Abstract
Excessive perioperative bleeding remains a substantial problem. Factor XIII (FXIII) contributes to clot stability, and it has therefore been suggested that supplementation with FXIII concentrate may improve perioperative hemostasis. We evaluated the effects of increasing doses of FXIII, alone or in combination with fibrinogen or platelet concentrate, in blood samples from 2 considerably different groups of surgical patients: cardiac and scoliosis surgery patients. Whole-blood samples were collected immediately after operation from cardiac and scoliosis surgery patients. The samples were supplemented with 3 clinically relevant doses of FXIII concentrate (+20%, +40%, and +60%), alone or in combination with a fixed dose of fibrinogen concentrate (+1.0 g/L) or fresh apheresis platelets (+92 × 109/L). Clot formation was assessed with rotational thromboelastometry (ROTEM). When the highest dose of FXIII concentrate was added, EXTEM clotting time was shortened by 10% in both cardiac and scoliosis surgery patients (95% confidence intervals: 2.4%-17% and 3.3%-17%, respectively), and FIBTEM maximum clot firmness was increased by 25% (9.3%-41%) in cardiac patients, relative to baseline. When fibrinogen was added, the dose-dependent effect of FXIII on clot stability was maintained, but the total effect was markedly greater than with FXIII alone, +150% (100%-200%) and +160% (130%-200%) for the highest FXIII dose in cardiac and scoliosis patients, respectively. Ex vivo supplementation with clinically relevant doses of FXIII improved clot formation moderately in blood samples from cardiac and scoliosis surgery patients, both alone and when given in combination with fibrinogen or platelet concentrate.
Keywords: factor XIII, fibrinogen, platelets, coagulation, surgery
Introduction
Excessive blood loss remains a significant problem during and after major surgical procedures such as spine and cardiac surgery.1–3 The bleeding is multifactorial and may depend on patient factors such as poor liver and kidney function and antithrombotic medication, procedural factors, and impaired perioperative hemostasis caused by large bleeding and fluid replacement with crystalloids and colloids, that is, hemodilution. In cardiac surgery, the use of cardiopulmonary bypass (CPB) with exposure of blood to nonendothelial surfaces may also contribute.
Bleeding and hemodilution reduce the plasma concentration of coagulation factors. It has previously been shown that fibrinogen is the first coagulation factor to reach critically low plasma levels in patients with ongoing bleeding,4 but the concentration of other coagulation factors may also be insufficient when the hemostasis is challenged from major bleeding. We, and others, have previously shown a significant correlation between coagulation factor XIII (FXIII) plasma activity and bleeding volume after cardiac surgery,5–7 and it has been demonstrated that FXIII activity is reduced in surgical patients with unexpected bleeding.8 In another study, patients were randomized to receive either FXIII substitution or placebo at the start of gastrointestinal cancer surgery. The decrease in clot stability and the blood loss during surgery were significantly less in the group that received FXIII concentrate.9 Accordingly, current guidelines on the treatment of perioperative bleeding state that FXIII supplementation can be considered in patients with ongoing bleeding.10,11
However, little is known about the functional effects of FXIII supplementation in surgical patients and the relationship between dose and response. Furthermore, it is not known whether potential effects are reduced, maintained, or enhanced when FXIII is combined with other prohemostatic blood products such as fibrinogen and platelet concentrates. The aim of the present ex vivo study was to investigate the effects of FXIII concentrate on clot formation in blood samples collected immediately after surgery in 2 groups of surgical patients with major differences, but both of which have a relatively high risk of large perioperative bleeding—cardiac and scoliosis surgery patients.
Materials and Methods
Patients
Nine patients undergoing cardiac surgery with CPB and 10 patients undergoing scoliosis surgery were included from February to October 2012 and from February to May 2014. Patient characteristics are given in Table 1. All patients gave written informed consent. The inclusion criteria were elective coronary artery bypass grafting procedure for the cardiac patient group and idiopathic scoliosis in otherwise healthy individuals for the scoliosis patient group. Exclusion criteria for the cardiac patients were preoperative medication affecting coagulation, P2Y12 platelet inhibition within 5 days before surgery, liver or kidney dysfunction, and age <18. Exclusion criterion for the scoliosis patients was ongoing medication affecting hemostasis. The study was approved by the regional research ethics committee and performed according to the World Medical Association Declaration of Helsinki.
Table 1.
Patient Demographics and Results of Preoperative and Postoperative Blood Analyses.a
| Parameter | Cardiac (n = 9) | Scoliosis (n = 10) | |
|---|---|---|---|
| Female/male gender | 0/9 | 8/2 | |
| Age (years) | 67 (62-71) | 17 (13-20) | |
| Body mass index (kg/m2) | 28 (25-30) | 19 (16-21) | |
| ASA score (1/2/3) | 0/1/8 | 9/1/0 | |
| Smoker (former/no) | 7/2 | 1/9 | |
| Hemoglobin concentration (g/L) | Preoperatively | 128 (120-136) | 118 (112-124) |
| Postoperatively | 110 (100-119)b | 107 (100-114)c | |
| Platelet count (× 109/L) | Preoperatively | 215 (173-256) | 256 (215-297) |
| Postoperatively | 145 (122-167)b | 236 (203-269) | |
| Fibrinogen concentration (g/L) | Preoperatively | 3.1 (2.7-3.5) | 2.4 (2.1-2.7) |
| Postoperatively | 2.7 (2.2-3.2) | 2.0 (1.7-2.3)c | |
| Factor XIII activity (%) | Preoperatively | 90 (80-101) | 76 (66-86) |
| Postoperatively | 78 (67-89)c | 64 (54-73)c | |
| EXTEM clotting time (seconds) | Preoperatively | 48 (41-55) | 54 (49-60) |
| EXTEM maximum clot firmness (mm) | Preoperatively | 65 (63-67) | 62 (59-64) |
| FIBTEM maximum clot firmness (mm) | Preoperatively | 18 (15-20) | 13 (11-15) |
Abbreviation: ASA score, American Society of Anesthesiologists score.
aMean and 95% confidence interval or number.
b P < .01, preoperatively versus postoperatively.
c P < .05.
Clinical Management
Cardiac patients
All cardiac surgery patients were operated with CPB. Before cannulation, heparin (Lövens, Ballerup, Denmark), 300 IU/kg, was given and supplemented as required to maintain an activated clotting time of more than 480 seconds. Cardiopulmonary bypass was performed with a hollow fiber membrane oxygenator. All patients received tranexamic acid, 2 g before surgery and 2 g after skin closure. Aprotinin was not used in any patient. Clopidogrel or ticagrelor was discontinued at least 5 days before surgery. The operations were performed with standard nonpulsatile CPB technique in normothermia and hemodilution (hematocrit 20%-30%). Cardioprotection was achieved with cold blood cardioplegia. Weaning off CPB was performed at a temperature of at least 36°C. Protamine (1 mg protamine per 100 U of heparin) was given to reverse the effect of heparin.
Scoliosis patients
All scoliosis surgery patients were operated under total intravenous anesthesia. None of the patients were on any medication that would influence coagulation or platelet function. Five patients received tranexamic acid perioperatively. All patients received thrombosis prophylaxis with dalteparin (Pfizer Inc, New York), 5000 IU, with the first dose being given the evening before surgery.
Study Design
Preoperative blood samples from cardiac surgery patients were collected after the induction of anesthesia but before the initiation of surgery, and postoperative samples were collected when the patient was weaned from CPB and after heparin had been neutralized with protamine. Blood samples from scoliosis surgery patients were collected preoperatively after the induction of anesthesia but before the initiation of surgery and postoperatively immediately after surgery was completed. All samples were collected from an arterial line, except in 2 scoliosis surgery patients where sampling was performed from a peripheral vein catheter. Samples were collected in citrated tubes (0.129 M citrate, volume 2.7 mL) for analysis of fibrinogen plasma concentration and FXIII activity. The blood was centrifuged for 20 minutes at 2000g within 30 minutes of collection, and the plasma was stored at −80°C in polypropylene cryotubes for later analysis. Additional blood was collected in EDTA tubes for the measurement of hemoglobin concentration and platelet count.
Preoperatively, clot formation was analyzed in citrated whole blood (0.129 M citrate) without any additives. Postoperatively, 10 different samples were prepared for each study patient: 1 baseline and 3 with increasing doses of FXIII concentrate (Fibrogammin; CSL Behring, Marburg, Germany; +20%, +40%, and +60%), alone or in combination with a fixed dose of fibrinogen concentrate (+1.0 g/L, Riastap; CSL Behring) or freshly prepared apheresis platelets (+92 × 109/L) from the institutional blood bank. The doses of FXIII, fibrinogen, and platelet concentrates correspond to clinically relevant doses of approximately 1050 to 3150 IU FXIII concentrate, 3 g fibrinogen concentrate, and 3 U single-donor apheresis platelets to a patient weighing 70 kg.12 Phosphate-buffered saline (140 mM NaCl, 10 mM Na3PO4, pH 7.4) was used in different volumes to maintain the same hemodilution in the samples. All samples for clot formation analysis had a total volume of 987 µL, 760 µL of which was citrated whole blood.
Analyses
Clot formation was assessed with rotational thromboelastometry (ROTEM; Pentapharm GmbH, Munich, Germany). Technical details have been given elsewhere.13 The ROTEM assays, EXTEM and FIBTEM, were used for each sample. EXTEM analyzes clot formation activated with tissue factor. FIBTEM uses the same activator but eliminates the platelet contribution by adding cytochalasin D to the sample, in order to highlight the effect of fibrin polymerization. Clotting time and maximum clot firmness are reported for the EXTEM analysis, and maximum clot firmness is reported for the FIBTEM analysis.
Fibrinogen concentration and FXIII activity were analyzed at the accredited coagulation laboratory at Sahlgrenska University Hospital. The laboratory participates in the external quality assessment program of the ECAT Foundation (www.ecat.nl). Plasma concentration of fibrinogen (reference range: 2.0-4.5 g/L) was measured by the modified method of Clauss.14 Plasma activity of FXIII (reference range: 70%-140%) was analyzed with the Berichrom FXIII assay (Dade Behring, Marburg, Germany) on the BCS XP instrument (Siemens Healthcare GmbH, Erlangen, Germany). Hemoglobin concentration, hematocrit, and platelet count were analyzed using standard clinical methods.
Statistics
A sample size of 8 patients would give us a power of 80% with a 2-sided paired test to detect a difference of 2 mm in FIBTEM maximum clot firmness, with a significance level of 0.05 and a standard deviation of 2 mm. A 2-mm increase corresponds to an increase of approximately 20% in FIBTEM maximum clot firmness in postoperative samples from cardiac surgery patients.15,16 Patient characteristics, baseline parameters, and thromboelastometry results are presented as mean with 95% confidence interval, unless stated otherwise. Related-samples Wilcoxon signed rank test was used for comparisons of baseline parameters within groups. To evaluate the difference in thromboelastometry results between the blood samples prepared with various concentrations of FXIII concentrate, alone or together with fibrinogen or platelet concentrate, a mixed model was used. Treatment and baseline value were defined as fixed effects, and dose was included as a random effect. Hence, the repeated effect of dose was estimated with a random slope. Any P value of <.05 was considered to be statistically significant. Statistical calculations were done with SPSS 20.0 (IBM Corp, Armonk, New York) and SAS 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
General
All patients recovered as expected after surgery. None of the patients received intraoperative transfusion of blood products. The median amount of perioperative bleeding was 880 mL (range: 510-1410 mL) in the cardiac group and 695 mL (range: 470-1185 mL) in the scoliosis group.
Baseline Variables
Hemoglobin concentration and FXIII activity were reduced in both groups of patients after surgery (Table 1). Platelet count was only significantly reduced in cardiac surgery patients, and fibrinogen concentration was only significantly reduced in scoliosis surgery patients.
Factor XIII Supplementation
Cardiac surgery patients—Clotting time
In the mixed-model analysis, supplementation with FXIII concentrate alone resulted in a shortened EXTEM clotting time for the highest FXIII dose by 8.2 seconds (95% confidence interval: 1.9-15 seconds; P = .001), compared to baseline, and a significant decrease in clotting time between the lowest and highest dose of FXIII of 6.2 seconds (1.1-14 seconds; P = .013; Figure 1A and Table 2). Supplementation with FXIII in combination with a fixed dose of fibrinogen or platelets shortened the clotting time further compared to FXIII alone: The highest dose resulted in a decrease of 11 seconds (7.1-15 seconds) and 14 seconds (7.1-21 seconds), respectively, compared to baseline (both P < .001; Figure 1A and Table 2).
Figure 1.
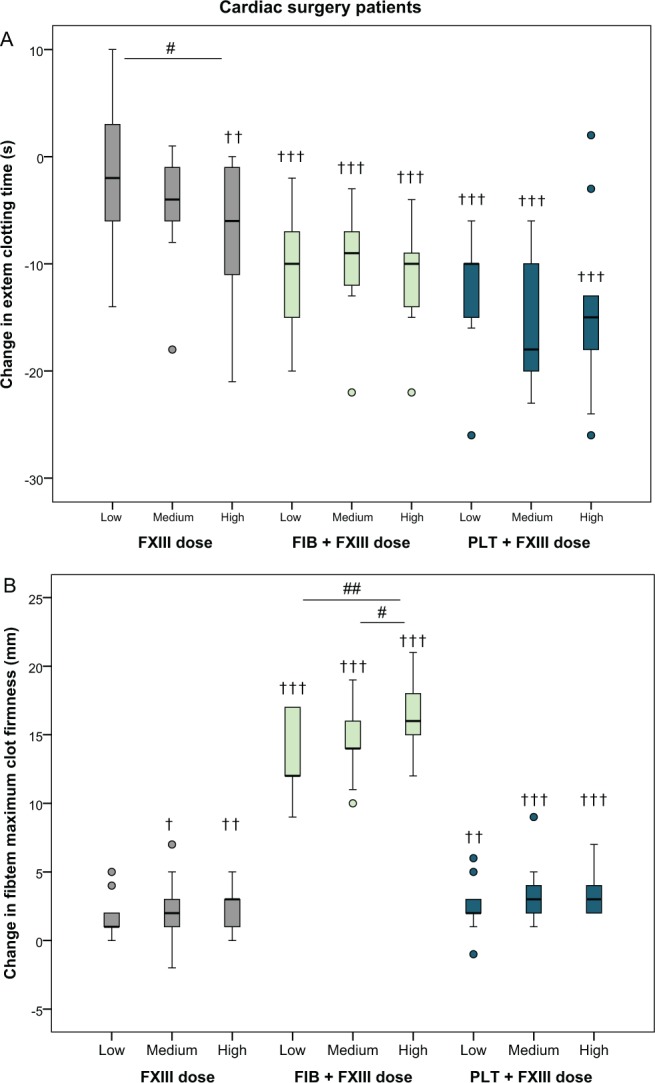
Absolute changes in EXTEM clotting time and FIBTEM maximum clot firmness after the addition of increasing doses of factor XIII (+20%, +40%, and +60%) in blood samples from cardiac surgery patients (n = 9), alone or in combination with a fixed dose of fibrinogen or platelets. See Methods section for details. † P < .05, †† P < .01, ††† P < .001, relative to baseline. # P < .05, ## P < .01 compared to another dose of FXIII (within treatment). Outliers (values more than 1.5 times the interquartile range away from the lower or upper quartile) are shown with circles.
Table 2.
Results of Clot Formation Analyses of Blood Samples From Cardiac and Scoliosis Surgery Patients.a
| Sample | Cardiac Surgery Patients (n = 9) | Scoliosis Surgery Patients (n = 10) | ||||
|---|---|---|---|---|---|---|
| EXTEM Clotting Time (s) | EXTEM Maximum Clot Firmness (mm) | FIBTEM Maximum Clot Firmness (mm) | EXTEM Clotting Time (s) | EXTEM Maximum Clot Firmness (mm) | FIBTEM Maximum Clot Firmness (mm) | |
| Baseline | 78 (69-88) | 55 (52-58) | 12 (9.2-15) | 69 (61-78) | 55 (51-58) | 8.4 (6.6-10) |
| FXIII low | 76 (67-85) | 56 (53-58) | 14 (11-16) | 63 (58-69) | 55 (52-58) | 8.5 (6.8-10) |
| FXIII medium | 73 (66-81) | 56 (53-58) | 14 (12-16) | 64 (56-71) | 55 (52-58) | 8.9 (7.5-10) |
| FXIII high | 70 (62-78) | 57 (54-60) | 15 (12-17) | 62 (55-69) | 55 (52-58) | 9.6 (7.9-11) |
| FXIII low + fibrinogen | 68 (60-77) | 62 (60-64) | 25 (24-27) | 53 (47-58) | 62 (59-64) | 19 (17-21) |
| FXIII medium + fibrinogen | 68 (60-76) | 61 (59-64) | 26 (24-28) | 52 (47-56) | 62 (59-65) | 20 (18-22) |
| FXIII high + fibrinogen | 67 (61-73) | 63 (61-65) | 28 (26-30) | 54 (49-58) | 62 (59-65) | 21 (19-24) |
| FXIII low + platelets | 66 (59-73) | 63 (60-65) | 14 (12-16) | 58 (52-64) | 61 (58-64) | 10 (8.5-12) |
| FXIII medium + platelets | 62 (55-70) | 63 (61-65) | 15 (13-17) | 57 (51-62) | 61 (58-64) | 11 (8.8-13) |
| FXIII high + platelets | 64 (55-73) | 63 (61-65) | 15 (13-18) | 56 (50-61) | 61 (59-64) | 11 (9.0-13) |
aThe blood samples were either supplemented with factor XIII (FXIII; +20%, +40%, and +60%), alone or in combination with a fixed dose of fibrinogen or platelets. Values are presented as mean and 95% confidence interval.
Cardiac surgery patients—Clot stability
There was a significant increase in FIBTEM maximum clot firmness for the medium dose and the highest dose of FXIII alone compared to baseline (+2.0 mm [−0.14 to 4.1 mm], P = .021 and +2.7 mm [1.3-4.0 mm], P = .003, respectively; Figure 1B and Table 2). Simultaneous supplementation with fibrinogen enhanced the dose effect of FXIII. The increase in FIBTEM maximum clot firmness between the lowest and highest dose of FXIII when added together with fibrinogen was +2.8 mm (1.9-3.7 mm, P = .002).
Scoliosis surgery patients—Clotting time
Supplementation with increasing doses of FXIII concentrate resulted in a significant decrease in EXTEM clotting time of 7.5 seconds (1.8-13 seconds, P = .018, mixed-model analysis) with the highest dose of FXIII compared to baseline (Figure 2A and Table 2). Supplementation with FXIII in combination with a fixed dose of fibrinogen or platelets shortened the clotting time more than with FXIII alone (−16 seconds [−7.5 to −24 seconds], P < .001 and −14 seconds [−5.9 to −22 seconds], P < .001, respectively, compared to baseline; Figure 2A and Table 2).
Figure 2.
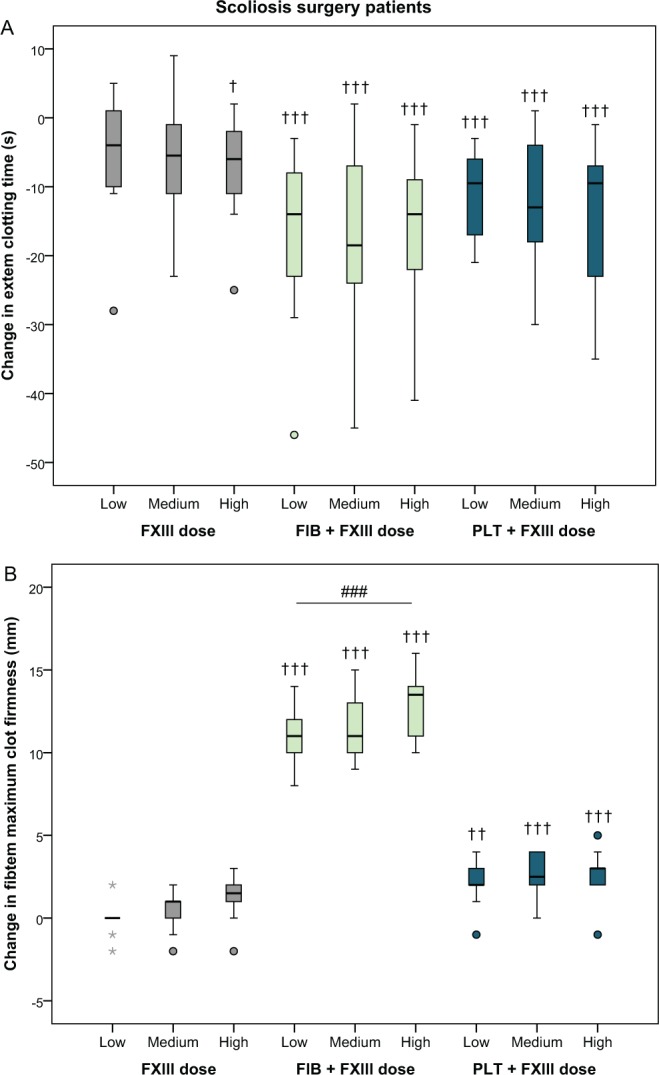
Absolute changes in EXTEM clotting time and FIBTEM maximum clot firmness after the addition of increasing doses of factor XIII (+20%, +40%, and +60%) in blood samples from scoliosis surgery patients (n = 10), alone or in combination with a fixed dose of fibrinogen or platelets. See Methods section for details. † P <.05, †† P <.01, ††† P < .001, relative to baseline. ### P <.001 compared to another dose of FXIII (within treatment). Outliers are denoted with circles and extreme values with stars. Outliers and extreme values are values more than 1.5 and 3 times the interquartile range, respectively, away from the lower or upper quartile.
Scoliosis surgery patients—Clot stability
In FIBTEM maximum clot firmness, fibrinogen enhanced the effect of FXIII compared to FXIII alone (Figure 2B and Table 2). There was no significant change between the lowest and highest dose of FXIII alone (+1.1 mm [0.1-2.1 mm], P = .082). However, when fibrinogen was added together with the FXIII, the increase between the lowest and highest dose of FXIII was 2.2 mm (1.1-3.3 mm, P < .001; Figure 2B and Table 2).
Discussion
The main findings of the present study were as follows. (1) Clinically relevant doses of FXIII improved clot formation in blood samples from cardiac surgery patients and scoliosis surgery patients. (2) The effects of FXIII alone were markedly lower than when FXIII was combined with fibrinogen or platelet concentrate. (3) The dose-dependent effect of FXIII on FIBTEM maximum clot firmness was enhanced when increasing doses of FXIII were added together with fibrinogen.
Several previous studies have investigated the effect of FXIII supplementation on clot formation in whole-blood samples.17–31 The present study differs from the previous studies in 2 important aspects. Firstly, we used whole-blood samples from 2 categories of patients with a high risk of bleeding, cardiac surgery patients and scoliosis surgery patients. The samples were collected immediately after surgery, that is, when the coagulation would be expected to be most disturbed. Most previous studies have used samples from healthy volunteers or blood donors and then diluted the samples with crystalloids or colloids to induce coagulopathy—and/or added tissue plasminogen activator to cause fibrinolysis.18,20–24,26,27,29,31 Secondly, we tested clinically relevant doses of FXIII, +20% to +60%, added alone or in combination with 2 other hemostatic agents, fibrinogen and platelets. These are blood products that may be used in the clinical setting to treat bleeding complications when impairment of clot stability is confirmed or suspected. This setup differs from other studies where supraphysiological doses of FXIII concentrate have been added to blood samples from intensive care patients17 or to diluted samples from healthy volunteers.18,22,23,27
The previous studies that have investigated the effect of FXIII supplementation of blood samples on clot formation have shown divergent results. Some studies have shown a positive effect on clotting time or clot stability with FXIII alone17,20,23–25 or an improved effect when FXIII was added simultaneously with fibrinogen as compared to fibrinogen alone.18,22,26,27,29 Other studies have not been able to demonstrate any effect of FXIII on these parameters.19,21,28,30,31 One possible explanation for such variation could be dissimilarities in the baseline activity level of FXIII in the blood sample. If the FXIII activity is normal, the addition of more FXIII may not be able to improve clot formation further. Other possible explanations might be the large differences in study design, grade and type of hemodilution, and analysis device used.
In some of the studies already mentioned, the influence of FXIII supplementation of blood samples on fibrinolysis was investigated.17,23,25,27,28,31 These 6 studies demonstrated that the addition of FXIII concentrate had a protective effect on fibrinolysis by improving various thromboelastometry variables linked to fibrinolysis, for example, maximum lysis or lysis onset time. In the present study, we were unable to detect any effect on fibrinolysis, simply because none of the study participants had any thromboelastometric signs of fibrinolysis in postoperative baseline samples (data not shown).
The effect of FXIII supplementation in the present study was limited. With the highest dose of FXIII, EXTEM clotting time was shortened by approximately 10% (in both groups) and FIBTEM clot firmness increased by 25% in the cardiac surgery group, compared to baseline. Furthermore, the effects of FXIII alone were markedly less than when fibrinogen or platelets were added simultaneously to the samples (Figures 1 and 2 and Table 2). This corroborates current guidelines suggesting that FXIII supplementation should only be considered in patients with diffuse bleeding and adequate fibrinogen levels or when fibrinogen supplementation is not successful.10,11 In fact, the present results in combination with previous observations on the dose-dependent improvement in clot firmness after the addition of increasing doses of fibrinogen and/or platelets32 suggest that higher doses of fibrinogen or platelet concentrates would be more effective in restoring clot firmness than adding FXIII concentrate. This is also supported by the only large-scale randomized trial with FXIII supplementation in cardiac surgery patients, which was unable to show any effect on bleeding and transfusions with recombinant FXIII.33
Interestingly, the effect of FXIII—both when given as monotherapy and in combination with fibrinogen or platelets—was comparable in both cardiac and scoliosis surgery patients despite the large differences between the groups. The groups in fact differed in most patient characteristics and laboratory tests, including, for example, age, gender, body mass index, and fibrinogen concentration (Table 1). This strengthens the validity of our results and indicates that they may also be relevant in other surgical populations.
The study had important limitations. The one that is inherent in any ex vivo study of clot formation is whether the results can be related to the clinical setting. The ex vivo model did not include the influence of the vascular wall and the endothelium, and of blood flow, on clot formation. Another limitation was that the samples were collected from normal postoperative cardiac and scoliosis surgery patients without excessive bleeding. The absolute thromboelastometry values cannot be directly compared to those in other studies performed with undiluted samples. The strengths of the study were that the samples were collected from patients (and not healthy volunteers) and that we measured changes in clot formation before and after FXIII, fibrinogen, and platelet supplementation in the same blood samples and that the same degree of hemodilution was maintained in all samples.
In conclusion, the results of this ex vivo study show that supplementation with clinically relevant doses of FXIII improves clot formation moderately in samples from cardiac and scoliosis surgery patients in a dose-dependent manner. The effect of FXIII alone was markedly less than the effect that was achieved with FXIII in combination with fibrinogen or platelets.
Acknowledgments
The authors thank the staff of the Regional Blood Bank for the preparation of the platelet concentrates.
Authors’ Note: An earlier version of this manuscript was included in a doctoral thesis from Sahlgrenska Academy, University of Gothenburg, Sweden (https://gupea.ub.gu.se/handle/2077/39556). The study sponsors had no influence on the analysis and interpretation of the data, in the writing of the report, or in the decision to submit the report for publication. The authors have access to the data.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AJ has received honoraria for presentations and advisory boards from CSL Behring.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Swedish Heart and Lung Foundation (grant number 20140281 to AJ), Sahlgrenska University Hospital (ALF/LUA grant number 146281 to AJ and number 428161 to HB), and CSL Behring, Marburg, Germany.
References
- 1. Frankel TL, Stamou SC, Lowery RC, et al. Risk factors for hemorrhage-related reexploration and blood transfusion after conventional versus coronary revascularization without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2005;27(3):494–500. [DOI] [PubMed] [Google Scholar]
- 2. Carling MS, Jeppsson A, Wessberg P, Henriksson A, Baghaei F, Brisby H. Preoperative fibrinogen plasma concentration is associated with perioperative bleeding and transfusion requirements in scoliosis surgery. Spine. 2011;36(7):549–555. [DOI] [PubMed] [Google Scholar]
- 3. Yu X, Xiao H, Wang R, Huang Y. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine. 2013;38(4):350–355. [DOI] [PubMed] [Google Scholar]
- 4. Hiippala ST, Myllylä GJ, Vahtera EM. Hemostatic factors and replacement of major blood loss with plasma-poor red cell concentrates. Anesth Analg. 1995;81(2):360–365. [DOI] [PubMed] [Google Scholar]
- 5. Ternström L, Radulovic V, Karlsson M, et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126(2):e128–e133. [DOI] [PubMed] [Google Scholar]
- 6. Chandler WL, Patel MA, Gravelle L, et al. Factor XIIIA and clot strength after cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2001;12(2):101–108. [DOI] [PubMed] [Google Scholar]
- 7. Shainoff JR, Estafanous FG, Yared JP, DiBello PM, Kottke-Marchant K, Loop FD. Low factor XIIIA levels are associated with increased blood loss after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1994;108(3):437–445. [PubMed] [Google Scholar]
- 8. Wettstein P, Haeberli A, Stutz M, et al. Decreased factor XIII availability for thrombin and early loss of clot firmness in patients with unexplained intraoperative bleeding. Anesth Analg. 2004;99(5):1564–1569. [DOI] [PubMed] [Google Scholar]
- 9. Korte WC, Szadkowski C, Gähler A, et al. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology. 2009;110(2):239–245. [DOI] [PubMed] [Google Scholar]
- 10. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270–382. [DOI] [PubMed] [Google Scholar]
- 11. Society of Thoracic Surgeons Blood Conservation Guideline Task Force Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. [DOI] [PubMed] [Google Scholar]
- 12. Karlsson M, Ternström L, Hyllner M, Baghaei F, Skrtic S, Jeppsson A. Prophylactic fibrinogen infusion in cardiac surgery patients: effects on biomarkers of coagulation, fibrinolysis, and platelet function. Clin Appl Thromb Hemost. 2011;17(4):396–404. [DOI] [PubMed] [Google Scholar]
- 13. Lang T, Bauters A, Braun SL, et al. Multi-centre investigation on reference ranges for ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2005;16(4):301–310. [DOI] [PubMed] [Google Scholar]
- 14. Clauss A. Rapid physiological coagulation method in determination of fibrinogen[in German]. Acta Haematol. 1957;17(4):237–246. [DOI] [PubMed] [Google Scholar]
- 15. Vonk AB, Veerhoek D, van den Brom CE, van Barneveld LJ, Boer C. Individualized heparin and protamine management improves rotational thromboelastometric parameters and postoperative hemostasis in valve surgery. J Cardiothorac Vasc Anesth. 2014;28(2):235–241. [DOI] [PubMed] [Google Scholar]
- 16. Petricevic M, Biocina B, Milicic D, et al. Bleeding risk assessment using whole blood impedance aggregometry and rotational thromboelastometry in patients following cardiac surgery. J Thromb Thrombolysis. 2013;36(4):514–526. [DOI] [PubMed] [Google Scholar]
- 17. Theusinger OM, Baulig W, Asmis LM, Seifert B, Spahn DR. In vitro factor XIII supplementation increases clot firmness in Rotation Thromboelastometry (ROTEM). Thromb Haemost. 2010;104(2):385–391. [DOI] [PubMed] [Google Scholar]
- 18. Schlimp CJ, Cadamuro J, Solomon C, Redl H, Schöchl H. The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33% diluted blood with albumin, gelatine, hydroxyethyl starch or saline in vitro. Blood Transfus. 2013;11(4):510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fenger-Eriksen C, Tønnesen E, Ingerslev J, Sørensen B. Mechanisms of hydroxyethyl starch-induced dilutional coagulopathy. J Thromb Haemost. 2009;7(7):1099–1105. [DOI] [PubMed] [Google Scholar]
- 20. Hanna J, Winstedt D, Schött U. Fibrinogen and FXIII dose response effects on albumin-induced coagulopathy. Scand J Clin Lab Invest. 2013;73(7):553–562. [DOI] [PubMed] [Google Scholar]
- 21. Schramko AA, Kuitunen AH, Suojaranta-Ylinen RT, Niemi TT. Role of fibrinogen-, factor VIII- and XIII-mediated clot propagation in gelatin haemodilution. Acta Anaesthesiol Scand. 2009;53(6):731–735. [DOI] [PubMed] [Google Scholar]
- 22. Haas T, Fries D, Velik-Salchner C, Reif C, Klingler A, Innerhofer P. The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg. 2008;106(5):1360–1365. [DOI] [PubMed] [Google Scholar]
- 23. Shenkman B, Livnat T, Lubetsky A, et al. The in-vitro effect of fibrinogen, factor XIII and thrombin-activatable fibrinolysis inhibitor on clot formation and susceptibility to tissue plasminogen activator-induced fibrinolysis in hemodilution model. Blood Coagul Fibrinolysis. 2012;23(5):370–378. [DOI] [PubMed] [Google Scholar]
- 24. Nielsen VG, Gurley WQ, Burch TM. The impact of factor XIII on coagulation kinetics and clot strength determined by thrombelastography. Anesth Analg. 2004;99(1):120–123. [DOI] [PubMed] [Google Scholar]
- 25. Grossmann E, Akyol D, Eder L, et al. Thromboelastometric detection of clotting Factor XIII deficiency in cardiac surgery patients. Transfus Med. 2013;23(6):407–415. [DOI] [PubMed] [Google Scholar]
- 26. Winstedt D, Tynngård N, Olanders K, Schött U. Free oscillation rheometry monitoring of haemodilution and hypothermia and correction with fibrinogen and factor XIII concentrates. Scand J Trauma Resusc Emerg Med. 2013;21:20 doi:10.1186/1757-7241-21-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shenkman B, Einav Y, Livnat T, Budnik I, Martinowitz U. In vitro evaluation of clot quality and stability in a model of severe thrombocytopenia: effect of fibrinogen, factor XIII and thrombin-activatable fibrinolysis inhibitor. Blood Transfus. 2014;12(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rea CJ, Foley JH, Okaisabor O, Sørensen B. FXIII: mechanisms of action in the treatment of hemophilia A. J Thromb Haemost. 2014;12(2):159–168. [DOI] [PubMed] [Google Scholar]
- 29. Kind SL, Spahn-Nett GH, Emmert MY, et al. Is dilutional coagulopathy induced by different colloids reversible by replacement of fibrinogen and factor XIII concentrates? Anesth Analg. 2013;117(5):1063–1071. [DOI] [PubMed] [Google Scholar]
- 30. Hvas AM, Andreasen JB, Christiansen K, Ravn HB. Ex-vivo response to blood products and haemostatic agents after paediatric cardiac surgery. Blood Coagul Fibrinolysis. 2013;24(6):587–592. [DOI] [PubMed] [Google Scholar]
- 31. Dirkmann D, Görlinger K, Gisbertz C, et al. Factor XIII and tranexamic acid but not recombinant factor VIIa attenuate tissue plasminogen activator-induced hyperfibrinolysis in human whole blood. Anesth Analg. 2012;114(6):1182–1188. [DOI] [PubMed] [Google Scholar]
- 32. Shams Hakimi C, Fagerberg Blixter I, Hansson EC, Hesse C, Wallén H, Jeppsson A. Effects of fibrinogen and platelet supplementation on clot formation and platelet aggregation in blood samples from cardiac surgery patients. Thromb Res. 2014;134(4):895–900. [DOI] [PubMed] [Google Scholar]
- 33. Karkouti K, von Heymann C, Jespersen CM, et al. Efficacy and safety of recombinant factor XIII on reducing blood transfusions in cardiac surgery: a randomized, placebo-controlled, multicenter clinical trial. J Thorac Cardiovasc Surg. 2013;146(4):927–939. [DOI] [PubMed] [Google Scholar]


