Abstract
The prognostic impact of nutritional status in patients with pulmonary embolism (PE) is poorly understood. A well-accepted nutritional status parameter, prognostic nutritional index (PNI), which was first demonstrated to be valuable in patients with cancer and gastrointestinal surgery, was introduced to patients with PE. Our aim was to evaluate the predictive value of PNI in outcomes of patients with PE. We evaluated the in-hospital and long-term (53.8 ± 5.4 months) prognostic impact of PNI on 251 patients with PE. During a median follow-up of 53.8 ± 5.4 months, 27 (11.6%) patients died in hospital course and 31 (13.4%) died in out-of-hospital course. The patients with lower PNI had significantly higher in-hospital and long-term mortality. The Cox proportional hazard analyses showed that PNI was associated with an increased risk of all-cause death for both unadjusted model and adjusted for all covariates. Our study demonstrated that PNI, calculated based on serum albumin level and lymphocyte count, is an independent prognostic factor for mortality in patients with PE.
Keywords: pulmonary embolism, prognostic nutritional index, mortality
Introduction
Venous thromboembolism, which covers pulmonary embolism (PE), is the third most common cardiovascular disease following acute myocardial infarction and stroke.1 Pulmonary embolism is a life-threatening disease, and adjusted PE hospitalization rate is 302/100 000 in the United States, even higher in black patients.1 The long-term mortality of PE is effected by many factors including age, comorbidities, type and time of treatment, right ventricular dilatation, right ventricular ejection fraction, tricuspid valve insufficiency, and pulmonary hypertension (HT).2 Many prognostic factors and laboratory biomarkers have been put forward to correlate long-term survival in PE. Creatinine kinase isoenzyme–MB and N-terminal pro-B-type natriuretic peptide were reported as prognostic laboratory biomarkers in PE.3 In addition, recent operation or fracture, shock during hospitalization, and mechanical ventilation were also defined as prognostic factors.4 The recent PE guideline insistently suggests Pulmonary Embolism Severity Index (PESI) to estimate long-term survival.5 Despite many valuable prognostic factors, PE still requires a cheap and reliable index revealing patient’s nutritional status. Several studies demonstrated correlation between a single nutritional indicator such as albumin and poor outcomes in patients with cancer.6 Whereas to determine nutritional status more complex and in detail, prognostic nutritional index (PNI) has been postulated.7 The PNI was first established to assess perioperative immunonutritional status and surgical risk in patients undergoing gastrointestinal surgery.8 We introduce this simple risk index to patients with PE, which was first adapted and examined in patients with malnutrition. The PNI, which reflects albumin concentration and lymphocyte count, is a newly established inflammation-based nutritional score.9 Inexpensive and simple blood biomarkers are required to determine these 2 parameters.10 Nevertheless, several nutritional indices such as controlling nutritional status and geriatric nutritional index have been reported to have prognostic value in patients with congestive heart failure and cancer.10 Among nutritional indices, PNI is the most well-accepted index whose value has been documented many times in the literature. The aim of our retrospective observational study was to evaluate the impact of admission PNI on outcome of patients with PE at a median follow-up of 53.8 ± 5.4 months.
Materials and Methods
Patient and Study Design
Between May 2009 and July 2011, 291 consecutive confirmed patients with PE, who were admitted to Dr Siyami Ersek Cardiovascular and Thoracic Surgery Training and Research Hospital, were evaluated retrospectively. A total of 29 patients who did not have albumin measurement or lymphocyte count during the hospitalization were excluded. An additional 11 patients were excluded from the study secondary to missing data after hospitalization. Pulmonary multislice computed tomography (CT) angiography (SOM-ATOM Sensation 64; Siemens, Erlangen, Germany) was used to diagnose PE.
The study population was divided into tertiles according to their admission PNI starting with the lowest PNI. The PNI was calculated using the following formula: 10 × serum albumin value (g/dL) + 0.005 × total lymphocyte count in the peripheral blood (per mm3). The optimal cutoff value for the continuous PNI was calculated by applying a receiver operating curve (ROC) analysis to test all possible cutoffs that would predict the in-hospital survival. A PNI of 38 was identified through an ROC analysis as an optimal cutoff value to predict the in-hospital survival (Figure 1).
Figure 1.
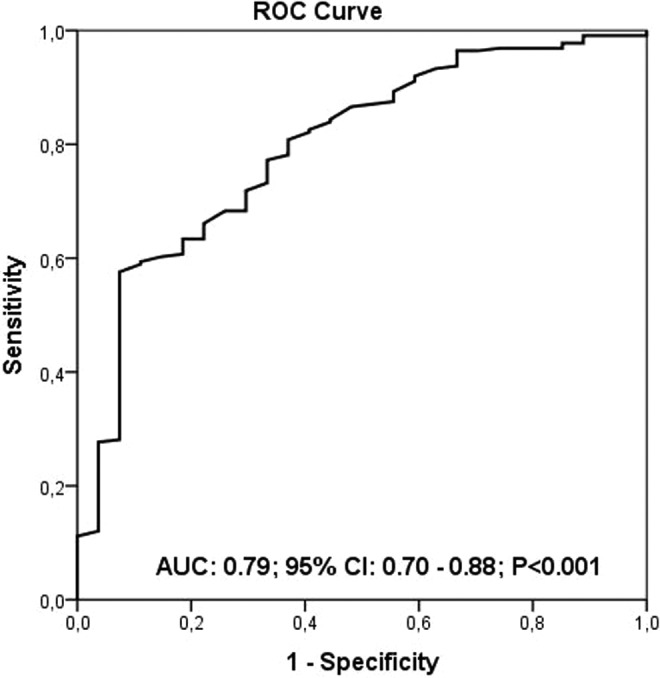
The prognostic nutritional index (PNI) level of 38 was identified as an effective cutoff point for in-hospital survival rate (area under the curve = 0.79; 95% confidence interval: 0.70-0.88, P < .001), and it had a sensitivity of 53% and specificity of 93%.
Analysis of Patient Data
A clinical history of risk factors, such as age, sex, HT, diabetes mellitus (DM), hyperlipidemia, and chronic lung and kidney disease, was determined from the hospital’s medical database. Echocardiographic and pulmonary CT angiography findings were also obtained from the same database. Echocardiogram was performed using a Vivid 7 system (GE Vingmed Ultrasound AS, Horten, Norway) in 96% of patients at first 48 hours in the coronary care unit, and left ventricular ejection fraction was calculated by using the Simpson method.11 The pulmonary arterial peak systolic pressure was calculated using the simplified Bernoulli equation.12 The occurrences of in-hospital and long-term events were evaluated by a trained study coordinator. The estimated glomerular filtration rate was calculated by using CKD-EPI equation. Blood values obtained from venous blood samples at hospital admission were recorded from the medical reports. White blood cell (WBC) count, hemoglobin level, and lymphocyte count were measured as part of the automated complete blood count using a Coulter LH 780 Hematology Analyzer (Beckman Coulter Ireland, Inc, Galway, Ireland). Biochemical measurements were performed using Siemens Healthcare Diagnostic Products kits and calibrators (Marburg, Germany). Creatinine kinase isoenzyme–MB levels were measured using an immune-inhibition method (Architect C 8000; Abbott Inc). The drugs were administered during the hospitalization according to the European Society of Cardiology Guidelines.13
Definitions
The primary end points were the incidence of in-hospital and long-term mortality. In-hospital mortality was defined as death from any cause during hospitalization. Long-term mortality was defined as death from any cause after discharge. Hypertension was defined as systolic pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg. Diabetes mellitus was defined as the use of insulin or antidiabetic agents in the patient’s medical history or a fasting glucose level greater than 126 mg/dL. Hyperlipidemia was defined as serum total cholesterol ≥240 mg/dL, serum triglyceride ≥200 mg/dL, low-density lipoprotein cholesterol ≥130 mg/dL, and previously diagnosed hyperlipidemia. Heart failure was defined as having typical symptoms (breathlessness, fatigue, and ankle swelling) and signs (elevated jugular venous pressure and pulmonary crackles) resulting from an abnormality of cardiac function.14 Shock was defined as systolic pressure less than 90 mm Hg or systolic pressure drop greater than or equal to 40 mmHg for more than 15 minutes without new-onset arrhythmia, hypovolemia, or sepsis.13 Major bleeding was defined as a decline in the hemoglobin level of 20 g/L or more or transfusion of 2 or more units of red cells. Altered mental status was defined as signs of disorientation, lethargy, stupor, or coma. Syncope was defined as a transient, self-limited loss of consciousness due to transient global cerebral hypoperfusion characterized by rapid onset, short duration, and spontaneous complete recovery.15
Follow-Up
All follow-up data were obtained from hospital records or by interviewing (directly or by telephone) patients, their families, or their personal physicians. The primary end point was death. Recurrent PE, major and minor bleeding, use of fresh frozen plasma, and history of international normalized ratio greater than 5 were noted. Patients without cancer were all prescribed and followed up with warfarin treatment for 3 months following their acute PE. Patients with cancer were followed up with enoxaparin treatment for 3 months.
Statistical Analysis
In a first step, the study population was divided into tertiles according to admission PNI. Three groups were formed accordingly: one with 84 patients (tertile 1), other with 84 patients (tertile 2), and the last with 83 patients (tertile 3). In a second step, baseline characteristics were compared among these 3 groups. Quantitative variables were expressed as mean ± SD. Kolmogorov-Smirnov test was used for testing normality. All continuous variables showed skewed distributions and compared using the Mann-Whitney U test. Categorical variables were expressed as number and percentages and Pearson χ2 or Fisher exact test was used to evaluate the differences. Logistic regression was used to assess the independent relationship between PNI and in-hospital mortality. After follow-up periods of 53.8 ± 5.4 months, the median survival times of 3 groups were compared using the Kaplan-Meier survival method. Overall survival was calculated from the day of diagnosis to the day of death. Differences between the groups were analyzed by the log-rank test. Univariate analysis and multivariate analysis with Cox proportional hazard regression were used to identify predictors of 53.8 ± 5.4 months of mortality. Three Cox multivariable models were used: model I, unadjusted; model II, age and sex adjusted; and model III, fully adjusted. The variables covariated in model III were demographics (age, sex); first measurement of systolic blood pressure and heart rate; first measurement during hospitalization of the following laboratory values (admission glomerular filtration rate calculated by CKD-EPI, blood urea nitrogen, WBC count, hematocrit, platelet count); creatine kinase–MB, troponin I, d-dimer, and brain-type natriuretic peptide; comorbidities (diabetes, chronic kidney disease, HT, stroke, heart failure, cancer, chronic lung disease, and atrial dysrhythmia), and medications (use of oral contraceptives, warfarin, and steroid). Analyses were performed using Statistical Package for Social Sciences software, version 20.0 (SPSS; IBM, Armonk, New York).
Results
Baseline Characteristics
Baseline characteristics, categorized by admission PNI, are listed in Table 1. A total of 251 patients (mean age 64 ± 15 years; men 46%) with PE were included. The patients in tertile 1 had higher age compared with other tertiles (P = .091). The 3 groups were similar in terms of HT, DM, hyperlipidemia, previous myocardial infarction, previous PCI, previous coronary artery bypass graft, chronic kidney disease, heart failure, chronic lung disease, cerebrovascular disease, prior PE, cancer, use of oral contraceptives, steroids, and warfarin. Whereas, tertile 1 had significantly higher PESI score (P < .001). All types of admission symptoms were similar between groups. Considering admission electrocardiography sinus tachycardia, right bundle branch block, T-wave inversion, atrial dysrhythmia, and S1Q3T3 were detected statistically similarly in all tertiles. Table 2 summarizes laboratory and echocardiography findings of the patients. Albumin, lymphocyte, and blood urea nitrogen were significantly lower in tertile 1 (P < .001). Whereas other laboratory parameters such as troponin, d-dimer, creatinine kinase-MB, brain-type natriuretic peptide, WBCs, and neutrophils were similar between groups. All of the echocardiographic parameters were statistically similar between tertiles. The mean PNI of patients with and without cancer was 39.1 and 38.1, respectively.
Table 1.
Baseline Characteristics.a,b
| Prognostic Nutritional Index | ||||
|---|---|---|---|---|
| T1 (n = 84) | T2 (n = 84) | T3 (n = 83) | P | |
| Age, years | 68 ± 12 | 65 ± 13 | 64 ± 17 | .091 |
| Male gender | 39 (46.4) | 35 (41.7) | 44 (53.0) | .337 |
| In-hospital stay, days | 9 ± 6 | 10 ± 5 | 10 ± 6 | .063 |
| Comorbidities | ||||
| Hypertension | 39 (46.4) | 35 (41.7) | 32 (38.6) | .583 |
| Diabetes mellitus | 27 (32.1) | 18 (21.4) | 27 (32.5) | .197 |
| Hyperlipidemia | 14 (16.7) | 16 (19.0) | 11 (13.3) | .596 |
| Current smoking status | 23 (27.4) | 24 (28.6) | 28 (33.7) | .635 |
| Previous MI | 9 (10.7) | 3 (3.6) | 7 (8.4) | .202 |
| Previous PCI | 4 (4.8) | 4 (4.8) | 3 (3.6) | .916 |
| Previous CABG | 6 (7.1) | 4 (4.8) | 2 (2.4) | .358 |
| Chronic kidney disease | 11 (13.1) | 5 (6.0) | 7 (8.4) | .265 |
| Heart failure | 5 (6.0) | 4 (4.8) | 4 (4.8) | .926 |
| Chronic lung disease | 10 (11.9) | 10 (11.9) | 7 (8.4) | .706 |
| Cerebrovascular disease | 7 (8.3) | 4 (4.8) | 2 (2.4) | .220 |
| Cancer | 8 (9.5) | 10 (11.9) | 11 (13.3) | .747 |
| DVT history | 22 (26.2) | 25 (29.8) | 24 (28.9) | .866 |
| Prior PE | 6 (7.1) | 4 (4.8) | 5 (6.0) | .809 |
| Use of OCS | 5 (6.0) | 2 (2.4) | 3 (3.6) | .486 |
| Use of warfarin | 4 (4.8) | 5 (6.0) | 1 (1.2) | .277 |
| Use of steroid | 1 (1.2) | 0 (0.0) | 4 (4.8) | .068 |
| At admission | ||||
| Systolic blood pressure, mm Hg | 129 ± 20 | 126 ± 25 | 126 ± 27 | .375 |
| Altered mental status | 7 (8.4) | 3 (3.6) | 2 (2.4) | .163 |
| Heart rate, beats per minute | 99 ± 19 | 100 ± 22 | 110 ± 24 | .088 |
| Respiratory rate, beats per minute | 20 ± 3 | 19 ± 3 | 20 ± 6 | .298 |
| Temperature | 36.5 ± 0.3 | 36.5 ± 0.2 | 36.5 ± 0.3 | .115 |
| O2 saturation, % | 91 ± 7 | 92 ± 5 | 94 ± 4 | .117 |
| PESI score | 115 ± 43 | 97 ± 40 | 76 ± 38 | <.001 |
| Intensive care unit stay, days | 5 ± 6 | 4 ± 2 | 4 ± 3 | .943 |
| Admission blood gas analysis | ||||
| pH | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.4 ± 0.1 | .950 |
| Lactate | 3.7 ± 2.7 | 3.3 ± 2.7 | 2.7 ± 1.8 | .593 |
| HCO3 | 21 ± 5 | 21 ± 4 | 21 ± 4 | .981 |
| Admission symptoms | ||||
| Syncope | 23 (27.7) | 20 (24.1) | 20 (24.4) | .838 |
| Chest pain | 7 (8.5) | 14 (17.1) | 18 (22.0) | .059 |
| Fatigue | 4 (4.9) | 8 (9.8) | 9 (11.0) | .335 |
| Dyspnea | 64 (78.0) | 74 (90.2) | 71 (86.6) | .081 |
| Lower limb pain | 28 (24.4) | 30 (44.8) | 42 (60.0) | .116 |
| Lower limb edema | 27 (43.5) | 27 (41.5) | 37 (52.9) | .369 |
| USG findings of DVT | 25 (54.3) | 25 (47.2) | 42 (68.9) | .057 |
| Electrocardiography | ||||
| Sinus tachycardia | 57 (91.9) | 62 (92.5) | 58 (87.9) | .604 |
| Right bundle branch block | 23 (41.1) | 24 (38.1) | 24 (40.0) | .945 |
| T-wave inversion (leads V1-3) | 24 (42.1) | 28 (44.4) | 24 (40.0) | .883 |
| Atrial dysrhythmia | 5 (8.8) | 10 (15.4) | 5 (8.3) | .367 |
| S1Q3T3 | 36 (64.3) | 38 (58.5) | 36 (60.0) | .798 |
| Localization of thrombosis in PCTA | ||||
| Common pulmonary artery | 12 (14.2) | 14 (16.6) | 14 (16.8) | |
| Right pulmonary artery | 11 (13.0) | 12 (14.2) | 11 (13.2) | |
| Left pulmonary artery | 6 (7.1) | 4 (4.7) | 4 (4.8) | |
| Left and right pulmonary artery | 8 (9.5) | 7 (8.3) | 8 (9.6) | |
| Segmental pulmonary artery | 2 (2.3) | 2 (2.3) | 1 (1.2) | |
| Subsegmental pulmonary artery | 48 (57.1) | 42 (50.0) | 45 (54.2) | |
Abbreviations: CABG, coronary artery bypass graft; DVT, deep vein thrombosis; HCO3, bicarbonate; MI, myocardial infarction; OCS, oral contraception; PCI, percutaneous coronary intervention; PCTA, pulmonary CT angiography; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; SD, standard deviation; USG, ultrasonography.
aContinuous variables are presented as mean ± SD.
bNominal variables are presented as frequency (%).
Table 2.
Laboratory and Echocardiography Findings.a,b
| Prognostic Nutritional Index | ||||
|---|---|---|---|---|
| T1 (n = 84) | T2 (n = 84) | T3 (n = 83) | P | |
| Laboratory variables | ||||
| Glomerular filtration rate (CKD-EPI) | 62 ± 24 | 64 ± 28 | 69 ± 26 | .072 |
| Albumin, mg/dL | 2.3 ± 0.4 | 2.9 ± 0.4 | 3.4 ± 0.5 | <.001 |
| Troponin I, ng/mL | 1.2 ± 2.2 | 0.6 ± 0.9 | 0.8 ± 1.6 | .171 |
| Creatinine, mg/dL | 1.2 ± 0.8 | 1.1 ± 0.5 | 1.0 ± 0.3 | .089 |
| d-Dimer, ng/mL | 5002 ± 5856 | 4679 ± 5519 | 5110 ± 4474 | .513 |
| Creatine kinase–MB, U/L | 32 ± 25 | 29 ± 18 | 24 ± 20 | .098 |
| Brain-type natriuretic peptide, pg/mL | 785 ± 959 | 409 ± 287 | 482 ± 449 | .680 |
| White blood cell count, cells/µL | 11.8 ± 3.8 | 11.6 ± 4.4 | 12.2 ± 3.8 | .354 |
| Lymphocyte, cells/µL | 1.2 ± 0.5 | 1.8 ± 0.7 | 2.4 ± 1.0 | <.001 |
| Neutrophil, cells/µL | 10.0 ± 5.6 | 8.8 ± 4.1 | 14.9 ± 5.2 | .108 |
| Hemoglobin, g/dL | 12.6 ± 2.1 | 12.9 ± 1.8 | 12.8 ± 2.2 | .431 |
| Blood urea nitrogen mg/dL | 30 ± 19 | 23 ± 11 | 19 ± 8 | .001 |
| Echocardiography parameters | ||||
| LVEF, % | 55 ± 8 | 57 ± 5 | 59 ± 4 | .103 |
| RV TAPSE | 18 ± 3 | 19 ± 4 | 20 ± 4 | .053 |
| RV S’ velocity, cm/s | 10 ± 2 | 10 ± 2 | 10 ± 2 | .207 |
| PASP, mm Hg | 54 ± 14 | 56 ± 15 | 54 ± 13 | .354 |
| LV diameter, mm | 4.7 ± 0.4 | 5.1 ± 0.4 | 5.0 ± 0.4 | .129 |
| RV diameter, mm | 5.0 ± 0.8 | 4.6 ± 0.8 | 4.4 ± 0.7 | .062 |
| LA diameter, mm | 3.9 ± 0.4 | 3.7 ± 0.5 | 3.9 ± 0.7 | .153 |
| RV dilatation | 67 (97.1) | 66 (95.1) | 66 (95.3) | .852 |
| Presence of thrombus | 8 (14.5) | 7 (10.9) | 3 (4.7) | .185 |
Abbreviations: LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; PASP, pulmonary artery systolic pressure; RV, right ventricle; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion.
aContinuous variables are presented as mean ± SD.
bMann-Whitney U test was used for continuous variables.
In-Hospital and Long-Term Outcomes
Table 3 summarizes the in-hospital and long-term clinical outcomes. In-hospital mortality was significantly higher in tertile 1 compared to other tertiles (22.6%, 7.2%, and 2.4%, respectively, P < .001). Patients in tertile 1 group also had a higher incidence, asystole, hypotension, and cardiogenic shock. All-cause mortality was significantly higher in tertile 1 in long-term follow-up (26.2%, 12.8%, and 4.9%, P < .001). The PNI level of 38 was identified as an effective cutoff point for in-hospital survival rate (area under curve = 0.79; 95% confidence interval [CI]: 0.70-0.88, P < .001), and it had a sensitivity of 53% and specificity of 93% (Figure 1). The patients were followed up for a mean period of 53.8 ± 5.4 months. The 5-year Kaplan-Meier overall survivals for the 3 groups were 84.3% and 97.9%, respectively. The Kaplan-Meier cumulative survival curve is shown in Figure 2. The 5-year Kaplan-Meier survivals for T1, T2, and T3 were 77.4%, 92.9%, and 97.6%, respectively.
Table 3.
In-Hospital and Long-Term Outcomes.a,b,c
| Prognostic Nutritional Index | ||||
|---|---|---|---|---|
| T1 (n = 84) | T2 (n = 84) | T3 (n = 83) | P | |
| In-hospital course | ||||
| All-cause mortality | 19 (22.6) | 6 (7.1) | 2 (2.4) | <.001 |
| Major bleeding | 13 (15.5) | 10 (11.9) | 13 (15.7) | .736 |
| Intracranial hemorrhage | 1 (1.2) | 0 (0.0) | 1 (1.2) | .602 |
| Use of fresh frozen plasma | 1 (1.2) | 1 (1.2) | 1 (1.2) | 1.000 |
| Red cell transfusion | 10 (11.9) | 4 (4.8) | 7 (8.5) | .248 |
| Hemodialysis | 5 (6.0) | 2 (2.4) | 2 (2.4) | .360 |
| Minor bleeding | 35 (41.7) | 35 (41.7) | 28 (33.7) | .480 |
| Asystole | 18 (21.4) | 7 (8.3) | 3 (3.6) | <.001 |
| Hypotension, <90 mm Hg | 22 (26.2) | 9 (10.7) | 5 (6.0) | <.001 |
| Cardiogenic shock | 13 (15.5) | 8 (9.5) | 2 (2.4) | .014 |
| Out-hospital course | ||||
| All-cause mortality | 17 (26.2) | 10 (12.8) | 4 (4.9) | <.001 |
| Recurrent pulmonary embolism | 3 (4.6) | 2 (2.6) | 2 (2.5) | .714 |
| Major bleeding | 2 (3.1) | 2 (3.1) | 3 (3.7) | .918 |
| Minor bleeding | 4 (6.2) | 1 (1.3) | 6 (7.4) | .174 |
| History of INR >5 | 9 (13.8) | 11 (14.1) | 16 (19.8) | .528 |
| Use of fresh frozen plasma | 2 (3.1) | 4 (5.3) | 7 (8.8) | .339 |
Abbreviation: IRN, international normalized ratio.
aNominal variables presented as frequency (%).
bContinuous variables are presented as median and 25 to 75 percentiles; nominal variables are presented as frequency (%).
cMann-Whitney U test used for continuous variables and Pearson χ2 test used for nominal variables.
Figure 2.
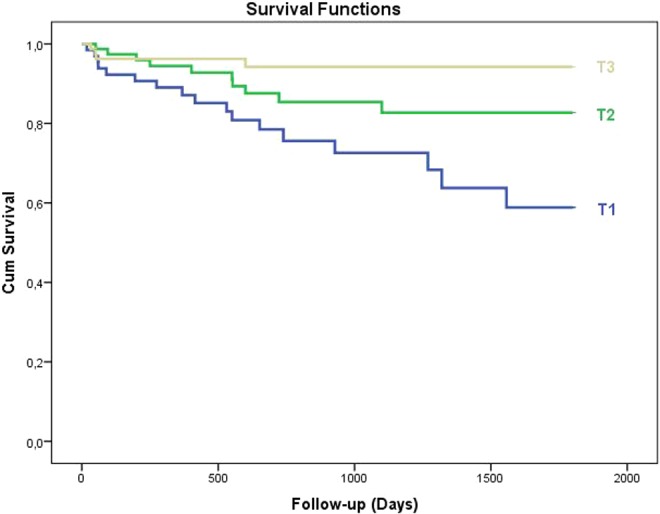
The Kaplan-Meier cumulative survival curve is shown. The 5-year Kaplan Meier survival for T1, T2, and T3 was 77.4%, 92.9%, and 97.6% respectively.
Table 4 lists unadjusted and adjusted logistic regression for in-hospital events (mortality and major adverse cardiac events) and Cox proportional regression analysis for long-term mortality categorized by tertiles. The in-hospital mortality of tertile 1 had 8.1 times higher mortality rates (95% CI: 2.1-27.1) than tertile 3, which had lower rates and was used as the reference. The long-term mortality also had the higher rates at tertile 1 and had 4.6 times higher mortality rates (95% CI: 2.6-10.9) than tertile 1, which had the lower rates and was used as the reference.
Table 4.
In-Hospital Event Rates and Logistic Regression Models for Mortality by Prognostic Nutritional Index and Cox Proportional Analysis and Long-Term Mortality by Prognostic Nutritional Index.
| Prognostic Nutritional Index | |||
|---|---|---|---|
| T1 | T2 | T3 | |
| In-hospital mortality | |||
| Number of deaths | 19 | 6 | 2 |
| Mortality, % | 22.6 | 7.1 | 2.4 |
| Mortality, OR (95% CI) | |||
| Model 1: unadjusted | 11.8 (2.6-52.6) | 3.1 (0.6-15.9) | 1 (Reference) |
| Model 2: adjusted for age and sex | 11.9 (2.4-38.2) | 2.9 (0.8-12.5) | 1 (Reference) |
| Model 3: adjusted for all covariatesa | 8.1 (2.1-27.1) | 2.5 (0.9-10.4) | 1 (Reference) |
| Long-term mortality | |||
| Number of deaths | 17 | 10 | 4 |
| Mortality, % | 26.2 | 12.8 | 4.9 |
| Mortality, HR (95% CI) | |||
| Model 1: unadjusted | 5.5 (1.8-16.4) | 2.4 (0.7-7.9) | 1 (Reference) |
| Model 2: adjusted for age and sex | 5.2 (2.1-13.3) | 2.1 (0.9-6.8) | 1 (Reference) |
| Model 3: adjusted for all covariatesa | 4.6 (2.6-10.9) | 1.8 (1.0-5.5) | 1 (Reference) |
Abbreviations: CI, confidence interval; HR, hazard ratio; OR, odds ratio.
aDemographics (age and sex); first measurement of systolic blood pressure and heart rate; first measurement during hospitalization of the following laboratory values (admission glomerular filtration rate calculated by CKD-EPI, blood urea nitrogen, white blood cell count, hematocrit, and platelet count); creatine kinase–MB, troponin I, d-dimer, and brain-type natriuretic peptide; comorbidities (diabetes, chronic kidney disease, hypertension, stroke, heart failure, cancer, chronic lung disease, and atrial dysrhythmia), and medications (use of oral contraceptives, warfarin, and steroid).
Discussion
This is a pilot study presenting nutritional status in patients with PE by the help of internationally accepted nutritional index, PNI. Nutritional status determines the patients’ general medical conditions comprising immune competence, protein turnover, and physical condition. Our retrospective observational study revealed a significant correlation between admission PNI and long-term survival. Malnutrition, which is easily adjusted using PNI, manifests a state of decreased food intake and protein turnover despite the regular necessities of the human metabolism. As a matter of fact, it leads decreased response to acute disease, exacerbations of chronic diseases, and decreased long-term survivals. Considering cardiovascular diseases, PNI was first adopted in ST-segment elevation myocardial infarction recently.16 Thus, for the first time, we introduce association with PNI and PE with a median follow-up of 53.8 ± 5.4 months. The predictor value of PNI in PE is up-and-coming. In- and out-hospital PE mortality was strongly correlated with patients’ PNI.
Pulmonary embolism is a major cause of mortality, morbidity, and hospitalization in Europe. Numerous prediction indices originated from clinical status have been shown in patients with PE. For instance, PESI is one of the most extensively validated score.17 Pulmonary Embolism Severity Index score includes age, gender, cancer, chronic heart failure, chronic pulmonary disease, pulse rate, systolic blood pressure, respiratory rate, body temperature, altered mental status, and arterial oxyhemoglobin status.5 Its strength underlies in the reliable identification of patients for 30-day mortality5 Despite its serious strength, access to admission data and calculation may sometimes be problematic. Therefore, we introduced a simple calculation, PNI, whose validity was proved with long-term follow-up. Admission albumin and lymphocyte count were enough to determine PNI, whose long-term predictive role is valuable. The mortality in acute PE is strongly correlated with PNI score similar to myocardial infarction.16,18 The decreased response of albumin and lymphocytes to acute disease indicates low immune-nutritional status. Therefore, the PNI score should be considered as a clinical element and indicator of disease severity in acute PE. Its role can be defined as an identifier for high-risk patients who may benefit long duration of anticoagulation therapy or close follow-up. Whereas considering chronic diseases, the prognostic power of PNI has not been tested. Thus, it is difficult to correlate chronic PE with PNI at the present moment.
Albumin is the most abundant plasma protein produced by hepatocytes. During inflammation, there are decreases in plasma albumin concentration and albumin synthesis.19 When isolated hepatocytes are incubated in the presence of pro-inflammatory cytokines, negative acute phase proteins such as albumin are reduced.20 Moreover, lymphocyte count is influenced in inflammatory events due to increased apoptosis and is a frequent finding secondary to increased levels of corticosteroids in acute stress conditions such as acute coronary syndrome and PE.21 Therefore, complete blood cell subgroups (neutrophils, lymphocytes, and platelets) are used as inflammation markers with ease.22 As we explained in detail, a novel predictive parameter, PNI, was introduced to patients with PE, which includes both albumin and lymphocyte counts. Prognostic nutritional index brings these 2 important measurements together and presents a noteworthy prognostic value.
According to the ROC curve analysis of the current study, an optimal cutoff value of 38 was obtained. The only cardiovascular study in the literature, which was performed in patients with ST-segment elevation myocardial infarction, also found the same cutoff value of 38.16 From this view, this increases the predictive value of PNI not only in PE but also in overall cardiovascular patients.
Study Limitations
The current study has several limitations. First, this was a single-center, retrospective, observational study. Second, PNI levels after discharge were not recorded; thus, the effect of post discharge PNI level on clinical outcomes could not be determined. Third, although PNI level might be influenced by hormonal changes, we could not measure hormones such as serum catecholamine and cortisol. Fourth, because albumin is not a standard laboratory test during PE follow-up, we are lacking in follow-up PNI score. Finally, because of methodological limitations of retrospective analysis, it is not possible to define the exact causal relationship between PNI level and mortality. These limitations require further examinations to validate our present findings; a prospective study in a larger number of patients is needed.
Conclusion
Our pilot study indicated that PNI, calculated based on serum albumin level and lymphocyte count, is an independent prognostic factor for survival of patients with PE. This result confirmed that nutritional and immunological situations are important when considering the long-term outcome in patients with PE. Patients with low PNI were demonstrated to have poor prognosis. Further investigations on independent multicenter cohorts should be performed in order to validate our findings.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Minges KE, Bikdeli B, Wang Y, et al. National trends in pulmonary embolism hospitalization rates and outcomes for adults aged ≥65 years in the United States (1999 to 2010). Am J Cardiol. 2015;116(9):1436–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gussoni G, Frasson S, La Regina M, Di Micco P, Monreal M; RIETE Investigators Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb Res. 2013;131(1):24–30. [DOI] [PubMed] [Google Scholar]
- 3. Bozbay M, Uyarel H, Avsar S, et al. Creatinine kinase isoenzyme-MB: a simple prognostic biomarker in patients with pulmonary embolism treated with thrombolytic therapy. J Crit Care. 2015;30(6):1179–1183. [DOI] [PubMed] [Google Scholar]
- 4. Xu NL, Xue H, Chen YS, Li HR, Hong RJ. Establishment and evaluation of a prognostic model for acute pulmonary embolism [in Chinese]. Zhonghua Jie He He Hu Xi Za Zhi. 2016;39(4):304–310. [DOI] [PubMed] [Google Scholar]
- 5. Righini M, Roy PM, Meyer G, Verschuren F, Aujesky D, Le Gal G. The Simplified Pulmonary Embolism Severity Index (PESI): validation of a clinical prognostic model for pulmonary embolism. J Thromb Haemost. 2011;9(10):2115–2117. [DOI] [PubMed] [Google Scholar]
- 6. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang L, Xia L, Wang Y, et al. Low prognostic nutritional index (PNI) predicts unfavorable distant metastasis-free survival in nasopharyngeal carcinoma: a propensity score-matched analysis. PLoS One. 11(7):e0158853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan AW, Chan SL, Wong GL, et al. Prognostic nutritional index (PNI) predicts tumor recurrence of very early/early stage hepatocellular carcinoma after surgical resection. Ann Surg Oncol. 2015;22(13):4138–1448. [DOI] [PubMed] [Google Scholar]
- 9. Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. [DOI] [PubMed] [Google Scholar]
- 10. Narumi T, Arimoto T, Funayama A, et al. The prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol. 2013;62(5):307–313. [DOI] [PubMed] [Google Scholar]
- 11. Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. [DOI] [PubMed] [Google Scholar]
- 12. Oh JK, Hagler DJ, Cabalka A, Reeder GS, Cetta F, Jr, Seward JB. Transesophageal and intracardiac echocardiography In: Oh JK, Seward JB, Tajik AJ, eds. The Echomanual. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2006:29–58. [Google Scholar]
- 13. Konstantinides SV, Torbicki A, Agnelli G, et al. ; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3069. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJ, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. [DOI] [PubMed] [Google Scholar]
- 15. Moya A, Sutton R, Ammirati F, et al. ; Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS). Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30(21):2631–2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basta G, Chatzianagnostou K, Paradossi U, et al. The prognostic impact of objective nutritional indices in elderly patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Int J Cardiol. 2016;221:987–992. [DOI] [PubMed] [Google Scholar]
- 17. Chan CM, Woods C, Shorr AF. The validation and reproducibility of the Pulmonary Embolism Severity Index. J Thromb Haemost. 2010;8(7):1509–1514. [DOI] [PubMed] [Google Scholar]
- 18. Keskin M, Hayıroğlu ML, Keskin T, et al. A novel and useful predictive indicator of prognosis in ST-segment elevation myocardial infarction; prognostic nutritional index. Nutr Metab Cardiovasc Dis. 2017; 27(5):438–446. [DOI] [PubMed] [Google Scholar]
- 19. Liao WSL, Jefferson LS, Taylor JM. Changes in plasma albumin concentration, synthesis rate and mRNA level during acute inflammation. Am J Physiol. 1986;251(6 pt 1):C928–C934. [DOI] [PubMed] [Google Scholar]
- 20. Castell JV, Gómez-Lechón MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12(5):1179–1186. [DOI] [PubMed] [Google Scholar]
- 21. Hoffman M, Blum A, Baruch R, Kaplan E, Benjamin M. Leukocytes and coronary heart disease. Atherosclerosis. 2004;172(1):1–6. [DOI] [PubMed] [Google Scholar]
- 22. Karataş MB, İpek G, Onuk T, et al. Assessment of prognostic value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in patients with pulmonary embolism. Zhonghua Minguo Xin Zang Xue Hui Za Zhi. 2016;32(3):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]


