Abstract
The present study is the premier clinical attempt to scrutinize the practicability of prophylactic fibrinogen infusion in patients undergoing heart transplantation (HT). A total of 67 consecutive patients who had undergone HT between January 2012 and December 2014 were assessed. After exclusion of some patients, 23 patients were given preoperative 2 g fibrinogen concentrate over a period of 15 minutes after the termination of cardiopulmonary bypass pump and complete reversal of heparin, and 30 patients were not given. Some laboratories were measured before general anesthesia and at 6 and 24 hours after surgery. In addition, major adverse events were also evaluated during hospitalization. The mean age of the patients was 39.5 ± 11.4 years, with a predominance of male sex (77.4%). All laboratories at baseline were comparable between groups. The length of hospital stay was longer in the control group compared to the fibrinogen group (20 [16-22] vs 16 [12-19] days; P = .005). There was a trend for patients in the fibrinogen group to have more acute kidney injury (AKI) after surgery (10% vs 30.4%) and less reoperation for bleeding (20% vs 8.7%). The amount of postoperative bleeding was significantly higher in the control group compared to the fibrinogen group (P < .001). The number of packed red blood cell transfused during 24 hours after surgery was significantly lower in the fibrinogen group (P < .001). The transfusion of fibrinogen in patients undergoing HT may be associated with reductions in postoperative bleeding, the number of packed red blood cells, and hospital length of stay; however, it may enhance postoperative AKI.
Keywords: heart transplantation, fibrinogen, bleeding, transfusion, acute kidney injury
Introduction
It has been proposed that bleeding after heart transplantation (HT) may be caused by impairment of the coagulation cascades or technical problems in surgery.1 Patients undergoing HT may be required to receive blood products due to excessive perioperative blood loss. Excess bleeding and subsequent transfusion of blood products increase the possibility of right ventricular failure, infection, and rejection rate.2 Meanwhile, it enhances the length of intensive care unit stay, the length of hospital stay, subsequent renal failure due to right ventricular failure,3 need to renal replacement therapy,4 and overall morbidity and mortality.5
In order to assess major bleeding following cardiac surgery, the platelet dysfunction, coagulation pathway derangements, and fibrinolysis should be taken into consideration after excluding the surgical-related issues.6 Fibrinogen has an essential role in maintaining hemostasis by stabilization of the platelet plug and a subsequent clot formation. It has also been found to be the first factor that dropped to critical low levels in the major postoperative bleeding.7
For managing the postoperative bleeding conventionally, fresh frozen plasma (FFP) and cryoprecipitate are used as the first line of standard replacement,7 but their efficacy in controlling the bleeding is not clear.6 In addition, it may be associated with some complications such as overload, transfusion-related lung injury, and infection.7 Although plasma-derived fibrinogen concentrate has been used for many years for patients with congenital fibrinogen deficiency, it has recently become popular for acquired fibrinogen deficiency, particularly in cases with bleeding following cardiac surgery.6
In many European countries with the exception of United Kingdom, the fibrinogen concentrate is a part of standard care in postoperative cardiac surgery bleeding,7 but its implementation in managing the postoperative cardiac surgery bleeding has not been approved in the United States and United Kingdom.7 Some previous studies have shown that the prophylactic infusion of fibrinogen concentrate reduces the bleeding after coronary artery bypass surgery and other types of cardiac surgery.8 To the best of our knowledge, the present study is the first one to scrutinize the practicability and efficacy of the prophylactic fibrinogen infusion in patients undergoing HT.
Materials and Methods
Study Protocol and Population
A total of 67 consecutive patients who had undergone HT from January 2012 to December 2014 in Masih Daneshvari Hospital, Tehran, Iran, were assessed retrospectively. All operations were performed by 1 cardiac surgeon, his assistants, and a cardiac anesthetist. This study was approved by the institutional review board of the National Research Institute of Tuberculosis and Lung disease (NRITLD) and the Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
The main inclusion criteria included patients older than 18 years undergoing isolated HT. Patients with liver and renal failure, bleeding propensity, patients on clopidogrel therapy, patients transferred to intensive care unit on extracorporeal membrane oxygenation or intra-aortic balloon pump, and patients who developed surgical bleeding after transplantation were excluded from the study. All patients in whom the international normalized ratio (INR) was >1.5 preoperatively and there was no time for reversal were also excluded from the study. In addition, in order to evaluate the effect of fibrinogen on effective clot formation, all patients with postoperative surgical-related bleeding were excluded.
Surgical Techniques and Interventions
Anesthesia was induced using 10 to 15 μg/kg of intravenous (IV) fentanyl, 0.2 mg/kg of IV etomidate, and 0.1 mg/kg of IV pancuronium bromide. Before the commencement of the operation, a percutaneous central venous line and Swan-Ganz catheter were placed. Fentanyl, midazolam, and atracurium were used to maintain general anesthesia. Before cardiopulmonary bypass (CPB), a baseline activated clotting time (ACT) was measured, and an IV bolus dose of heparin (400 Units/kg) was administered to all patients. The next dose of heparin was targeted at maintaining ACT values above 480 seconds. For myocardial protection, Belzer UW (Bridge to Life, Europe Ltd, London, United Kingdom) was used in all cases. The technique of operation was orthotopic bicaval for all patients. The same amount of prime solution of the oxygenator was used in all patients of our cohort, which included 1500 cm3 of Ringer solution and 100 cm3 of 20% albumin. For all patients, hypothermia was induced till 32°C. Similar fluid therapy was also used in both the groups. Donors for a specific recipient was selected from the registry center of the Iran’s Ministry of Health and acceptance of the transplant team. Human leukocyte antigen (HLA) typing was not done for any patient with HT, and all the donors and recipients were ABO identical or compatible.
In the fibrinogen group, all patients received 2 g of fibrinogen (Haemocomplettan P; CSL Behring, Pennsylvania) over a period of 15 minutes after the termination of CPB and complete heparin reversal by administration of protamine (1 mg protamine/100 Units heparin) to maintain the ACT below 120 seconds. In controls, all the interventions were same except for not administering fibrinogen.
Attending cardiac anesthesiologist and cardiac surgeon decided about all transfusion during early postoperative care. The amounts of intraoperative and postoperative transfusions were based on the Society of Thoracic Surgeons guidelines.9 Hematocrit level was maintained between 20% and 30% during operation. Hematocrit value <25% was considered as transfusion cut point in the intensive care unit. In addition, FFP and platelets were transfused when INR was >1.5 and platelet count <100 × 109/L, respectively.
Laboratory Measurements
Some serum biomarkers were evaluated, including hemoglobin, hematocrit, platelet count, prothrombin time, partial thromboplastin time, and INR. These values were measured prior to induction of the general anesthesia and were repeated at 6 and 24 hours postoperatively. We monitored these parameters in relation to the levels of postoperative bleeding.
Outcome Evaluation
The overall chest tube drainage during the first postoperative day was measured. The amount of transfused packed red blood cells within first day after surgery was also evaluated. Some major adverse events were reported during hospitalization, including cardiovascular events, sepsis, acute kidney injury (AKI), and death. The diagnosis of AKI was based on the AKI network criteria,10 and abrupt (within 48 hours) reduction in renal function as an absolute increase in serum creatinine of 0.3 mg/dL was considered as postoperative AKI.
Statistical Analysis
Data analysis was performed using SPSS software version 20 (IBM Co, New York). Continuous variables were expressed as mean ± standard deviation or median (interquartile range), and these values were compared by Student t test or Mann-Whitney U test, respectively. Nominal variables were expressed as number (percentage) and were analyzed using χ2 or Fisher exact test. Correlation analysis was used to identify the correlation between laboratories and the postoperative bleeding volume. Repeated-measures analysis of variance was also used to compare continuous variables at different times between study groups. Statistical significance is considered for 2-tailed values of P < .05.
Result
Of 53 patients who underwent isolated HT, 23 patients were given human fibrinogen concentrate at the end of operation and 30 patients were not. The patients’ mean age was 39.5 ± 11.4 years, and there was no significant difference between groups (P = .069). Male sex was predominant, and it was comparable between groups (P = .235). The major cause of heart failure was dilated cardiomyopathy (84.9%), and one case had invasive myxoma (1.9%). All laboratories at baseline were comparable between groups. Other characteristics are summarized in Table 1.
Table 1.
Baseline Characteristics and Surgical-Related Factors Based on Study Groups.a
| Total | Control, n = 30 | Fibrinogen, n = 23 | P Value | |
|---|---|---|---|---|
| Age, years | 39.5 ± 11.4 | 37 ± 10.9 | 42.7 ± 11.5 | .069 |
| Male | 41 (77.4%) | 7 (30.4%) | 16 (69.6%) | .235 |
| BMI, kg/m2 | 23.1 (20.3-25.1) | 22.2 (20.1-24.5) | 23.9 (21.8-25.1) | .323 |
| Prior histories | ||||
| Diabetes mellitus | 2 (3.8%) | 2 (6.7%) | 0 (0%) | .499 |
| Hypertension | 5 (9.4%) | 3 (10%) | 2 (8.7%) | .872 |
| Myocardial infarction | 5 (9.4%) | 3 (10%) | 2 (8.7%) | .872 |
| Cerebrovascular events | 1 (1.9%) | 1 (3.3%) | 0 (0%) | 1 |
| Smoking | 5 (9.4%) | 4 (13.3%) | 1 (4.3%) | .374 |
| Causes of heart failure | .083 | |||
| Dilated CM | 45 (84.9%) | 28 (93.3%) | 17 (73.9%) | |
| Ischemic CM | 5 (9.4%) | 1 (3.3%) | 4 (17.4%) | |
| Restrictive CM | 1 (1.9%) | 1 (3.3%) | 0 (0%) | |
| Others | 2 (3.8%) | 0 (0%) | 2 (8.7%) | |
| Surgical factors | ||||
| CPB time, minutes | 130 (119.5-141) | 129 (117-141) | 132 (120-144) | .478 |
| ACC time, minutes | 101 (92-116.5) | 97.5 (90-108) | 110 (100-129) | .019 |
| Anesthesia time, hours | 6.1 (5.3-8) | 6.2 (5.3-7.1) | 6.1 (5.3-9) | .353 |
| Baseline laboratories | ||||
| Hemoglobin, mg/dL | 14.1 (2.1) | 14.5 (2.7) | 13.8 (1.8) | .297 |
| Hematocrit, % | 41.8 (5.3) | 42.1 (5.6) | 41.4 (5) | .687 |
| Platelet count, ×109/L | 218.7 (66.5) | 214.2 (48) | 222.1 (78) | .701 |
| PT, seconds | 14.1 (3.1) | 14.9 (4.1) | 13.3 (1.3) | .114 |
| PTT, seconds | 32 (8.5) | 33.5 (9) | 30.3 (7.9) | .245 |
| Creatinine, mg/dL | 1.5 (0.6) | 1.5 (0.7) | 1.6 (0.5) | .562 |
Abbreviations: BMI, body mass index; CM, cardiomyopathy; CPB, cardiopulmonary bypass; ACC, aortic cross clamp; PT, prothrombin time; PTT, partial thromboplastin time; SD, standard deviation.
aValues in the table are presented as number (%), mean (SD), or median (interquartile range).
Intra-aortic balloon pump and extracorporeal membrane oxygenation were implemented in 2 (3.8%) cases in the control group and 1 (1.9%) case in the fibrinogen group, respectively (both P values > .05). The length of stay in hospital was longer in the control group than that in the fibrinogen group (20 [16-22] vs 16 [12-19] days; P = .005). Of the in-hospital events listed in Table 2, there was a trend among patients in fibrinogen group to have more AKI after surgery (10% vs 30.4%) and less reoperation for bleeding (20% vs 8.7%). Other postoperative features are summarized in Table 2.
Table 2.
Postoperative Features Based on Study Groups.a
| Total | Control, n = 30 | Fibrinogen, n = 23 | P Value | |
|---|---|---|---|---|
| IABP after surgery | 2 (3.8%) | 2 (6.7%) | 0 (0%) | .207 |
| ECMO after surgery | 2 (3.8%) | 1 (3.3%) | 1 (4.3%) | .848 |
| ICU stay, day | 4 (3-5) | 4 (3-5) | 4 (3-5) | .647 |
| Hospital stay, day | 18 (15-21) | 20 (16-22) | 16 (12-19) | .005 |
| Post-CPB ACT, seconds | 130 (120-140) | 130 (119-140) | 130 (120-140) | .684 |
| Packed RBC, number | 2 (0-2) | 2 (2-3) | 0 (0-1) | <.001 |
| Reoperation for bleeding | 8 (15.1%) | 6 (20%) | 2 (8.7%) | .255 |
| In-hospital events | ||||
| AKI | 10 (18.9%) | 3 (10%) | 7 (30.4%) | .059 |
| Sepsis | 2 (3.8%) | 2 (6.7%) | 0 (0%) | .207 |
| Myocardial infarction | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Cerebrovascular events | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Death | 7 (13.2%) | 4 (13.3%) | 3 (13%) | .975 |
Abbreviations: IABP, intra-aortic balloon pump; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; CPB, cardiopulmonary bypass; ACT, anticoagulation clotting time; RBC, red blood cell count; AKI, acute kidney injury.
aValues are presented as number (%) or median (interquartile range).
Based on the correlation analysis, the levels of hemoglobin and hematocrit at 6 hours were inversely correlated with bleeding volume at 12 hours (r = −.408, P = .026 and r = −.389, P = .037, respectively). In addition, hemoglobin and hematocrit at 6 hours correlated with bleeding volume at 24 hours (r = −.394, P = .035 and r = −.372, P = .047, respectively). Platelet count at 6 hours and inversely correlated with bleeding at 24 hours (r = −.408, P = .026) and preoperative prothrombin time directly correlated with bleeding at 24 hours (r = .316, P = .047). Other correlations were not significant (not reported).
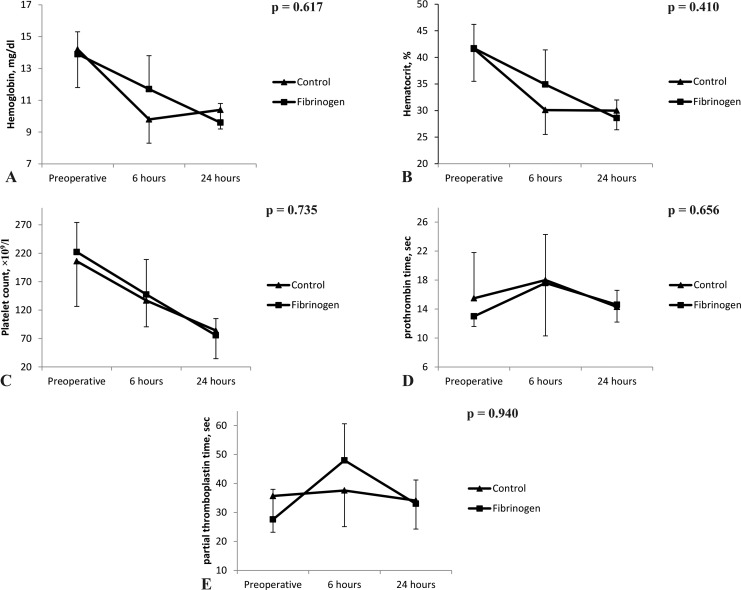
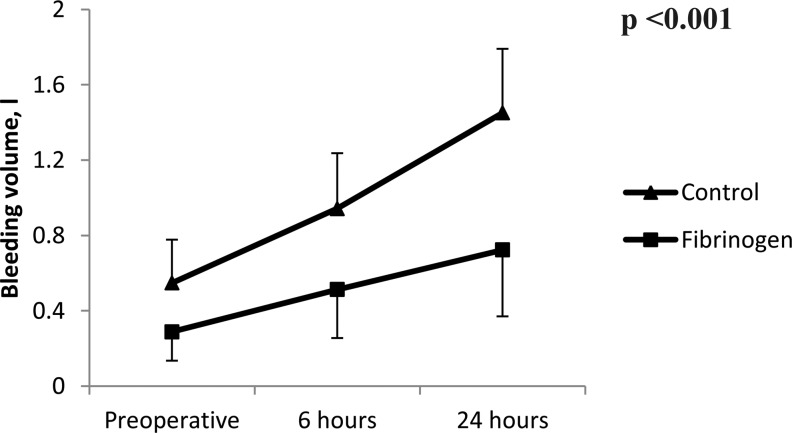
When evaluating postoperative changes in laboratories (from baseline to 24 hours after surgery), there were no significant differences regarding the between-patient effects (P > .05 for all laboratories; Figure 1). However, the amount of postoperative bleeding was significantly higher in control compared with the fibrinogen group (Figure 2). Furthermore, the number of packed red blood cells transfused during 24 hours after surgery was significantly lower in patients receiving fibrinogen compared to the control group (0 [0-1] vs 2 [2-3] packed cells; P < .001). However, the number of patients undergoing reoperation for bleeding was comparable between groups; none of the bleeding etiologies leading to reoperations were surgical related.
Figure 1.
Changes in laboratories (A) hemoglobin, (B) hematocrit, (C) platelet count, (D) prothrombin time, and (E) partial thromboplastin time from baseline to 24 hours postoperative time in both groups.
Figure 2.
The bleeding volume at postoperative day.
When evaluating factors associated with the development of AKI, patients with postoperative AKI compared to those without AKI had less bleeding at 24 hours (0.8 ± 0.45 vs 1.2 ± 0.5 L; P = .024) and less number of packed red blood cells transfused (0.8 ± 1.1 vs 1.7 ± 1.2 number; P = .034). The levels of hemoglobin and hematocrit and the platelet counts were significantly lower in patients with AKI compared to those without AKI. In addition, patients with AKI were older (48.7 ± 9.1 vs 37.3 ± 10.8 years; P = .003), and there was a trend toward cases with AKI to have longer duration of aortic cross-clamp time (117.5 ± 30.9 vs 105.3 ± 17.1 minutes; P = 0.093).
Discussion
In this retrospective and single-center study, we showed for the first time that the infusion of fibrinogen concentrate after reversal of heparin in HT may be beneficial to reduce the development of postoperative bleeding. In addition, the administration of fibrinogen decreased the hospital length of stay and the number of packed red blood cells transfused. In addition, there was a trend among patients receiving fibrinogen to have more AKI compared to controls.
Perioperative bleeding remained a major complication following open heart surgeries, owing to the fact that the excessive postoperative bleeding has been associated with an increased cost of care and a significant morbidity and mortality.11,12 The excess transfusion of blood products as the main management of such events increases the risk of right ventricular failure2 and decreases patients’ survival.5 About 5% to 10% of patients undergoing HT especially in redo sternotomies may have more than 2 L of blood loss at first 24 hours after operation.5 The excessive blood loss may be attributed to a decline in coagulation factors (ie, hyperfibrinolysis) and an impaired platelet function.12,13 The use of appropriate technique for precise evaluation of platelet function, fibrinogen levels, and coagulation factors aids physicians in rapid assessment of the postoperative bleeding events. Moreover, it helps surgeons to implement optimal treatment such as administration of pharmacological and/or blood products and to identify patients requiring further exploration to ameliorate the morbidity and mortality.
Some of CPB-related factors, including decreases in coagulation factors and platelets, led to hemodilution due to the prime solution of oxygenator and the activation of fibrinolysis. Moreover, hypothermia is known as the main causes of the nonsurgical bleeding after all cardiac surgeries.12,14 However, in our study, the amount of prime solution in the oxygenator in all patients was the same (ie, 1000 cm3 of Ringer, 100 cm3 of Albumin 20%, and 200 cm3 of Mannitol solution 20%). Hypothermia was induced till 32°C for all patients. None of these factors could interfere with the coagulation pathways in the study cohort due to the implementation of similar interventions in all patients.
The serum level of fibrinogen may be reduced significantly during major surgical blood loss. In an animal model of dilutional coagulopathy, even in moderate blood loss, increased breakdown may be greater than the increase in compensative fibrinogen synthesis.15 The significance of hypothesis that patients with higher serum fibrinogen levels experience fewer bleeding complications further highlights the role of fibrinogen in the maintenance of postoperative homeostasis and prevention of excessive bleeding.16,17 Some recent studies have focused on the role of hyperfibrinolysis as the etiology for abnormal bleeding after CPB.18 A large study involving 894 patients showed a significant association of hemostatic parameters assessment such as platelet count, prothrombin time, activated partial thromboplastin time, fibrinogen, fibrin degradation products, ACT, and 16-hour chest tube drainage. Of these parameters, only postoperative fibrinogen level was significantly different in bleeders and nonbleeders.19 We did not measure the fibrinogen level in our patients, and we are unable to conclude whether our findings are influenced by the fibrinogen levels.
Karlsson et al found a significant correlation between plasma fibrinogen level and postoperative bleeding volume, but no correlation was observed between bleeding and prothrombin time, and a significant inverse correlation was also demonstrated between bleeding and platelet count in coronary artery bypass graft surgery.20 Sadeghi et al showed that fibrinogen in patients undergoing coronary artery bypass graft surgery was associated with a significant lower blood loss (P = .0001) but no significant difference in transfusion of blood products. Moreover, there were no significant differences between the control and fibrinogen groups regarding the preoperative and postoperative laboratories, including prothrombin time, platelet count, hemoglobin, and partial thromboplastin time. In addition, postoperative fibrinogen level was comparable between the groups.21 Moreover, Kindo et al reported that prothrombin time was an important predictor of excess bleeding.22 In lines with some parts of these findings, we found that preoperative prothrombin time and platelet counts at 6 hours after surgery were associated with more postoperative bleeding at 24 hours.
In a study by Ranucci et al,23 the use of fibrinogen concentrate decreased postoperative bleeding and blood product transfusions in patients undergoing complex cardiac surgery. In contrast, patients who underwent high-risk cardiac surgeries with intraoperative bleeding (blood volume between 60 and 250 mL suctioned from the thoracic cavity in a period of 5 minutes) were given either fibrinogen concentrate or nothing. They found that there was no significant difference in the amount of intraoperative blood loss and more rates of postoperative cardiovascular events in fibrinogen group.24 In addition, in the latest meta-analysis up to the end of 2016, it was concluded, the use of fibrinogen significantly reduced the bleeding volume (mean difference −127 mL; P = .002; I2 = 54%) and the number of packed red blood cell units (mean difference −0.9; P < .001; I2 = 42%). None of the meta-analyzed studies was powered to estimate the survival and adverse events, but the rate of mortality was lower in the fibrinogen group.25 It seems that further large-scale trials are required to have a more robust conclusion regarding the beneficial effect of perioperative fibrinogen in the setting of cardiac surgery.
Two main factors may impact the effect of fibrinogen, including fibrinogen level and fibrinogen dose. Based on a pooled correlation coefficient in a meta-analysis, it has been found that there was weak to moderate correlations between preoperative (−0.40) and postoperative (−0.23) fibrinogen levels and postoperative bleeding.26 In contrast, some studies showed no association between preoperative fibrinogen level and postoperative bleeding.27 However, the latest trial has shown that the fibrinogen level increased in both groups (fibrinogen and control groups) at 24 hours, which can be explained by the increased fibrinogen production by liver on postoperative day. It should be mentioned that fibrinogen concentration has a dose-dependent effect on platelet aggregation, clot formation, and maintaining hemostatic process.24Rahe-Meyer et al28 infused 8 g fibrinogen concentrate with a guide of thromboelastometry which results in the cessation of intraoperative bleeding during thoracoabdominal aortic aneurysm surgery and reduced transfusion and 24-hour drainage volume. However, in the majority of previous studies, the fibrinogen dose has been 2 to 4 g.24,25 It has been found that the fibrinogen level of 2.2 g/L was the best cutoff point to predict bleeding.22 On the other hand, in the latest trial, the targeted level to achieve a postinfusion plasma fibrinogen level was 2.5 g/L, which did not result in the reduction of bleeding and blood product transfusion.24 It may be assumed that small doses of fibrinogen have attenuated effects on controlling nonsurgical bleeding, while with higher doses its impact became augmented.
Of main concern in postoperative care of patients undergoing cardiac surgeries is AKI. None of the previous studies have reported the effect of fibrinogen on postoperative AKI in patients undergoing cardiac surgeries except for 2 studies in which there was no difference between patients receiving fibrinogen and comparator with regard to the development of AKI requiring dialysis 29 and renal insufficiency or failure.24 A study has shown that higher fibrinogen level was associated with higher contrast-induced AKI in patients undergoing coronary intervention.30 Fibrinogen is significantly upregulated in the kidney after development of AKI.31 Moreover, in a study of patients with AKI, fibrinogen deposition was observed in the glomerular basement membrane, leading to the reduction in glomerular filtration was found.32 Our finding regarding AKI may be caused by the higher level of fibrinogen in patients receiving fibrinogen. Moreover, we found that cases with AKI had less bleeding and therefore received less transfusion, which support the hypothesis mentioned here.
Some limitations to our study should be considered. Firstly, it is a small retrospective study. Secondly, the lack of perioperative thromboelastometric monitoring and measurement of fibrinogen level preclude us to correlate our findings with coagulation status and patients’ outcomes.
Conclusion
The transfusion of preoperative fibrinogen concentrate in patients undergoing HT may be associated with reductions in postoperative bleeding volume at 24 hours, the number of packed red blood cells transfused at 24 hours, and hospital length of stay; however, it may lead to enhancement of postoperative AKI.
Acknowledgments
The authors gratefully thank Yadollah Mafhumi and Zahra Faghih, the perfusionists of the team, and all the nurses of the operation room and ICU for their hardworking and dedication in care of the patients.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Shams Hakimi C, Fagerberg Blixter I, Hansson EC, Hesse C, Wallén H, Jeppsson A. Effects of fibrinogen and platelet supplementation on clot formation and platelet aggregation in blood samples from cardiac surgery patients. Thromb Res. 2014;134(4):895–900. [DOI] [PubMed] [Google Scholar]
- 2. Haglund NA, Davis ME, Tricarico NM, et al. Perioperative Blood Product Use: A Comparison Between HeartWare and HeartMate II Devices. Ann Thorac Surg. 2014;98(3):842–849. [DOI] [PubMed] [Google Scholar]
- 3. Simon MA. My approach to the patient with right ventricular failure. Trends Cardiovasc Med. 2015;25(5):471–472. [DOI] [PubMed] [Google Scholar]
- 4. Mirhosseini SM, Fakhri M, Asadollahi S, et al. Continuous renal replacement therapy versus furosemide for management of kidney impairment in heart transplant recipients with volume overload. Interact Cardiovasc Thorac Surg. 2013;16(3):314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollis AL, Lowery AV, Pajoumand M, et al. Impact on postoperative bleeding and cost of recombinant activated factor VII in patients undergoing heart transplantation. Ann Card Anaesth. 2016;19(3):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rahe-Meyer N. Fibrinogen concentrate in the treatment of severe bleeding after aortic aneurysm graft surgery. Thromb Res. 2011;128(suppl 1):S17–S19. [DOI] [PubMed] [Google Scholar]
- 7. Miceli A, Ranucci M, Glauber M. Fibrinogen concentrate as first-line hemostatic treatment for the management of bleeding in complex cardiac surgery. J Thorac Cardiovasc Surg. 2016;151(2):383–384. [DOI] [PubMed] [Google Scholar]
- 8. Karlsson M, Ternstrom L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. Thromb Haemost. 2009;102(1):137–144. [DOI] [PubMed] [Google Scholar]
- 9. Ferraris VA, Brown JR, Despotis GJ, et al. ; Society of Thoracic Surgeons Blood Conservation Guideline Task Force; International Consortium for Evidence Based Perfusion. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. [DOI] [PubMed] [Google Scholar]
- 10. Mehta RL, Kellum JA, Shah SV, et al. Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2): R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44(10):1453–1462. [DOI] [PubMed] [Google Scholar]
- 12. Birati EY, Rame JE. Post–heart transplant complications. Crit Care Clin. 2014;30(3):629–637. [DOI] [PubMed] [Google Scholar]
- 13. Hatton KW, Flynn JD, Lallos C, Fahy BG. Integrating evidence-based medicine into the perioperative care of cardiac surgery patients. J Cardiothorac Vasc Anesth. 2011;25(2):335–346. [DOI] [PubMed] [Google Scholar]
- 14. Ranucci M, Baryshnikova E, Castelvecchio S, Pelissero G. Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann Thorac Surg. 2013;96(2):478–485. [DOI] [PubMed] [Google Scholar]
- 15. Martini WZ, Chinkes DL, Pusateri AE, et al. Acute changes in fibrinogen metabolism and coagulation after hemorrhage in pigs. Am J Physiol Endocrinol Metab. 2005;289(5):14. [DOI] [PubMed] [Google Scholar]
- 16. Blome M, Isgro F, Kiessling AH, et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb Haemost. 2005;93(6):1101–1107. [DOI] [PubMed] [Google Scholar]
- 17. Ucar HI, Oc M, Tok M, et al. Preoperative fibrinogen levels as a predictor of postoperative bleeding after open heart surgery. Heart Surg Forum. 2007;10(5): E392–E396. [DOI] [PubMed] [Google Scholar]
- 18. Paramo JA, Rifon J, Llorens R, Casares J, Paloma MJ, Rocha E. Intra- and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery. Haemostasis. 1991;21(1):58–64. [DOI] [PubMed] [Google Scholar]
- 19. Gravlee GP, Arora S, Lavender SW, et al. Predictive value of blood clotting tests in cardiac surgical patients. Ann Thorac Surg. 1994;58(1):216–221. [DOI] [PubMed] [Google Scholar]
- 20. Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Nilsson S, Jeppsson A. Plasma fibrinogen level, bleeding, and transfusion after on-pump coronary artery bypass grafting surgery: a prospective observational study. Transfusion. 2008;48(10):2152–2158. [DOI] [PubMed] [Google Scholar]
- 21. Sadeghi M, Atefyekta R, Azimaraghi O, et al. A randomized, double blind trial of prophylactic fibrinogen to reduce bleeding in cardiac surgery. Braz J Anesthesiol. 2014;64(4):253–257. [DOI] [PubMed] [Google Scholar]
- 22. Kindo M, Hoang Minh T, Gerelli S, et al. Plasma fibrinogen level on admission to the intensive care unit is a powerful predictor of postoperative bleeding after cardiac surgery with cardiopulmonary bypass. Thromb Res. 2014;134(2):360–368. [DOI] [PubMed] [Google Scholar]
- 23. Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4(6):e002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bilecen S, de Groot JA, Kalkman CJ, et al. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high-risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317(7):738–747. [DOI] [PubMed] [Google Scholar]
- 25. Fominskiy E, Nepomniashchikh VA, Lomivorotov VV, et al. Efficacy and safety of fibrinogen concentrate in surgical patients: a meta-analysis of randomized controlled trials. J Cardiothorac Vasc Anesth. 2016;30(5):1196–1204. [DOI] [PubMed] [Google Scholar]
- 26. Gielen C, Dekkers O, Stijnen T, et al. The effects of pre- and postoperative fibrinogen levels on blood loss after cardiac surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014;18(3):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jalali A, Ghiasi M, Aghaei A, Khaleghparast S, Ghanbari B, Bakhshandeh H. Can plasma fibrinogen levels predict bleeding after coronary artery bypass grafting? Res Cardiovasc Med. 2014;3(3):e19521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138(3):694–702. [DOI] [PubMed] [Google Scholar]
- 29. Galas FR, de Almeida JP, Fukushima JT, et al. Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: a randomized pilot trial. J Thorac Cardiovasc Surg. 2014;148(4):1647–1655. [DOI] [PubMed] [Google Scholar]
- 30. Celik IE, Kurtul A, Duran M, et al. Elevated serum fibrinogen levels and risk of contrast-induced acute kidney injury in patients undergoing a percutaneous coronary intervention for the treatment of acute coronary syndrome. Coron Artery Dis. 2016;27(1):13–18. [DOI] [PubMed] [Google Scholar]
- 31. Krishnamoorthy A, Ajay AK, Hoffmann D, et al. Fibrinogen beta-derived Bbeta(15-42) peptide protects against kidney ischemia/reperfusion injury. Blood. 2011;118(7):1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Koffler D, Paronetto F. Fibrinogen deposition in acute renal failure. Am J Pathol. 1966;49(2):383–395. [PMC free article] [PubMed] [Google Scholar]