Abstract
Disseminated intravascular coagulation (DIC) is a major pathophysiological mechanism of sepsis and greatly increases the risk of death in septic patients. Disseminated intravascular coagulation is a complex physiological phenomenon that involves inappropriate activation of coagulation, inflammation, and endothelial processes. The purpose of this study was to analyze the levels of inflammatory cytokines in the plasma of patients with DIC in order to compare the measured levels with those from healthy individuals, draw correlations, and provide a basis for further biomarker panel development. The inflammatory biomarkers interleukin (IL) 1β, IL-6, IL-8, IL-10, interferon (IFN) γ, vascular endothelial growth factor (VEGF), tumor necrosis factor (TNF) α, monocyte chemoattractant protein (MCP)-1, and epidermal growth factor (EGF) showed significant (P < .05) elevation in patients with DIC. Interestingly, while numerous correlations were present between IL-β, IL-6, IL-8, IL-10, IFN-γ, TNF-α, MCP-1, and many of the inflammatory cytokines measured, VEGF and EGF exhibited much less extensive correlation, suggesting that their involvement in DIC may be independent of the other investigated inflammatory markers.
Keywords: disseminated intravascular coagulation, sepsis-associated coagulopathy, biochip, inflammatory cytokines
Introduction
Sepsis is a severe systemic inflammatory response to infection and is the most common cause of death in critically ill patients. Epidemiological information about sepsis is highly variable due to the spectrum of severity of the disease1; however, it is clear that sepsis is a major cause of morbidity and mortality worldwide. Septicemia was recognized by the Centers for Disease Control as the 11th most common cause of death in the United States in 2010,2 and the rate of in-hospital death due to sepsis is increasing.3 Sepsis is also a significant financial burden; an average hospital stay with sepsis costs in excess of US$500 000 and in 2011 it was estimated that the annual cost of sepsis in the United States is greater than US$20 billion.4
Disseminated intravascular coagulation (DIC) is one of the major pathophysiological mechanisms of sepsis, and the development of DIC doubles a septic patient’s risk of death.5,6 Disseminated intravascular coagulation is characterized, seemingly paradoxically, by both bleeding and thrombotic complications.6 Inappropriate and widespread activation of the coagulation cascade leads to fibrin deposition, vascular occlusion, and multiple organ failure. This coagulation process consumes platelets and coagulation factors, leading to a potential for severe and potentially fatal bleeding complications.
The pathophysiology of DIC is complex and involves numerous interactions between the coagulation cascade, the host immune and inflammatory system, and the damaged vascular endothelium. The interactions between these systems are multidirectional and multifaceted, and much remains to be understood about these processes. Due to the complexity of sepsis-associated DIC, in vitro and animal models may not accurately replicate the disease state. Accordingly, analysis of blood plasma obtained from patients with DIC can provide valuable insight into the disease process and provide a framework for the development of improved diagnostic, prognostic, and research tools.
The diagnosis of sepsis-associated DIC is a complex clinical problem. The diagnosis of sepsis (or another condition associated with DIC) is a prerequisite for the diagnosis of DIC. Diagnosis of sepsis is often difficult as sepsis and associated conditions occur across a range of severities, and diagnosis is based not on a single laboratory test or test panel but on a range of clinical and laboratory parameters.4,7 After a diagnosis of sepsis has been established, the diagnosis of DIC still presents a clinical problem. Again, there is no gold standard biomarker or laboratory test for DIC, and diagnosis is based on a score computed from clinical data. Several scoring systems have been proposed and tested,8–11 with the system proposed by the International Society of Thrombosis and Hemostasis (ISTH) being the most widely accepted.8 The ISTH scoring system categorizes patient scores on a scale of 0 to 5, classifying a score of 3 to 4 as nonovert (compensated) and a score of 5 or higher as overt (decompensated). A condition associated with DIC, such as sepsis, trauma, or obstetrical calamity, is a prerequisite for use of the scoring system. Points are awarded for reduced platelet count, elevation in a fibrin-related marker (ie, D-dimer), prolonged prothrombin time, and reduced fibrinogen level.8
Multiple attempts have been made to develop simpler tests for sepsis and DIC; over 150 biomarkers have been investigated for sepsis alone.12 No single marker has thus far been shown to be diagnostic for sepsis or DIC. It is therefore likely that any meaningful biomarker profile used for evaluation of patients with sepsis with the added complication of DIC will have to take into account a panel of biomarkers. This marker panel will likely be a representative of the multiple processes and systems dysregulated during the disease process, such as inflammation, endothelial dysfunction, and coagulation. Before a biomarker panel can be devised, biomarker candidates must be screened in order to identify the most promising candidates. Accordingly, high-throughput techniques allowing for the rapid evaluation of levels of numerous factors in a small volume of a single sample are desirable. The use of this type of biomarker screening approach will facilitate the identification of the optimal markers to use to represent a given system, such as inflammation, in a more comprehensive biomarker profile for DIC. The biomarker profiling of this complex syndrome requires an understanding of the pathogenesis of the components of the hemostatic system including coagulation, fibrinolysis, platelets, and endothelial abnormalities. Since the activation of the hemostatic system results in marked inflammatory responses, a multipanel biochip array profiling of these samples provides additional insight into the interface between hemostatic abnormalities and inflammatory processes.
Biochip technology allows for the simultaneous measurement of numerous analytes in a single sample in an efficient, high-throughput manner, allowing for the rapid collection of a large amount of data. Biochips specific for proteins have important implications in drug discovery, diagnostics, and basic research13; biochips are a current focus of study as diagnostic tools for sepsis.14 Current commercially available biochips allow for the simultaneous measurement of numerous commonly related factors, such as the major inflammatory markers addressed in this study: interleukin (IL) 2, IL-4, IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), interferon (IFN) γ, tumor necrosis factor (TNF) α, IL-1α, IL-1β, and epidermal growth factor (EGF).
Inflammatory cytokines contribute to the dysregulation of the procoagulant and anticoagulant pathways that is observed in sepsis-associated DIC. Although the immune and inflammatory responses are imperative for pathogen clearance and the ultimate resolution of infection, this overwhelming inflammatory response can be detrimental to the host, in part, by inducing the development of coagulopathy. Multiple inflammatory cytokines, including IL-6,15,16 IL-1,17–21 and TNF-α,20–23 have long been known to contribute to the development of a hypercoagulable state, largely through induction of tissue factor (TF) expression. Additional cytokines, such as IL-2,24,25 have also been shown to promote the hypercoagulable state, whereas some cytokines, such as IL-1018,26,27 and IL-4,18,26,28 have been shown to oppose the coagulation induced by other pro-inflammatory factors.
In this study, the levels of the inflammatory cytokines IL-2, IL-6, IL-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1α, IL-1β, and EGF were measured in the plasma of patients with sepsis-associated DIC. Cytokine levels in DIC patients were compared to levels in plasma from healthy individuals. Cytokines with the greatest observed change between normal individuals and DIC patients may be the most relevant to the pathophysiology of DIC and may be appropriate targets for future study. Additionally, comparisons were made between patients with overt and nonovert DIC to determine which factors may have the greatest relevance to the development of coagulopathy. Finally, correlations were also assessed between markers.
Materials and Methods
Blood Samples
De-identified blood samples from 50 patients with sepsis-associated DIC were used throughout this study. All patients met the criteria for sepsis, defined according to the 1992 American College of Chest Physicians/Society of Critical Care Medicine definition.7 Patients with potential DIC were identified on the basis of reduced platelet count and elevated international normalized ratio (INR). Citrated plasma samples were collected from patients with potential DIC and final diagnosis of DIC was made using the ISTH scoring algorithm for overt DIC, shown in Table 1.8 Patients with a DIC score of 3 to 4 were classified as having nonovert DIC while patients with a score of 5 or greater were classified as having overt DIC. In this population, 6 patients had overt DIC and 44 had nonovert DIC. Plasma was stored at −80ºC prior to analysis. Control plasma samples from 48 healthy, nonsmoking volunteers, male and female, aged 18 to 55 were purchased a commercial laboratory (George King Biomedical, Overland Park, Kansas).
Table 1.
DIC Scoring Algorithm.
| Variable | Value | Points |
|---|---|---|
| Platelets (K/μL) | >100 | 0 |
| 50-100 | 1 | |
| <50 | 2 | |
| INR | <1.3 | 0 |
| 1.3-1.7 | 1 | |
| >1.7 | 2 | |
| D-dimer (ng/mL) | <400 | 0 |
| 400-4000 | 2 | |
| >4000 | 3 | |
| Fibrinogen (mg/dL) | >100 | 0 |
| <100 | 1 |
Abbreviations: DIC, disseminated intravascular coagulation; INR, international normalized ratio.
Biochip Assay
A Randox Cytokine and Growth Factors High-Sensitivity Array assay kit was used to profile IL-2, IL-4, IL-6, Il-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1α, IL-1β, MCP-1, and EGF (Randox, London, United Kingdom). This allowed quantification of all factors in a single patient sample simultaneously using a sandwich chemiluminescent immunoassay. Fifty DIC samples and 48 normal samples were analyzed. Briefly, each biochip provided in the kit contained 12 testing regions, each with a different immobilized antibody specific to a different cytokine. The chip was incubated with the patient sample. After washing, conjugate, consisting of horseradish peroxidase–labeled, analyte-specific antibodies, was incubated with the chip. Increased level of a bound cytokine increased binding of conjugate and thus the chemiluminescent signal emitted upon addition of the signal reagent. The luminescent signal generated from each region of the biochip was translated into analyte concentration by the Randox evidence investigator using a calibration curve generated based on controls of known concentration.
Statistical Analysis
Descriptive statistics were calculated for each cytokine for both normal and DIC groups. Outliers were excluded if the measured concentration was greater than the mean plus 2 standard deviations. Statistical analysis was performed using GraphPad Prism software (GraphPad Software, La Jolla, California). Statistical difference between the normal and the DIC group was evaluated using the Mann-Whitney t test for nonparametric data with α = .05 as the cutoff for significance.
Percentage change from the normal mean for each cytokine was also calculated for each DIC sample using the following equation: . Correlations were calculated between these values using Spearman correlation coefficients with α = .05 as the cutoff for significance.
Results
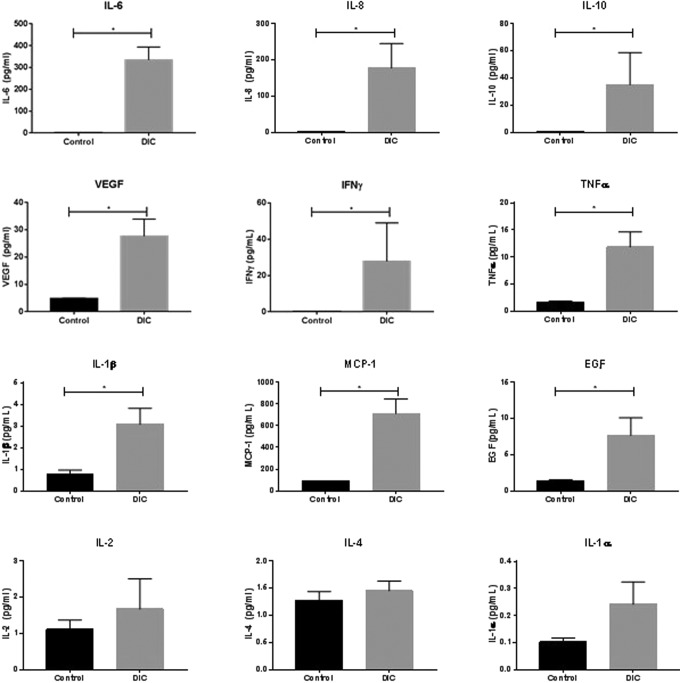
The levels of IL-2, IL-4, IL-6, IL-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1α, IL-1β, MCP-1, VEGF, and EGF were compared between the plasma from DIC patients and the plasma from healthy controls. As shown in Figure 1, the levels of IL-6, IL-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1β, MCP-1, and EGF were found to be significantly elevated in DIC patients compared to normal individuals (P < .005). The levels of IL-2, IL-4, and IL-1α were not found to be significantly different between healthy individuals and DIC patients (P > .05). Tabulated data, including median and range, are also shown in Table 2.
Figure 1.
Comparison of cytokine levels between DIC patients and healthy individuals. Comparisons were made using the Mann-Whitney t test for nonparametric data with α = .05 as the cutoff for statistical significance. Data are shown as mean ± SEM. DIC indicates disseminated intravascular coagulation; SEM, standard error of the mean.
Table 2.
Tabulated Biomarker Data in DIC Patients and Healthy Controls.
| Marker | Healthy Controls (n = 48) | DIC Patients (n = 50) | ||||
|---|---|---|---|---|---|---|
| Mean ± SEM (pg/mL) | Median (pg/mL) | Range (pg/mL) | Mean ± SEM (pg/mL) | Median (pg/mL) | Range (pg/mL) | |
| IL-2 | 1.11 ± 0.26 | 0 | 0-6.25 | 1.67 ± 0.8 | 0 | 0-39.2 |
| IL-4 | 1.28 ± 0.16 | 1.41 | 0-6.73 | 1.45 ± 0.2 | 1.05 | 0-6.0 |
| IL-6 | 1.25 ± 0.18 | 0.94 | 0.25-7.29 | 334.7 ± 58.7 | 172.5 | 4.3-1262 |
| IL-8 | 2.64 ± 0.13 | 2.59 | 0.98-5.74 | 177.9 ± 67.4 | 37.85 | 1.4-2463 |
| IL-10 | 0.68 ± 0.052 | 0.6 | 0-2.12 | 35.14 ± 23.7 | 3.85 | 0.3-1182 |
| VEGF | 4.78 ± 0.19 | 4.59 | 2.14-7.77 | 27.61 ± 6.32 | 14.3 | 1.3-260.3 |
| IFN-γ | 0.17 ± 0.045 | 0 | 0 -1.76 | 27.75 ± 21.25 | 0.25 | 0-1041 |
| TNF-α | 1.73 ± 0.15 | 1.77 | 0-6.55 | 11.87 ± 2.77 | 4.2 | 1.2-107 |
| IL-1α | 0.10 ± 0.15 | 0.1 | 0-0.53 | 0.24 ± 0.082 | 0.1 | 0-3.8 |
| IL-1β | 0.79 ± 0.18 | 0 | 0-5.63 | 3.08 ± 0.74 | 1.65 | 0-31.8 |
| MCP-1 | 88.6 ± 3.93 | 90.38 | 30.22-149.5 | 713.5 ± 132.2 | 412.5 | 10.9-4511 |
| EGF | 1.36 ± 0.17 | 1.27 | 0-5.42 | 7.67 ± 2.43 | 2.2 | 0-83.1 |
Abbreviations: DIC, disseminated intravascular coagulation; EGF, epidermal growth factor; IFN, interferon; IL, interleukin; SEM, standard error of the mean; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Percentage change was calculated between each individual DIC sample and the mean of the normal samples using the formula: , as shown in Figure 2. The degree of increase in DIC patients compared to normal was highly varied between cytokines. Interleukin 6 showed the highest average percentage change from the normal mean, averaging approximately a 10 000 percentage increase.
Figure 2.
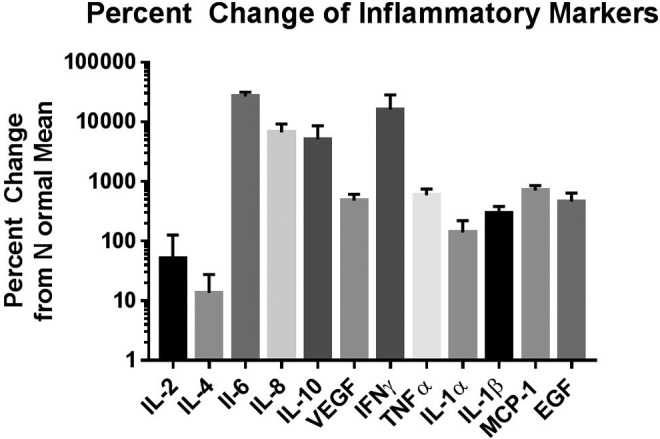
Percentage change of inflammatory markers in the blood of DIC patients compared to the normal mean. Data are shown as mean ± SEM on a logarithmic scale. DIC indicates disseminated intravascular coagulation; SEM, standard error of the mean.
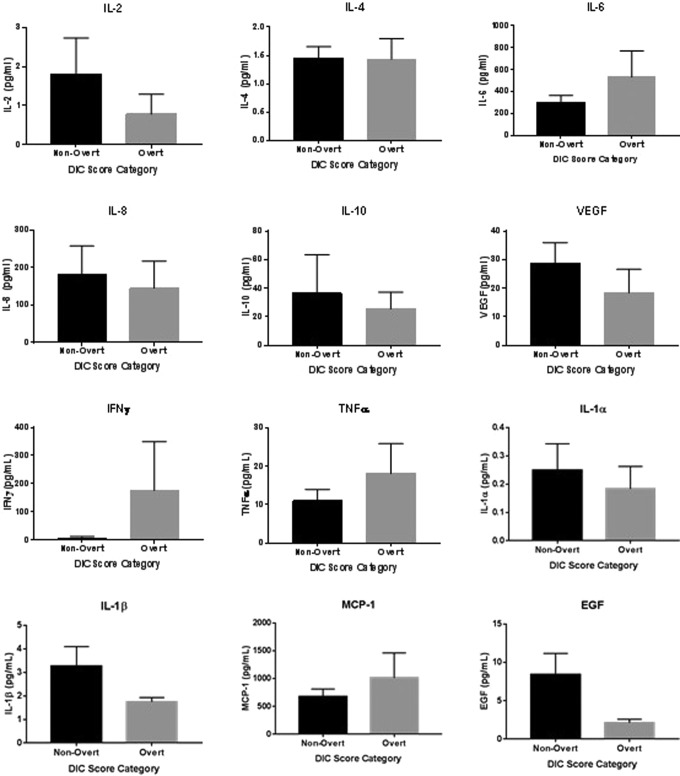
Disseminated intravascular coagulation patients were divided into 2 groups based on ISTH DIC score. Patients with a score of 3 to 4 were categorized as having nonovert DIC. Patients with a score of 5 or greater were categorized as having the more severe form of overt DIC. Of the 50 patients analyzed in this study, 44 had nonovert DIC and 6 had overt DIC. Inflammatory cytokine levels were compared between the overt and nonovert groups, as shown in Figure 3 and Table 3. No significant differences were observed between the nonovert and overt patients for any of the measured cytokines.
Figure 3.
Comparison of cytokine levels between patients with nonovert DIC and overt DIC. Comparisons were made using the Mann-Whitney t test for nonparametric data with α = .05 as the cutoff for statistical significance. Data are shown as mean ± SEM. DIC indicates disseminated intravascular coagulation; SEM, standard error of the mean.
Table 3.
Tabulated Biomarker Data in Patients With Overt or Nonovert DIC.
| Marker | Nonovert DIC (n = 44) | Overt DIC (n = 6) | ||||
|---|---|---|---|---|---|---|
| Mean ± SEM (pg/mL) | Median (pg/mL) | Range (pg/mL) | Mean ± SEM (pg/mL) | Median (pg/mL) | Range (pg/mL) | |
| IL-2 | 1.80 ± 0.95 | 0 | 0-39.2 | 0.78 ± 0.51 | 0 | 0-2.8 |
| IL-4 | 1.46 ± 0.20 | 1 | 0-6.0 | 1.43 ± 0.36 | 1.65 | 0-2.4 |
| IL-6 | 307.4 ± 58.49 | 168.5 | 6.5-1262 | 535.1 ± 238.6 | 334.1 | 4.3-1262 |
| IL-8 | 182.3 ± 76.09 | 37.85 | 1.4-2463 | 145.7 ± 71.91 | 62.3 | 6-424.3 |
| IL-10 | 36.44 ± 26.94 | 3.85 | 0.3-1182 | 25.63 ± 11.62 | 17.15 | 0.6-64.4 |
| VEGF | 28.85 ± 8.20 | 14.3 | 1.3-260.3 | 18.45 ± 8.20 | 12.85 | 2.5-57.2 |
| IFN-γ | 7.41 ± 5.59 | 0.2 | 0-244.8 | 7.41 ± 5.59 | 1.05 | 0-1041 |
| TNF-α | 11.02 ± 2.97 | 4 | 1.2-107 | 18.13 ± 7.85 | 11.8 | 2.3-54.9 |
| IL-1α | 0.25 ± 0.093 | 0.1 | 0-3.8 | 0.18 ± 0.079 | 0.15 | 0-0.5 |
| IL-1β | 3.26 ± 0.84 | 1.6 | 0-31.8 | 1.75 ± 0.18 | 1.8 | 1.1-2.3 |
| MCP-1 | 672.7 ± 138.3 | 389.9 | 10.9-4511 | 1013 ± 449.1 | 641.4 | 229.4-3174 |
| EGF | 8.43 ± 2.75 | 2.2 | 0-83.1 | 2.117 ± 0.45 | 2.05 | 0.8-3.9 |
Abbreviations: DIC, disseminated intravascular coagulation; EGF, epidermal growth factor; IFN, interferon; IL, interleukin; SEM, standard error of mean; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Correlations were calculated between the percentage change from the normal mean for each cytokine for each DIC sample using Spearman correlation coefficients with α = .05 as the cutoff for significance. Spearman correlation coefficients for significant correlations between inflammatory cytokines are shown in Table 4. Significant correlations were observed between the markers that were found to be elevated in the DIC patients. Interleukin 1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α, and MCP-1 were found to be positively correlated with each other in varying combinations. The strongest correlation observed was between IL-6 and IL-4, with a Spearman correlation coefficient of .874. Numerous other correlations were moderately strong, with correlation coefficients between .4 and .6. Vascular endothelial growth factor and EGF were found to have significant correlation only with each other, with a correlation coefficient of .395.
Table 4.
Spearman Correlation Coefficients for Inflammatory Cytokines.a
| IL-2 | IL-4 | IL-6 | IL-8 | IL-10 | VEGF | IFN-γ | TNF-α | IL-1α | IL-1β | MCP-1 | EGF | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-2 | NS | NS | NS | NS | NS | 0.447 | 0.461 | 0.429 | 0.352 | NS | NS | |
| IL-4 | NS | 0.874 | 0.316 | 0.295 | NS | NS | NS | NS | 0.442 | 0.557 | NS | |
| IL-6 | NS | 0.874 | NS | NS | NS | NS | NS | NS | NS | 0.529 | NS | |
| IL-8 | NS | 0.316 | NS | 0.403 | NS | NS | 0.362 | 0.594 | 0.496 | 0.594 | NS | |
| IL-10 | NS | 0.295 | NS | 0.403 | NS | NS | 0.466 | 0.357 | 0.288 | 0.452 | NS | |
| VEGF | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.395 | |
| IFN-γ | 0.447 | NS | NS | NS | NS | NS | 0.514 | NS | 0.357 | 0.359 | NS | |
| TNF-α | 0.461 | NS | NS | 0.362 | 0.466 | NS | 0.514 | 0.325 | 0.401 | 0.584 | NS | |
| IL-1α | 0.429 | NS | NS | 0.594 | 0.357 | NS | NS | 0.325 | 0.517 | 0.448 | NS | |
| IL-1β | 0.352 | 0.442 | NS | 0.496 | 0.288 | NS | 0.357 | 0.401 | 0.517 | 0.599 | NS | |
| MCP-1 | NS | 0.557 | 0.529 | 0.594 | 0.452 | NS | 0.359 | 0.584 | 0.448 | 0.599 | NS | |
| EGF | NS | NS | NS | NS | NS | 0.395 | NS | NS | NS | NS | NS |
Abbreviations: EGF, epidermal growth factor; IFN, interferon; IL, interleukin; NS, nonsignificant correlation; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
aCompilation of the Spearman correlation coefficients. Statistical significance was determined using a cutoff value of α = .05. Spearman correlations for significant correlations (p < 0.05) are shown in the table.
Discussion
Disseminated intravascular coagulation has a complex biochemical basis involving inflammation, coagulation and fibrinolysis, endothelial dysfunction, and other physiologic processes including hemodynamic regulation. Accordingly, high-throughput methods such as the biochip assay utilized in this study are helpful in providing a comprehensive biochemical profile underlying this serious clinical scenario.
The current study was primarily carried out to profile the inflammatory cytokines in sepsis-associated coagulopathy/DIC patients. The biochip array provided a comprehensive approach for the quantitation of the important cytokines involved in inflammatory responses. In the current study, only 6 patients had overt DIC, and the remainder of the patients exhibited nonovert DIC in accordance with the ISTH classification system. It must be noted that despite the clear clinical distinction between patients with overt and nonovert DIC in accordance with the ISTH score, cytokine levels are highly variable and may not vary in accordance with this scoring system.
The significant elevation of numerous pro-inflammatory and anti-inflammatory factors, specifically IL-6, IL-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1β, MCP-1, and EGF, in patients with DIC compared to healthy individuals is not unexpected and is in line with previous research.12,29,30 Simultaneous testing of all markers in a small volume of plasma provides a broader view of the alterations of these markers and their correlations. The correlations calculated in this study, however, divided these factors into 2 clear groups.
The numerous and significant positive correlations among IL-1β, IL-6, IL-8, IL-10, IFN-γ, TNF-α, and MCP-1 indicate that this set of cytokines is jointly regulated by the same aspect of the disease process in DIC. Of these cytokines, MCP-1 showed the greatest number of correlations, with significant relationships with IL-4, IL-6, IL-8, IL-10, IFN-γ, TNF-α, IL-1α, and IL-1β, with correlation coefficients greater than 0.35 for all cytokines. Of these cytokines, TNF-α, IL-1β, IL-6, IL-8, IFN-γ, and MCP-1 have been previously shown to enhance TF expression, a key factor in the pathogenesis of DIC.31 Interleukin 10 is a classic anti-inflammatory cytokine with the ability to inhibit the synthesis of TF.18 Previous research has shown detectable amounts of anti-inflammatory cytokines, specifically IL-10 in the blood of patients with sepsis, and may serve in a protective capacity due to its ability to inhibit production of the potentially toxic pro-inflammatory cytokines.16 Due to the strong correlations between these factors, future biomarker panels developed for sepsis-associated DIC may be able to select one of these factors, such as IL-6 or TNF-α, as the representative marker for the inflammatory process.
Vascular endothelial growth factor and EGF, on the other hand, were demonstrated to correlate only with each other. This may indicate that these factors are governed by a separate aspect of DIC than the other inflammatory factors. Previous studies have demonstrated both increased19 and decreased20 levels of VEGF in DIC patients. Possible explanations of decreased VEGF levels include DIC-induced consumption of platelets and thus increased levels of soluble VEGF receptors.20 Studies have also indicated that low levels of VEGF may be predictive of organ dysfunction in DIC patients.20 The role of VEGF, important in the regulation of angiogenesis and vascular permeability, is not yet clear, nor is any potential association with patient outcome. Elevated VEGF has been observed in patients with DIC, attributed to the activation of platelets.19 However, other studies have found low levels of VEGF in patients with DIC correlating with low platelet counts, and attributing the decrease in VEGF levels to the loss of platelets and the VEGF stored within them.20 Accordingly, the role of VEGF in DIC, and other associated factors including EGF, merits further investigation.
No significant differences in cytokine levels were observed between patients with nonovert DIC and patients with overt DIC in this study. High levels of inflammation are known to play a role in the initiation of DIC in septic patients. A lack of correlation between cytokine levels and severity of DIC suggests that while inflammation plays a role in the initiation of coagulation dysfunction in these patients, it is not the main determinant of the severity of DIC that ultimately occurs. However, the number of patients in this cohort with overt DIC was low (n = 6), and the measured cytokine levels were highly variable between patients. Further studies involving larger cohorts of patients, particularly more patients with overt DIC, are necessary to draw conclusions. Therefore, the studies reported in this communication underscore the importance of profiling the plasma samples from patients recruited in sepsis-associated coagulopathy/DIC studies for both hemostatic dysfunction and inflammatory responses. Utilizing the new technology of biochip arrays provides a systemic approach to produce such data.
Sepsis-associated coagulopathy is a complex syndrome and the differentiation between patients exhibiting only sepsis without any coagulopathy can be readily made by including the DIC score to classify these patient groups. In the current study, the DIC scores were calculated for the entire cohort. The patients included in this study all exhibited coagulopathy, and regardless of the overt or nonovert classification, had an elevated DIC score of 3 or greater. Thus, all of these patients were predisposed to coagulopathy, which was associated with elevation of various inflammatory biomarkers. Therefore, simultaneous activation of coagulation and inflammation is a characteristic of this syndrome.
In order to understand the biochemical basis of DIC, numerous factors representative of different but highly interactive physiological processes must be studied. The individual assessment of each of these factors may be prohibitive in terms of time, cost, and sample availability. As demonstrated in this study, the use of biochip assays may be able to overcome some of these practical limitations and allow for the investigation of the relationships between markers implicated in DIC. Ultimately, this may allow for the development of a biochip optimized for investigation of this disease and its diagnosis and treatment.
Acknowledgments
The authors have acknowledged the skillful assistance of the staff of the Hemostasis Research Laboratories of the Department of Pathology and the Loyola University Medical Center. The authors are thankful to Dr Eva Wojick, Chair of the Department of Pathology, for her support in facilitating this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41(5):1167–1174. [DOI] [PubMed] [Google Scholar]
- 2. Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. Natl Vital Stat Rep. 2013;61(4):1–117. [PubMed] [Google Scholar]
- 3. Hall MJ, Levant S, DeFrances CJ. Trends in inpatient hospital deaths: national hospital discharge survey 2000-2010. NCHS Data Brief. 2013;(118):1–8. [PubMed] [Google Scholar]
- 4. Hawiger J, Veach R, Zienkiewicz J. New paradigms in sepsis: from prevention to protection of failing microcirculation. J Thromb Haemost. 2015;13(10):1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saracco P, Vitale P, Scolfaro C, Pollio B, Pagliarino M, Timeus F. The coagulopathy in sepsis: significance and implications for treatment. Pediatr Rep. 2011;3(4):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levi M, Ten Cate H. Disseminated intravascular coagulation. N Engl J Med. 1999;341(8):586–592. [DOI] [PubMed] [Google Scholar]
- 7. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644–1655. [DOI] [PubMed] [Google Scholar]
- 8. Taylor FB, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 9. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11(4):761–767. [Google Scholar]
- 10. Bakhtiari K, Meijers JCM, de Jonge E, Levi M. Prospective validation of the international society of thrombosis and haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–2421. [DOI] [PubMed] [Google Scholar]
- 11. Hayakawa M, Gando S, Hoshino H. A prospective comparison of new Japanese criteria for disseminated intravascular coagulation: new Japanese criteria versus ISTH criteria. Clin Appl Thromb Hemost. 2007;13(2):172–181. [DOI] [PubMed] [Google Scholar]
- 12. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14(1):R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bodovitz S, Joos T, Bachmann J. Protein biochips: the calm before the storm. Drug Discov Today. 2005;10(4):283–287. [DOI] [PubMed] [Google Scholar]
- 14. Kemmler M, Sauer U, Schleicher E, Preininger C, Brandenburg A. Biochip point-of-care device for sepsis diagnostics. Sens Actuators B Chem. 2014;192(2014):205–215. [Google Scholar]
- 15. Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109(22):2698–2704. [DOI] [PubMed] [Google Scholar]
- 16. van der Poll T, Levi M, Hack CE, et al. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179(4):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nawroth PP, Handley DA, Esmon CT, Stern DM. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986;83(10):3460–3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Osnes L, Westvik A, Joo GB, Okkenhaug C, Kierulf P. Inhibition of IL-1 induced tissue factor (TF) synthesis and procoagulant activity (PCA) in purified human monocytes by IL-4, IL-10 and IL-13. Cytokine. 1996;8(11):822–827. [DOI] [PubMed] [Google Scholar]
- 19. Abraham E. Coagulation abnormalities in acute lung injury and sepsis. Am J Respir Cell Mol Biol. 2000;22(4):401–404. [DOI] [PubMed] [Google Scholar]
- 20. Esmon CT. The impact of the inflammatory response on coagulation. Thromb Res. 2004;114(5-6):321–327. [DOI] [PubMed] [Google Scholar]
- 21. Bevilacqua MP, Pober JS, Majeau GR, Fiers W, Cotran RS, Gimbrone MA., Jr Recombinant tumor necrosis factor induces procoagulant activity in cultured human vascular endothelium: characterization and comparison with the actions of interleukin 1. Proc Natl Acad Sci. 1986;83(12):4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Poll T, Büller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322(23):1620–1626. [DOI] [PubMed] [Google Scholar]
- 23. Hezi-Yamit A, Wong PW, Bien-Ly N, et al. Synergistic induction of tissue factor by coagulation factor Xa and TNF: evidence for involvement of negative regulatory signaling cascades. Proc Natl Acad Sci U S A. 2005;102(34):12077–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleischmann JD, Shingleton WB, Gallagher C, et al. Fibrinolysis, thrombocytopenia, and coagulation abnormalities complicating high-dose interleukin-2 immunotherapy. J Lab Clin Med. 1991;117(1):76–82. [PubMed] [Google Scholar]
- 25. Locker G, Kapiotis S, Veitl M, et al. Activation of endothelium by immunotherapy with interleukin-2 in patients with malignant disorders. Br J Haematol. 1999;105(4):912–919. [DOI] [PubMed] [Google Scholar]
- 26. Lindmark E, Tenno T, Chen J, Siegbahn A. IL-10 inhibits LPS-induced human monocyte tissue factor expression in whole blood. Br J Haematol. 1998;102(2):597–604. [DOI] [PubMed] [Google Scholar]
- 27. Pajkrt D, van der Poll T, Levi M, et al. Interleukin 10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89(8):2701–2705. [PubMed] [Google Scholar]
- 28. Herbert J, Savi P, Laplace MC, et al. IL-4 and IL-13 exhibit comparable abilities to reduce pyrogen-induced expression of procoagulant activity in endothelial cells and monocytes. FEBS Lett. 1993;328(3):268–270. [DOI] [PubMed] [Google Scholar]
- 29. Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2(3):e2010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levi M, de Jonge E, van der Poll T. Rationale for restoration of physiological anticoagulant pathways in patients with sepsis and disseminated intravascular coagulation. Crit Care Med. 2001;29(suppl 7):S90–S94. [DOI] [PubMed] [Google Scholar]
- 31. van der Poll T, de Jonge E, Levi M. Regulatory role of cytokines in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27(6):639–651. [DOI] [PubMed] [Google Scholar]