeding and 30-day mortality.6 There are multiple clinically relevant reasons for an altered aPTT in an acutely ill patient.3 Moreover, there are significant issues with standardization of aPTTs to anti-Xa concentrations, given the variability between reagents and laboratory detection equipment used in the aPTT assay. Ultimately, it was the discordance seen in our own study which prompted the decision to convert from aPTT to anti-Xa monitoring for all IV UFH nomograms used at UPMC, a network of 39 hospitals.
Discordance and costs are among several factors that must be weighed when evaluating the decision to switch from aPTT to anti-Xa monitoring. The aPTT offers clinical convenience; however, its accuracy is more often affected by preanalytic, analytic, and biologic variables.3,5 Anti-Xa testing, however, is subject to certain limitations as well including inaccuracy in the settings of hypertriglyceridemia (triglyceride level >360 mg/dL), hyperbilirubinemia (total bilirubin level >6.6 mg/dL), and recent use of low-molecular-weight heparins, fondaparinux, and oral selective factor Xa inhibitors.3,13
The strength of our study is seen in the evaluation of multiple IV UFH nomograms used for a variety of therapeutic indications. Our institution uses 4 nomograms (Table 1) which were designed for different therapeutic uses and corresponding aPTT and anti-Xa targets. Our DVT/pulmonary embolism (PE) nomogram was derived from the weight-based nomogram published by Raschke et al.14 The unstable angina/non-ST-segment elevation myocardial infarction (UA/NSTEMI) nomogram was adapted from the American Heart Association/American College of Cardiology treatment guidelines.15 The other 2 nomograms (atrial fibrillation/postoperative [Afib/Post-Op] and stroke/electrophysiology/ventricular assist device/high-risk bleed) were based on institutional expert opinion and consensus. The latter nomogram, in particular, was designed for patients determined to be at high risk of bleeding by the physician but still in need of anticoagulation. The decision to select a particular nomogram was based on provider discretion. Also, 3 of the 4 nomograms (DVT/PE, UA/NSTEMI, and Afib/Post-Op) may be ordered with or without a bolus (initial bolus and subsequent boluses for titration). This decision was also left to the prescriber but often is based upon a clinical assessment of bleeding and thromboembolic risk. Our study results were consistent when stratified by the presence or absence of a bolus and by nomogram. Although our study was not powered to compare the effectiveness between the different nomograms, further investigation into the use of a bolus and dosing strategies could help to improve safety outcomes.
The limitations to our single-center study are inherent to the retrospective design. The comparator aPTT arm was a retrospective analysis that could have resulted in missing or incomplete data, which was not documented in the electronic health record. This became evident with the increased number of reported bleeds within the prospectively monitored anti-Xa group, albeit not statistically different compared to the aPTT group. The bleeding and thrombotic outcomes reported were captured at any time while the patient was on IV UFH. We did not evaluate whether these outcomes occurred in the setting of a therapeutic result or a dose change. Other data that we were unable to collect included potential nonpharmacologic confounders at baseline (ie, coagulation abnormalities, critical illness, or other conditions associated with low antithrombin III activity). Finally, we did not perform a power calculation to detect differences in clinical outcomes prior to beginning data collection. Rather, our study design was based on a convenience sample needed to test feasibility of institution-wide conversion to anti-Xa monitoring and evaluation of time to therapeutic anticoagulation.
In conclusion, our study suggests a benefit with anti-Xa monitoring of IV UFH compared to the aPTT in terms of improved time to therapeutic anticoagulation and fewer dose adjustments for a variety of IV UFH indication-based nomograms. We also observed significant discordance between the aPTT and anti-Xa concentration. Whether these results translate to an improvement in clinical outcomes with anti-Xa monitoring needs to be further explored in a larger controlled study.
Acknowledgments
The authors acknowledge Dr Derek Pae for his contributions to data collection and management.
Authors’ Note: This material in this article has not been previously presented elsewhere.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e24S–e43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Olson JD, Arkin CF, Brandt JT, et al. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782–798. [PubMed] [Google Scholar]
- 3. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012;32(6):546–558. [DOI] [PubMed] [Google Scholar]
- 4. Cuker A, Ptashkin B, Konkle BA, et al. Interlaboratory agreement in the monitoring of unfractionated heparin using the anti-factor Xa-correlated activated partial thromboplastin time. J Thromb Haemost. 2009;7(1):80–86. [DOI] [PubMed] [Google Scholar]
- 5. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013;139(4):450–456. [DOI] [PubMed] [Google Scholar]
- 6. Price EA, Jin J, Nguyen H, Krishnan G, Bowen R, Zehnder JL. Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother. 2013;47(2):151–158. [DOI] [PubMed] [Google Scholar]
- 7. Rao AK, Pratt C, Berke A, et al. Thrombolysis in Myocardial Infarction (TIMI) Trial—phase I: hemorrhagic manifestations and changes in plasma fibrinogen and the fibrinolytic system in patients treated with recombinant tissue plasminogen activator and streptokinase. J Am Coll Cardiol. 1988;11(1):1–11. [DOI] [PubMed] [Google Scholar]
- 8. Rosborough TK. Monitoring unfractionated heparin therapy with antifactor Xa activity results in fewer monitoring tests and dosage changes than monitoring with the activated partial thromboplastin time. Pharmacotherapy. 1999;19(6):760–766. [DOI] [PubMed] [Google Scholar]
- 9. Guervil DJ, Rosenberg AF, Winterstein AG, Harris NS, Johns TE, Zumberg MS. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011;45(7-8):861–868. [DOI] [PubMed] [Google Scholar]
- 10. Fruge KS, Lee YR. Comparison of unfractionated heparin protocols using antifactor Xa monitoring or activated partial thrombin time monitoring. Am J Health-Syst Pharm. 2015;72(17 suppl 2):S90–S97. [DOI] [PubMed] [Google Scholar]
- 11. Hull RD, Raskob GE, Brant RF, et al. Relation between the time to achieve the lower limit of the APTT therapeutic range and recurrent venous thromboembolism during heparin treatment for deep vein thrombosis. Arch Intern Med. 1997;157(22):2562–2568. [PubMed] [Google Scholar]
- 12. Miller AE, Montague D, Rodgers JE, Sanghvi S, Whinna HC, Krumnacher H. Substitution of a heparin correlation value for activated partial thromboplastin time in heparin nomograms. Am J Health Syst Pharm. 2011;68(10):893–898. [DOI] [PubMed] [Google Scholar]
- 13. Faust AC, Kanyer D, Wittkowsky AK. Managing transitions from oral factor Xa inhibitors to unfractionated heparin infusions. Am J Health-Syst Pharm. 2016;73(24):2037–2041. [DOI] [PubMed] [Google Scholar]
- 14. Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993;119(9):874–881. [DOI] [PubMed] [Google Scholar]
- 15. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 ACC/AHA guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–e426. [DOI] [PubMed] [Google Scholar]


