Abstract
Development of inhibitors remains a major clinical complication in patients with hemophilia A receiving replacement therapy with factor VIII (FVIII). Understanding the immune mechanisms involved in the development of inhibitors can provide valuable information about pathways to human tolerance. Recent evidence indicates that B regulatory (Breg) cells play a pivotal role in controlling the production of antibodies (Abs) while promoting follicular T helper (Tfh) cells and monocytes, expressing the low-density lipoprotein receptor-related protein (LRP/CD91), which is involved in FVIII intake from the circulation. We studied circulating levels of Breg cells along with Tfh cells and the expression of LRP/CD91 on monocytes in patients with hemophilia A using 8-color flow cytometry and cell culture. Compared to healthy controls, patients with hemophilia A with inhibitors showed a severe reduction in levels of Breg cells and produced less interleukin-10 when activated via the CD40 signaling pathway. In addition, patients with hemophilia A with inhibitors exhibited an overexpression of LPR/CD91 on monocytes and normal levels of Tfh cells. Levels of Breg cells were not significantly related to LPR/CD91 although negative associations were evidenced. Collectively, these results provide new insights into the role of Breg cells and LPR/CD91 in the development of inhibitors in patients with hemophilia A.
Keywords: hemophilia A, inhibitors, B regulatory cells, LPR, follicular cells
Introduction
A major clinical challenge in the treatment of patients with hemophilia A is the development of neutralizing antibodies (Abs) or inhibitors, against factor VIII (FVIII). Indeed, inhibitors are observed in approximately 25% to 30% of severely affected patients and usually appeared within the first 50 days of treatment.1 They rapidly abrogate FVIII activity and/or its pharmacokinetic properties, rendering replacement therapy ineffective.2 As a result, patients with inhibitors are expected to be at increased risk of morbidity and mortality. Although, the mechanisms underlying generation of inhibitors were intensively studied, the question why some patients are more likely to develop inhibitors and other do not is still unanswered.
By analogy to other humoral immune responses, development of inhibitors involves a close interaction among antigen-presenting cells such as monocytes/dendritic cells, T cells, and B cells. In this view, monocytes/dendritic cells play a pivotal role given their capacity to internalize FVIII from the circulation, thereby initiating the primary immune response. Recently, the low-density lipoprotein receptor-related protein (LRP), also known as α-2-marcoglobulin receptor or CD91, was shown to be upregulated on monocytes of patients with hemophilia A.3 The LRP/CD91-dependent endocytic pathway was involved in the catabolism of FVIII and also in its plasmatic regulation.4–6 These studies suggest that the LRP/CD91 pathway might play an important role in the development of inhibitors by activating immune cells such as B cells, which differentiate into antibody-secreting cells and memory B cells.
More recently, a subset of B cells known as B regulatory (Breg) cells was identified. These cells exert their suppressive capacity through the production of interleukin 10 (IL-10), an inhibitory cytokine utilized also by T-cells to modulate immune responses.7 B regulatory cells were also found to play a key role in modulating the production of Abs in a variety of conditions.7 Therefore, it was suggested that any dysfunction of Breg cells may lead to the development of inhibitors in patients with hemophilia. On the other hand, B cells interact with follicular T helper (Tfh) cells to produce Abs. This process is highly regulated as evidenced in animal models, where depletion of these cells significantly impact on the development of Abs.8,9 Till recently, Tfh cells were found only in germinal centers of lymph nodes; however, recent studies have shown that these cells can also be identified in the circulation and directly contribute to the generation of high-avidity Abs after vaccination.10
To date, limited information on the circulating levels of Breg cells, Tfh cells, and their interrelationships with monocytes expressing the LRP/CD91 receptor has been reported in patients with hemophilia. Given that Breg cells can impact on the production of Abs, we hypothesized that they may be altered in patients with hemophilia who develop inhibitors. To test this hypothesis, we analyzed the phenotype and functional characteristics of circulating Breg cells, along with Tfh cells and monocytes expressing the LRP/CD91 receptor in patients with hemophilia with and without inhibitors.
Materials and Methods
Methods
Patients with hemophilia were recruited at the Pediatric Hematology Clinic, Sultan Qaboos University Hospital, Sultanate of Oman. In this cross-sectional study, participants were classified into patients with hemophilia A with or without active inhibitors based on the Bethesda assay. All patients were evaluated for the absence of clinical signs and symptoms of acute inflammatory or other overt diseases prior to blood sampling for inhibitor testing. Healthy age-matched male controls were also recruited for comparison. Both patients and healthy controls were negative for human immunodeficiency virus, hepatitis C virus, and hepatitis B virus. The study protocol was approved by the local ethics committee, and a signed informed consent was obtained from all patients and healthy controls.
Isolation of Peripheral Mononuclear Cells
Blood samples were drawn into citrate tubes and processed within 1 hour of collection. Plasma was used for inhibitor testing while peripheral blood mononuclear cells (PBMCs) were separated by Ficoll-Hypaque density centrifugation, washed, and re-suspended in phosphate buffer saline (PBS) containing heat-inactivated fetal calf serum (FCS). The freshly-prepared PBMC samples were either stained with appropriate monoclonal Abs or stored in liquid nitrogen until used.
Monoclonal Abs Used and Surface Immunostaining
Specific monoclonal Abs conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE-Texas Red (ECD), PECy-7, PE-CF594, allophycocyanin (APC), APC-Cy7, and peridin chlorophyllprotein-Cy5.5 (PerCP-Cy5.5) were used as surface or intracellular markers. Anti-CD3-ECD, anti-CD4-APC, anti-CD279-PECy-7, anti-CD278-PerCP5.5, anti-CD185-FITC, anti-CD19-APC, anti-CD24-FITC, anti-CD38-PE-CF594, anti-IL-10-PE, anti-CD14-APC, and anti-CD91-PE monoclonal Abs were obtained from Becton and Dickinson (BD Biosciences, New Jersey, USA). A total of 10 × 105 PBMCs were incubated with each cocktail of monoclonal Abs according to the manufacturer’s instructions. Briefly, in the 96-well polystyrene plate appropriate amounts of monoclonal Abs, including CD19/CD24/CD38 for Breg cells, CD3/CD4/CD185/CD278 /CD279 for Tfh cells, and CD14/CD91 for monocytes, and 100 μL PBMCs were incubated in the dark for 30 minutes at 4°C. The cells were then washed twice with PBS containing 1% FCS and were resuspended in 250 μL of 2% paraformaldehyde and stored at 4°C for up to 12 hours before being used for flow cytometry. Unstained cells and n-1 monoclonal Abs were used as controls in all experiments.
Immunophenotypic Analysis
The LSR Fortessa and FACS Diva software (BD) were used for cell acquisition and analysis, respectively, as previously reported.11 Briefly, the sensitivity of fluorescent detectors was set and monitored using calibrated beads according to the manufacturer’s recommendations (BD). Fluorescence voltages and compensation values were determined using singly fluorochrome-stained PBMC from a healthy donor. Lymphocytes or monocytes were first identified according to their light-scattering properties and then analyzed for expression of surface markers. B regulatory cells were defined as CD19+CD24hiCD38hi, Tfh cells as CD3+CD4+CD185+CD278+CD279+, and LRP-monocytes as CD14+CD91+. For each sample, a minimum of 50 × 105 events in live cell gate was accumulated. To discriminate the live from dead cells, LIVE/DEAD Stain Kit (Invitrogen, New York) was used in all experiments.
Functional Analysis of Breg Cells
A 24-well plate was used to culture cells for IL-10 production. For each sample 2 × 106 cells were used. Cells were culture in RPMI medium 1640 (ThermoFisher scientific, Paisley, United Kingdom) containing d-glucose, HEPES buffer, l-glutamine, sodium bicarbonate, sodium pyruvate, fetal bovine serum, and antibiotic. Two wells were used for each sample. In the first well, PBMCs were stimulated with 1 µg/mL lipopolysaccharide (Sigma, Missouri, USA) or 0.5 μg of mouse anti-CD40 mAb (Invitrogen) and others were left not stimulated. The cells were then incubated at 37°C in 5% CO2 for 48 hours. Blocking was done in the last 5 hours with 50 ng/mL phorbol myristate acetate, 1 µg/mL inomycin (Sigma), and 10 µg/mL brefeldin A (Sigma). After incubation period, cells were harvested and stained with 1 µL LIVE/DEAD Stain Kit (Invitrogen) on ice according to the manufacturer’s instructions. They were then stained extracellularly with anti-CD19-APC, anti-CD24-FITC, and anti-CD38-PECF594 for 20 minutes in the dark at room temperature. Cells were washed and fixed in BD CytofixTM fixation buffer (BD Biosciences) in the dark at room temperature and then BD CytopermTM permeabilization buffer plus (BD Biosciences) was used for permeabilization. Cells were washed and stained intracellularly with anti-IL-10-PE and then fixed for cell acquisition by flow cytometry.
Statistical Analysis
Quantitative data were summarized using mean, standard deviation, and range. Group differences in the circulating levels of Breg cells, Tfh cells, and LRP-monocytes were evaluated using the Kruskal-Wallis test. The unpaired nonparametric Mann-Whitney U test was used to identify differences between the 2 study groups when Kruskal-Wallis showed statistically significant results. Correlations among study variables were evaluated by Spearman correlation tests. All tests were 2-tailed with an α level of .05. Analyses were performed using the GraphPad PRISM 5.0 software.
Results
Study Population Characteristics
Patient characteristics at study enrollment are summarized in Table 1. Overall, the demographic and hematological data were similar between patients with or without inhibitors. A total of 27 (90%) patients without inhibitors were on prophylaxis with recombinant FVIII, whereas 10% were receiving plasma-derived FVIII. All patients with hemophilia with inhibitors were on demand therapy with recombinant FVIIa. The mean (standard deviation, SD) and range of anti-FVIII inhibitors was 6.5 (1.5 [1.6-12.5]) BU/mL. Two (6%) patients without inhibitors have sickle cell trait. No patient received depletion therapy or had a recent surgery. In both patients with or without inhibitors, the most common ABO group was O with frequencies of 50% and 56%, respectively, followed by group B.
Table 1.
Baseline Characteristics of Study Participants.
| Characteristics | Study Population | ||
|---|---|---|---|
| Patients With Hemophilia A Without Inhibitors (n = 30) | Patients With Hemophilia A With Inhibitors (n = 6) | Healthy Controls (n = 20) | |
| Age, years | 6 (1 [1-16]) | 7 (2 [1-14]) | 5 (1 [1-12]) |
| Male, n (%) | 30 (100) | 6 (100) | 20 (100) |
| Hemoglobin levels, g/dL | 12 (2 [10-15]) | 11 (2 [10-13]) | 12 (1 [10-14]) |
| Lymphocytes, % | 55 (4 [21-81]) | 51 (5 [40-70]) | 54 (3 [21-81]) |
| Monocytes, % | 8 (1 [4-17]) | 10 (2 [6-13]) | 8 (2 [6-12]) |
| FVIII concentration, IU/mL | 0.11 (0.04 [0.001-1.2]) | 0.08 (0.04 [0.004-0.01]) | – |
| Anti-FVIII inhibitor titer, BU/mL | ND | 6.5 (1.5 [1.6-12.5]) | ND |
Abbreviations: FVIII, factor VIII; ND: not detectable.
aResults are shown as mean (standard deviation [range]).
Numerical Analysis of Breg Cells
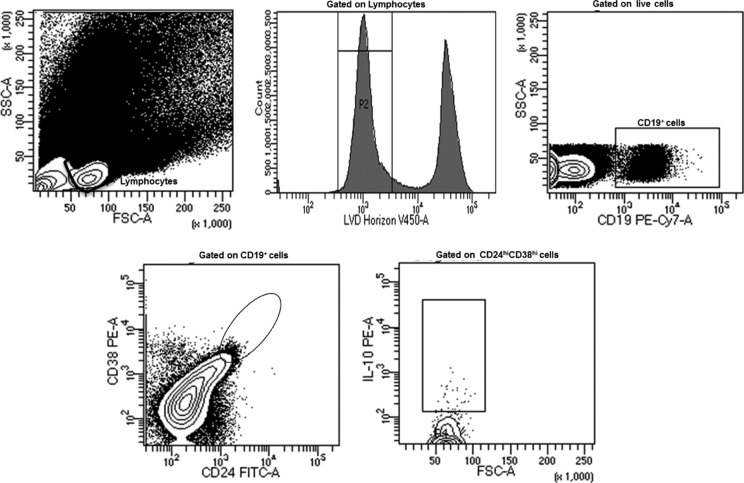
To phenotypically characterize Breg cells, a sequential gating strategy was used as described by Blair et al12 and shown in Figure 1. To determine whether levels of Breg cells vary among study groups, we first compared levels of total B-cells as defined by CD19+ cells. No significant differences in total B-cells were found among patients with hemophilia A with or without inhibitors and healthy controls, with frequencies of 7.1% (0.8%), 6.6% (0.7%), and 7.6% (0.5%; P = .18), respectively. We next compared the levels of Breg cells among the study groups. As shown in Figure 2, levels of Breg cells were substantially lower in patients with inhibitors (3.2% [0.3%]) as compared to healthy controls (7.3% [0.6%]; P = .001). Interestingly, levels of Breg cells were also reduced in patients with inhibitors compared to patients without inhibitors (P = .04). In contrast, levels of Breg cells were similar between patients with hemophilia A without inhibitors and healthy controls (P = .7). Collectively, these data suggest that patients with hemophilia A with inhibitors displayed a pronounced reduction in Breg cells, contrasting with patients with hemophilia without inhibitors.
Figure 1.
A representative gating strategy used to identify circulating regulatory B cells and their production of interleukin-10.
Figure 2.

Levels of regulatory B cells among study group. The mean, standard error of the mean, and P values are shown.
Analysis of Circulating Tfh Cells
To more precisely characterize circulating Tfh cells, a combination of homing markers and transcription factors was simultaneously used, including the chemokine (C-X-C motif) receptor 5 (CXCR5 or CD185), inducible co-stimulator (ICOS or CD278), and the programmed cell death protein 1 (PD-1 or CD279). This combination was shown to better mirror the Tfh cells found in lymph nodes.7,8 The levels of Tfh cells were similar among patients with hemophilia A with or without inhibitors and healthy controls; Figure 3 (0.8% [0.1%], 0.75% [0.08%], and 0.6% [0.09%]; P = .23, respectively). Overall, these data indicate that Tfh cells are preserved in both patients with or without inhibitors.
Figure 3.
Levels of follicular T helper cells among study group. The mean, standard error of the mean, and P values are shown.
Analysis of the Expression of LRP/CD91 on Monocytes
Because LRP/CD91 acts as a catabolic receptor for FVIII, we examined whether CD91 expression on monocytes differ among the study groups. Figure 4 showed that the frequency of monocytes expressing CD91 was markedly higher in patients with inhibitors as evidenced by a nearly 3-fold higher values of CD14+CD91+ in these patients (17.8% [2.5%]) compared to healthy controls (6.9% [1.9%]; P = .001). Similarly, levels of monocytes expressing CD91 were significantly increased in patients without inhibitors (18.9% [3.5%]) compared to healthy controls (6.9% [1.9%]; P = .001). Of note, levels of monocytes expressing CD91 were similar between patients with hemophilia A with and without inhibitors. These results suggest that patients with hemophilia A display greater levels of LRP/CD91 on monocytes than healthy individuals.
Figure 4.
Levels of LPR/CD91 expression on monocytes among study group. The mean, standard error of the mean, and P values are shown.
Functional Analysis of Breg Cells
We next investigated whether the different study groups displayed differences in IL-10 activation pathway by analyzing intracellular production of IL-10 in Breg cells after stimulation. Interestingly, Breg cells from patients with hemophilia A with inhibitors produced lower levels of IL-10 (2.2% [0.2%]) as compared to patients with hemophilia A without inhibitors (5.6% [0.7%], P = .04) or healthy controls (7.3% [0.5%], P < .001; Figure 5). Conversely, IL-10 production by Breg cells was similar between patients with hemophilia A without inhibitors and healthy controls (P = .09). Taken together, these findings suggest that Breg cells from patients with hemophilia A with inhibitors had impaired IL-10 production, contrasting with patients with hemophilia A without inhibitors and healthy controls.
Figure 5.
Levels of interleukin-10 production by regulatory B cells among study group. The mean, standard error of the mean, and P values are shown.
Associations Among Levels of Breg and Tfh Cells and LPR/CD91 on Monocytes
We assessed next, the relationships among levels of Breg cells, Tfh cells, LPR-monocytes and other study variables. Interestingly, levels of Breg cells in patients with hemophilia with inhibitors was negatively associated with LRP/CD91 on monocytes (ρ = −0.37, P = .09) and positively with Tfh cells (ρ = 0.41, P = .22) but did not reach statistical significance. Moreover, most of the other correlations among study variables were not statistically significant (data not shown).
Discussion
Replacement therapy with FVIII still constitutes the first-line treatment for patients with hemophilia A, but the development of inhibitors remains a major clinical complication for this therapy. Understanding the immune mechanisms involved in the development of inhibitors can provide valuable information on pathways to human tolerance. Several clinical and experimental studies have shown that B cells are key in maintaining and breaking tolerance to FVIII.13 In fact, the production of neutralizing Abs relies on the development FVIII specific memory B cells or long-lived plasma cells, on one hand, while generation of several distinct regulatory cells may prevent inhibitor formation on the other hand, as evidenced in animal models using anti-CD3 monoclonal antibody therapy.14 In this study, we explored whether these newly identified Breg cells might play a role, with other cells, in the development of neutralizing Abs in patients with hemophilia A. Interestingly, our findings showed that levels of circulating Breg cells were significantly reduced in hemophilia A patients with inhibitors compared to patients without inhibitors or healthy controls. Several mechanisms may account for the reduction of Breg cells in this group of patients. First, a decrease in the differentiation of naïve/memory B cells to Breg cells. This assumption is supported by observation that Breg cells might arise at any stage of B-cell development depending on the environment in which B cells are located.7 It has been shown that immature and mature B cells as well as memory B cells are endowed with the capacity to differentiate into Breg cells in both animal models and humans.7 However, other studies suggest that Breg cells might also derive from a specific progenitor independent of developmental pathways of conventional B cell subsets.15 Secondly, it might be due to an increase in the migration of Breg cells from the peripheral blood to lymph nodes and other tissues. This is supported by the fact that Breg cells home to secondary lymphoid organs in order to regulate immune responses and production of Abs.7 Finally, an increase in apoptosis of Breg cells could be another plausible explanation given that these cells control the exacerbation of various inflammatory processes.7,15 In addition to the decreased levels of Breg cells, we also found that patients with inhibitors display impaired IL-10 production when activated via the CD40 signaling pathway. Early studies have shown that CD40 signaling is pivotal for the generation and function of Breg cells.12 CD40 ligation results in the activation of multiple signaling cascades of which Janus family of kinases (JAK1) and signal transducer and activators of transcription family (STAT3) are involved in IL-10 production.12,16 Whether the decreased production of IL-10 is associated with deficits in JAK1 and STAT3 signaling cascades remains to be investigated. Because of insufficient quantities of cells, such functional analyses were not performed in the present study. It is, therefore, of importance to address these functional properties of Breg cells in patients with hemophilia A with inhibitors including more study participants.
Previous studies have shown that Tfh cells are critical for directing the development of Abs by B cells in the germinal centers.9 Limited studies have focused on associations between circulating Tfh cells and Breg cells in patients with hemophilia A. Using a combination of markers that better mimic Tfh cells in lymph nodes, their levels in the circulation were investigated and correlated with Breg cells and LPR/CD91 expression on monocytes in patients with hemophilia A with or without inhibitors. In this study, we used simultaneously 5 markers to accurately define the Tfh cell phenotype in the peripheral blood. Our results revealed that levels of Tfh cells do not differ significantly among patients with hemophilia A with or without inhibitors and healthy controls. In addition, our findings indicate that levels of Tfh cells are not significantly correlated with Breg cells, IL-10-producing Breg cells, and LRP/CD91 expression on monocytes in both patients with hemophilia with or without inhibitors. The lack of an ability to observe significant correlations might be explained in part by the fact that the Tfh cell phenotype measured in this study does not exactly reflect Tfh cells in germinal centers, although an extensive panel of monoclonal Abs was used to better define circulating Tfh cells. Whether these are truly insignificant associations among Tfh cells, Breg cells, and LRP/CD91 expression on monocytes, require further investigation along with the coculture assays using sorted cells.
The results of this study also extend to investigation on the role of LRP/CD91 expression on monocytes in patients with hemophilia A. They confirm other reports showing that LRP/CD91 is upregulated on monocytes of patients with hemophilia A.3 Interestingly, we further showed that LRP/CD91 is also overexpressed in patients with hemophilia A with inhibitors. Both patients with hemophilia A with or without inhibitors exhibited a nearly 3-fold increase in LRP/CD91 expression on monocytes compared to healthy controls. Additionally, our findings indicate that levels of LRP/CD91 expression on monocytes are negatively correlated with Breg cells and IL-10-producing Breg cells, but these associations did not reach statistical significance. These results are consistent with a model in which decreased levels of Breg cells might increase the expression of LRP/CD91 expression on monocytes that may take more FVIII from the circulation, thereby accelerating the development of inhibitors. This suggestion is supported by a recent study showing that FVII-pulsed human monocyte-derived dendritic cells can present peptides from several FVIII domains.17 Both mature and immature monocyte-derived dendritic cells were able to internalize efficiently FVIII peptides, retaining their capacity of presentation for 4 days and binding strongly to multiple HLA-DRB1 molecules.17 However, our ex vivo findings contrast with those observed in vitro indicating that CD91 expression on human monocyte-derived dendritic cells does not play a dominant role in FVIII intake.18 Therefore, further studies are needed to support our findings and to test whether Breg cells could suppress the LRP/CD91 overexpression on monocyte-derived dendritic cells via IL-10 production as this cytokine can induce changes in the presentation of antigens through several mechanisms.
One limitation of this study is the relatively small number of patients with hemophilia A with inhibitors included, but the low level of statistical dispersion of the results suggests that increasing the number of patients would not have a major effect on the significance of the obtained results. In addition, relatively few patients with hemophilia A patients develop inhibitors in our ongoing pediatric cohort, adding to that the overall small number of individuals who are being diagnosed and followed in our clinic, accumulating to 154 patients to date.19 Another limitation is that most enrolled patients were children. Therefore, it could be useful in future studies to include adult patients with hemophilia A with late-onset inhibitor to investigate whether Breg cells are also functionally impaired.
Conclusion
In conclusion, to our knowledge this is the first report to demonstrate that patients with hemophilia A with inhibitors displayed a reduction in levels of circulating Breg cells with a deficit in their IL-10 production, and an overexpression of LPR/CD91 on monocytes, along with normal levels of Tfh cells. Overall, these results provide new insights into the role of Breg cells, Tfh cells, and LPR/CD91 receptor in patients with hemophilia A with inhibitors. Further investigation of the interrelationships among these immune cells will help elucidate the mechanisms underlying the development of inhibitors in patients with hemophilia A.
Acknowledgments
The authors are thankful to all participants and also to the Departments of Hematology and Microbiology and Immunology staff for providing technical assistance with flow cytometry.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants from the Oman Research Council (#ORG/HSS/13/002) and the Sultan Qaboos University (#IG/MED/HAEM/14/01).
References
- 1. Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379(9824):1447–1456. [DOI] [PubMed] [Google Scholar]
- 2. Tellier Z, André MH, Polack B. Management of haemophilia A-inhibitor patients: clinical and regulatory perspectives. Clin Rev Allergy Immunol. 2009;37(2):125–134. [DOI] [PubMed] [Google Scholar]
- 3. Franchini M, Urbani S, Amadei B, et al. LRP1/CD91 is up-regulated in monocytes from patients with haemophilia A: a single-centre analysis. Haemophilia. 2013;19(3): e126–e132. [DOI] [PubMed] [Google Scholar]
- 4. Franchini M, Montagnana M. Low-density lipoprotein receptor-related protein 1: new functions for an old molecule. Clin Chem Lab Med. 2011;49(6):967–970. [DOI] [PubMed] [Google Scholar]
- 5. Lenting PJ, Neels JG, van den Berg BM, et al. The light chain of factor VIII comprises a binding site for low density lipoprotein receptor-related protein. J Biol Chem. 1999;274(34):23734–23739. [DOI] [PubMed] [Google Scholar]
- 6. Saenko EL, Yakhyaev AV, Mikhailenko I, Strickland DK, Sarafanov AG. Role of the low density lipoprotein-related protein receptor in mediation of factor VIII catabolism. J Biol Chem. 1999;274(53):37685–37692. [DOI] [PubMed] [Google Scholar]
- 7. Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity. 2015;42(4):607–612. [DOI] [PubMed] [Google Scholar]
- 8. Badell IR, Ford ML. T follicular helper cells in the generation of alloantibody and graft rejection. Curr Opin Organ Transplant. 2016;21(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol. 2014;35(9):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bentebibel SE, Khurana S, Schmitt N, et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci Rep. 2016;6:26494 doi:10.1038/srep26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boulassel MR, Mercier F, Gilmore N, Routy JP. Immunophenotypic patterns of CD8+ T cell subsets expressing CD8alphaalpha and IL-7Ralpha in viremic, aviremic and slow progressor HIV-1-infected subjects. Clin Immunol. 2007;124(2):149–157. [DOI] [PubMed] [Google Scholar]
- 12. Blair PA, Noreña LY, Flores-Borja F, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. [DOI] [PubMed] [Google Scholar]
- 13. Actor MA, Holley KC, Csencsits-Smith K. Role of B cells in breaking and maintaining tolerance to clotting factor VIII in congenital and acquired hemophilia A. Antibodies. 2014;3(2):192–204. [Google Scholar]
- 14. Waters B, Qadura M, Burnett E, et al. Anti-CD3 prevents factor VIII inhibitor development in hemophilia A mice by a regulatory CD4+CD25+-dependent mechanism and by shifting cytokine production to favor a Th1 response. Blood. 2009;113(1):193–203. [DOI] [PubMed] [Google Scholar]
- 15. Vitale G, Mion F, Pucillo C. Regulatory B cells: evidence, developmental origin and population diversity. Mol Immunol. 2010;48(1-3):1–8. [DOI] [PubMed] [Google Scholar]
- 16. Carey AJ, Tan CK, Ulett GC. Infection-induced IL-10 and JAK-STAT: a review of the molecular circuitry controlling immune hyperactivity in response to pathogenic microbes. JAKSTAT. 2012;1(3):159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Haren SD, Wroblewska A, Herczenik E, et al. Limited promiscuity of HLA-DRB1 presented peptides derived of blood coagulation factor VIII. PLoS One. 2013;8(11): e80239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dasgupta S, Navarrete AM, André S, et al. Factor VIII bypasses CD91/LRP for endocytosis by dendritic cells leading to T-cell activation. Haematologica. 2008;93(1):83–89. [DOI] [PubMed] [Google Scholar]
- 19. Nazir HF, Al Lawati T, Beshlawi I, et al. Mode of delivery and risk of intracranial haemorrhage in newborns with severe haemophilia A: a multicentre study in Gulf region. Haemophilia. 2016;22(3): e134–e138. [DOI] [PubMed] [Google Scholar]