Abstract
Heparin-induced thrombocytopenia (HIT) is one of the serious complications in patients who undergo cardiac surgery. However, there remains a major problem in diagnosing HIT because the current immunological assays for detection of HIT antibody have limitations. Furthermore, the clinical course of thrombocytopenia in this surgery makes it increasingly difficult to diagnose HIT. We investigated the relationship between platelet count and HIT antibody in 59 patients who underwent cardiac surgery using cardiopulmonary bypass (CPB). The number of postoperative HIT antibody-positive patients evaluated using enzyme-linked immunosorbent assay kit (polyanion IgG/IgA/IgM complex antibodies/antiplatelet factor 4 enhanced) was 37 (62.7%). In contrast, platelet activation by HIT antibody was evaluated using the serotonin release assay (SRA). More than 20% and 50% release of serotonin was obtained from 12 patients (20.3%) and 8 patients (13.6%), respectively. The levels of d-dimer were significantly different on postoperative day 14 between SRA-positive and SRA-negative groups; however, postoperative thrombus complication was not detected using sonography in the patients with positive serotonin release at all. After being decreased by the operation, their platelet count recovered within 2 weeks in both groups equally. In our study, although the patients were positive in the platelet activating HIT antibody assay, they remained free from thrombosis and their platelet count recovered after early postoperative platelet decrease. Therefore, in addition to the SRA, monitoring of platelet count might be still considered an indispensable factor to facilitate the prediction of HIT thrombosis prior to manifestation in the patients undergoing cardiac surgery using CPB.
Keywords: heparin-induced thrombocytopenia, platelet count monitoring, serotonin release assay, cardiac surgery, cardiopulmonary bypass
Introduction
Heparin use during cardiovascular surgery is known to cause the generation of antiplatelet factor 4 (PF4)/heparin complex antibodies, leading to multiple, systemic thromboses with concomitant thrombocytopenia (heparin-induced thrombocytopenia [HIT]) in some patients. Because only a subset of these patients will produce those antibodies that activate platelets, HIT is an unpredictable and critical complication of cardiovascular surgery.
Heparin administration is mandatory as a routine procedure in cardiac surgery to prevent thrombus formation during cardiopulmonary bypass (CPB). Therefore, HIT is always possible in these surgeries. Once it occurs, the management of patients becomes more difficult at once and their morbidity depends on the severity of thromboses irrespective of surgical procedure. Therefore, the prediction and prompt diagnosis of HIT and its thrombosis have been eagerly sought for by cardiac surgeons. Although antibody assay of HIT has been reported, the unsolved problem still exists. It does not establish a correlation between the commonly used serological diagnosis by the enzyme immunoassays for the PF4/heparin antibodies (HIT antibody) and clinical manifestations of thrombocytopenia with concomitant thrombosis.1 Thus, in the present study, we investigate the risk factors for HIT in patients who underwent cardiac surgery using CPB, and we focus on both the serotonin release assay (SRA)2 and its cutoff value in relation to the prediction of HIT.
Materials and Methods
Patients who underwent cardiac surgery using CPB in the University of Yamanashi, Department of Cardiovascular Surgery, from June 2005 to August 2006, were enrolled in this study. The study was performed in accordance with the Declaration of Helsinki, and its protocol was approved by the ethics committee of the University of Yamanashi. Our study included 59 patients (42 men and 17 women) with a mean age of 68.3 (8.6) years. All patients provided written informed consent before participating in this study. Unfractionated heparin (UFH; 300 U/kg) was administered just before cannulation for CPB to maintain an activated coagulation time of longer than 400 seconds during CPB. After cessation of CPB, heparin was neutralized by protamine, and additional UFH was not used until at least 14 days after the surgery. The most frequent disease was coronary artery disease (36 patients), followed by valve insufficiency (16 patients). The diagnosis of the patients’ conditions and the operations performed are presented in Table 1. Each patient was examined preoperatively and both 1 week and 2 weeks after the operation. The number of platelets was counted every day until 2 days postoperatively and every other day thereafter. The d-dimer levels were measured on the scheduled days. Heparin-induced thrombocytopenia antibody levels were measured by enzyme-linked immunosorbent assay, which can detect antiplatelet factor 4 (PF4)/polyanion IgG/IgA/IgM complex antibodies (GTI).1 Polyanion IgG/IgA/IgM complex antibodies titers were represented as optical density (OD) values, and a result was considered positive if the OD value was greater than 0.40.1 To measure the activated platelet function by the HIT antibody, the 14C SRA was performed.2 Briefly, platelet-rich plasma is labeled with 14C-serotonin,incubated, and resuspended to a count of 3.0 × 105 platelets/μL. The test serum is incubated with the platelet suspension, and 14C-serotonin released into the supernatant is measured using a scintillation counter. Each serum is designated as positive for heparin antibody if they cause the release of ≥20% serotonin with 0.1 U/mL heparin after platelet lysis, because less than 20% release of SRA is observed in healthy volunteers.3 Results were grouped by the following 3 phases: serotonin release (SR) <20% (negative), ≥20% (SR20 positive), or ≥50% (SR50 positive) under a low dose of heparin (0.1 U/mL). In addition, when the releasing rate of serotonin was changed from SR20 or SR50 to <20% or <50% under a high concentration of heparin (100 U/mL), the results were considered positive. Clinical assessment was performed daily after the operation to detect physical presence of deep vein thrombosis or pulmonary embolism. When thrombus formation was suspected clinically, duplex scan sonography was performed immediately, particularly focusing on the veins of the lower extremities, which is the most frequent site of postoperative thrombosis. When pulmonary embolism was suspected from respiratory distress, computed tomography (CT) with contrast enhancement was performed. The difference between the 2 groups was analyzed statistically by the Student t test or the chi-square test (IBM SPSS Statics, Version 22, IBM). A P value less than .05 indicates a statistically significant difference between the 2 groups.
Table 1.
Preoperative Diagnosis for the Patients and the Operations Performed.a
| Preoperative Diagnosis | Number of Patients | Operation Performed | Number of Operations |
|---|---|---|---|
| CAD | 33 | CABG | 33 |
| Valve disease | 13 | Valve replacement | 12 |
| CAD + valve disease | 3 | Valve plasty | 1 |
| ASD | 2 | Valve replacement + CABG | 3 |
| TAA | 4 | ASD closure | 2 |
| cAD | 2 | TAR | 5 |
| Cardiac tumor (myxoma) | 2 | Aorta reconstruction | 1 |
| Extirpation | 2 |
Abbreviations: CAD, coronary artery disease; ASD, atrium septum defect; TAA, thoracic aortic aneurysm; cAD, chronic aortic dissection; CABG, coronary artery bypass grafting; TAR, total arch replacement.
aEach patient received 300 U/kg body weight of unfractionated heparin, and the volume was adjusted to achieve an activating coagulation time (ACT) of over 400 seconds.
Results
Of the 59 patients, 53 had a history of heparin use prior to the operation, for coronary angiography or regular hemodialysis. Using clinical assessment, 10 patients were detected with thrombus formation by the administration of heparin in our cardiac surgery. However, no thrombi were detected on sonography or CT in the perioperative period, including SRA-positive or GTI-positive patients.
Eight patients were preoperatively SR20-positive, and all of them had a history of heparin use before cardiac surgery. All patients underwent cardiac surgery safely with CPB under the use of UFH, without thrombosis in the tubes or CPB device. After surgery, 12 patients were SR20-positive (12/59, 20.3%), whereas 37 patients were GTI-positive (37/59, 62.7%). These results are presented in Table 2; group A(20) represents SR20-positive patients, whereas group B(20) represents the patients whose serotonin releasing rate was lower than 20% (SR20-negative). Nearly half of the patients, namely, 2 patients in group A(20) and 27 patients in group B(20), showed discrepancy of the results between GTI and SR(20).
Table 2.
Patient Results When the SR Cut-Off Value Was Considered as 20%.a
| Group A(20) (n = 12) | Group B(20) (n = 47) | P Value | |
|---|---|---|---|
| Preoperative SR ≥20% | 2 (2/12, 16.7%) | 6 (6/47, 12.8%) | ns |
| GTI-positive, preoperation | 1 (1/12, 8.3%) | 1 (1/47, 2.1%) | ns |
| GTI-positive, postoperation | 10 (10/12, 83.3%) | 27 (27/47, 57.4%) | ns |
| Sex (M/F) | 10/2 | 32/5 | ns |
| Age (years) | 66.8 (9.3) | 68.7 (8.5) | ns |
| History of heparin use | 2 (2/12, 16.7%) | 4 (4/47, 8.5%) | ns |
| Platelet count (×104/μL) | |||
| Preoperation | 18.8 (5.8) | 19.4 (4.6) | ns |
| POD 7 | 17.6 (6.9) | 18.1 (6.8) | ns |
| POD 14 | 24.8 (11.5) | 31.7 (8.0) | ns |
| Lowest number | 7.0 (2.4) | 8.8 (3.7) | ns |
| Lowest POD | 2.2 (1.2) | 2.3 (0.9) | ns |
| Thrombocytopenia (POD 7) | 2 (2/12, 16.7%) | 7 (7/47, 8.5%) | ns |
| d-dimer (μg/mL) | |||
| Preoperation | 2.5 (4.7) | 2.2 (2.8) | ns |
| POD 7 | 10.7 (10.4) | 7.5 (5.5) | ns |
| POD 14 | 16.6 (11.2) | 8.4 (5.4) | 0.001 |
| Highest value | 16.7 (11.3) | 9.5 (5.9) | 0.003 |
| Thrombotic events after the operation | 0 | 0 | |
Abbreviations: GTI, Polyanion IgG/IgA/IgM complex antibodies; POD, postoperative day; ns, not significant; SR, serotonin release.
aThrombocytopenia: patients with platelet counts of less than 10 × 104 /μL or less than 70% of the preoperative count on postoperative day 7. Numbers are expressed as the mean (SD). Group A(20) comprises patients with a postoperative SR of ≥20%, whereas group B(20) comprises patients with a postoperative SR of <20% upon low-dose heparin-induced activation.
Recently, a higher cutoff of 50% instead of 20% SRA was selected to avoid false-positive results as suggested by a previous report4 (Table 3). Preoperatively, there were no patients showing SR ≥50%. Eight patients (7 men and 1 woman; mean age: 64.6 years) were SR50-positive after the surgery (group AA(50)). In group AA(50), there were 7 GTI-positive patients (7/8, 87.5%). On the other hand, in group BB(50), 51 patients (35 men and 16 women; mean age: 68.8 years) showed that serotonin releasing rate was below 50% (represented as SR(50)-negative). There were 30 GTI-positive patients in this group (30/51, 58.8%). Similar to the results of SR(20), half of the patients showed discrepancy of the results between GTI and SR(50).
Table 3.
Patient Results When the Cutoff Value Was Considered to Be 50%.a
| Group AA(50) (n = 8) | Group BB(50) (n = 51) | P Value | |
|---|---|---|---|
| Preoperative SR ≥50% | 0 | 0 | |
| GTI-positive, preoperation | 1 (1/8, 12.5%) | 1 (1/51, 2.0%) | ns |
| GTI-positive, postoperation | 7 (7/8, 87.5%) | 30 (30/51, 58.8%) | ns |
| Sex (M/F) | 7/1 | 35/16 | ns |
| Age (years) | 64.6 ± 8.4 | 68.8 ± 8.5 | ns |
| History of heparin use | 1 (1/8, 12.5%) | 5 (5/51, 9.8%) | ns |
| Platelet (×104/μL) | |||
| Preoperation | 18.4 ± 6.8 | 19.4 ± 4.5 | ns |
| POD 7 | 18.2 ± 5.6 | 17.9 ± 7.1 | ns |
| POD 14 | 30.3 ± 3.4 | 30.7 ± 9.2 | ns |
| Lowest number | 7.1 ± 1.9 | 8.6 ± 3.6 | ns |
| Lowest POD | 2.0 ± 0.9 | 2.3 ± 1.0 | ns |
| Thrombocytopenia (POD 7) | 2 (2/8, 25.0%) | 7 (7/51, 9.8%) | ns |
| d-dimer (μg/mL) | |||
| Preoperation | 1.1 ± 0.9 | 2.4 ± 3.4 | ns |
| POD 7 | 8.1 ± 5.7 | 8.1 ± 7.0 | ns |
| POD 14 | 15.9 ± 10.4 | 9.2 ± 6.7 | 0.018 |
| Highest value | 15.9 ± 10.4 | 10.2 ± 7.1 | 0.052 |
| Thrombotic events after the operation | 0 | 0 |
Abbreviations: GTI, polyanion IgG/IgA/IgM complex antibodies; POD, postoperative day; SR, serotonin release; ns, not significant; SR, serotonin release.
aGroup AA(50) comprises patients with a postoperative SR of ≥50%, whereas group BB(50) comprises patients with an SR of <50% upon low-dose heparin-induced activation.
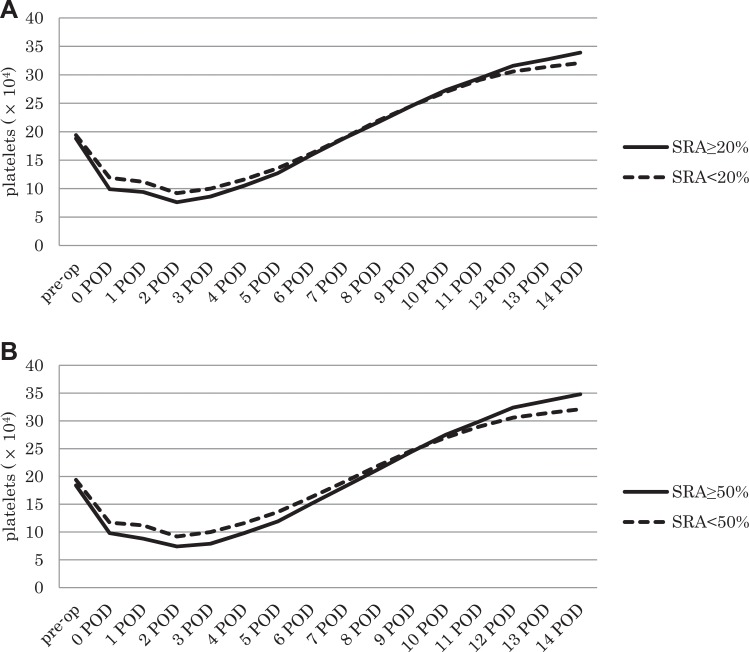
On postoperative day (POD) 7, thrombocytopenia was diagnosed using the platelet count; a platelet count below 10 × 104/μL or a greater than 30% decrease in platelet count compared with the preoperative measurement was considered to indicate thrombocytopenia.5 According to this criteria of platelet count (score = 0),4 2 patients of group AA(50) (2/8, 25.0%) and 7 patients of group BB(50) (7/51, 13.7%) were diagnosed with thrombocytopenia. In terms of the prevalence rate of thrombocytopenia, group A(20) and group B(20) showed similar trends to group AA(50) and group BB(50), respectively. The platelet count of the patients before surgery and on POD 7 and 14 did not significantly differ between the 2 groups, with postoperative counts presenting the lowest one. Therefore, the amount of SR was independent of the platelet count on each day.
Platelet counts showed similar patterns of changes in the SRA-positive and SRA-negative groups during the 2 weeks in each group of the cutoff level with 20% or 50% (Figure 1A and B). Although the platelet count decreased until 2 days after the operation and then started to increase, reaching preoperative numbers after approximately 1 week, it continued to increase until reaching values higher than preoperative numbers at 2 weeks. SRA-positive patients showed the tendency that platelet count was lower at POD 2 or 3 and higher at POD 14 than SRA-negative patients on each SR cutoff assay, although the difference was not significant.
Figure 1.
Time course of the changes in platelet count. A, Changes in the platelet count in both groups when 20% SR was used as the cutoff level. B, Changes in platelet count in both groups when 50% SR was used as the cutoff level. SR indicates serotonin release.
The d-dimer levels were significantly different between before surgery and on POD 14, as opposed to on POD 7 in both the SR20 groups and SR50 groups (Tables 2 and 3). In addition, there was a significant difference in the highest value of d-dimer between the SR20 groups (Table 2). Despite these results showing a significant difference, no thrombotic complications occurred in any groups during the study period.
Discussion
In this present study, we focused on the risk factors for HIT and the cutoff value of SRA to predict HIT and thrombotic event. Although HIT thrombus formation during or after the surgery sometimes could result in fatality, it is difficult to monitor the onset of HIT thromboses because the patients who undergo cardiac surgery present with thrombocytopenia frequently. In addition, the prediction of this complication remains difficult because serological diagnosis does not necessarily correlate with its clinical manifestation, that is, immunological detection of HIT antibody is more frequent than clinical manifestation,6 particularly in patients undergoing CPB surgery.7–9 Therefore, awareness of this complication from SRA and physical findings has been the most important factor for saving patients, by acting immediately after its occurrence.
We investigated 2 different methods to detect HIT serologically, namely, immunological examination using GTI assay and functional assessment with SRA. However, more than half of the patients (37/59, 62.7%) had positive results of GTI assay postoperatively in this study group. Considering that there was no patient with thrombosis in this study group, immunological assay seems to be too sensitive as in the previous reports, particularly in the patients undergoing CPB surgery.10 Thus, we consider that the functional activity of HIT antibody is more important than its quantity, as suggested by Trossaërt et al11 and Bakchoul et al.12 It was also reported that the functional assay was recommended if the seropositive patients need to undergo cardiac surgery.13 In terms of this evidence, SR was selected as a predictable marker of thrombosis in our surgery.
Classically, an SR of 20% has been selected as the cutoff value.14 However, as shown in Table 2, preoperative SR20-positive patients underwent successful cardiac surgery with CPB safely under an administration of UFH. This finding suggested that a 20% cutoff level is too sensitive. Warkentin et al recently proposed using 50% as a cutoff level.4,15 As shown in Table 3, when 50% was used as the cutoff level, there were no preoperative SR50-positive patients. Although we agree with this cutoff level (SR50) in comparison with 20% and it seems to be acceptable, contrary to our expectation, our patients could not develop postoperative thrombotic complications clinically.
Heparin-induced thrombocytopenia is generally defined as a clinicopathological syndrome with a significant fall in platelet count; however, patients who undergo cardiac surgery regularly show thrombocytopenia immediately after the surgery, which is irrelevant to HIT.16 Consumption and destruction of blood cells during operations accompanied with CPB is considered to be a major factor. Usually, the platelet count recovers approximately in 1 week after surgery and increases over the preoperative count by 2 weeks after surgery. As shown in Figure 1, platelet counts indicated a similar tendency of common findings in spite of the difference in patients with or without activated platelets antibody. Meanwhile, the 4Ts score has been proposed to detect HIT syndrome,5,17 which mainly focuses on the number of platelets. The lowest platelet count score, namely a score of 0, is defined as a >30% decrease in the preoperative platelet count or a platelet count of less than 10 × 104/μL4 This may be a useful clue for the possible occurrence of HIT in patients, except for those who undergo cardiac surgery. However, as mentioned above, patients undergoing surgery with the assistance of CPB regularly show a similar tendency in platelet count, and thus, platelet counts have limited value in such patients to predict HIT, especially in early postoperative days.16 By contrast, Warkentin focused on timing of platelet decrease, emphasizing the importance of the second decline in the platelet count, which began 5 to 10 days after heparin exposure.18 From this point of view, the changes in patterns of platelet count appear to be useful indications of development of HIT thrombosis. Lillo-Le Louët et al suggested specific criteria to diagnose HIT after CPB-assisted surgery.9 They focused on the changes in daily platelet numbers after surgery concomitantly with CPB duration time. Selleng et al also suggested that a platelet count drop of >50% between 5 and 10 postoperative days would be one of the criteria of HIT in place of early postoperative thrombocytopenia.16 These approaches seem to be promising in comparison with simple counting of the platelet numbers at the single, designated postoperative day. However, patients in both studies received an additional heparin treatment for 6 successive hours after the surgery or until the start of oral anticoagulant. After CPB surgery, heparin is used continuously only in specific and selected cases in Japan. Therefore, we hypothesized that both the prevalence of HIT and its thrombotic event would be different in our operating conditions from those in other countries, in addition to the sequential numbers of platelets postoperatively.
In this study, thrombus was also detected mainly by sonography of veins in the lower extremities when clinical examination suggested its occurrence, because the most possible sites of venous thrombus after surgery are around this area. Furthermore, since thrombus formation in other areas could not be excluded, we also examined d-dimer levels in order to diagnose thrombosis serologically. We found no significant difference between group AA(50) and group BB(50), except for d-dimer levels at POD 14, as shown in Table 3. However, thrombotic complications were not observed clinically in this study group in 14 days after surgery. Recently, HIT was discussed in association with disseminated intravascular coagulation (DIC).18 The elevated d-dimer in the patients in SRA-positive group may suggest minute and subclinical thrombosis, which was not detected by sonography nor manifested clinically. Although d-dimer was elevated in the SRA-positive group, undetected thrombus does not support the administration of additional anticoagulant. If we decided additional administration of heparin because of high levels of d-dimer at that situation, our patients who are SRA-positive might have been at risk of developing HIT. Elevation of d-dimer level in the SRA-positive group without clinical thrombotic event should be emphasized, because the relation between HIT and DIC could be a future study, expecting to give another insight of such relation focusing on the platelets.
Regarding possible causes of the discrepancies in SR values, d-dimer levels, and thrombus formation, we considered that a single use of heparin as shown in this study does not necessarily lead to clinical manifestations of thrombus. In patients of this study group, heparin was used only during the time of CPB. We neutralized heparin immediately after the cessation of CPB, and no additional heparin was administered for at least 14 days after the operation. Heparin-induced thrombocytopenia antibody and its reaction occurred by an immunological reaction. Repeated antigen stimulation would cause a more definite reaction than single use.9 Therefore, avoiding additional heparin use after cardiac surgery may also be an important factor for prevention of HIT thrombosis.7 Conversely, HIT thrombosis is known to occur in patients, although heparin use is limited during surgery. In the future, further study is needed to understand the risk factors for developing thromboses with a single use of heparin.
In conclusion, although the patients were positive in platelet-activating HIT antibody assay, they remained free from thrombosis and their platelet count recovered after CPB surgery without specific treatment. Therefore, it is important not only to perform the SRA but also to monitor platelet counts after cardiac surgery, for the purpose of facilitating the prediction of postoperative thrombotic complications by HIT. Although the functional assays of platelets activated by HIT antibody have been developing, monitoring platelet count is still considered to be the indispensable factor for HIT thrombosis after cardiac surgery, because such assays represented by SRA is too sensitive in the clinical condition at the present time. Especially, the timing and the pattern of decline of the platelet number should be carefully monitored to predict HIT occurrence.
Acknowledgments
Masahiko Matsumoto, MD, a former professor in the Department of Cardiovascular Surgery, Yamanashi Medical University, passed away in February 2014. He assisted in this study through his valuable discussions and support. We would like to express our sincere appreciation for his assistance and encouragement in performing this study and would like to dedicate this article to him. We would like to thank Keiko Wanaka, PhD (Kobe Research Projects on Thrombosis and Haemostasis, Kobe, Japan) and Jeanine M. Walenga, PhD (Cardiovascular Institute, Loyola University Medical Center, Maywood, IL, USA) for their kind assistance with the GTI and SRA measurement.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Matsuo T, Motohashi S, Wanaka K, Walenga JM. Production of anti-platelet factor 4/heparin complex antibodies after cardiovascular surgery. Clin Appl Thromb Hemost. 2015;21(2):177–180. [DOI] [PubMed] [Google Scholar]
- 2. Sheridan D, Caryer C, Kelton J. A diagnostic test for heparin-induced thrombocytopenia. Blood. 1986;67(1):27–30. [PubMed] [Google Scholar]
- 3. Asada R, Wanaka K, Walenga J, et al. Murine monoclonal antibody to platelet factor 4/heparin complexes as a potential reference standard for platelet activation assays in heparin-induced thrombocytopenia. Clin Appl Thromb Hemost. 2013;19(1):37–41. [DOI] [PubMed] [Google Scholar]
- 4. Warkentin TE, Greinacher A, Gruel Y, et al. Laboratory testing for heparin-induced thrombocytopenia: a conceptional framework and implications for diagnosis. J Thromb Haemost. 2011;9(12):2498–2500. [DOI] [PubMed] [Google Scholar]
- 5. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e495S–e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoon JH, Jang IK. Heparin-induced thrombocytopenia in cardiovascular patients. Cardiol Rev. 2011;19(3):143–153. [DOI] [PubMed] [Google Scholar]
- 7. Bauer TL, Arepally G, Konkle BA, et al. Prevalence of heparin-induced antibodies without thrombosis in patients undergoing cardiopulmonary bypass surgery. Circulation. 1997;95(5):1242–1246. [DOI] [PubMed] [Google Scholar]
- 8. Warkentin TE. New approaches to the diagnosis of heparin-induced thrombocytopenia. Chest. 2005;127(2 suppl):35S–45S. [DOI] [PubMed] [Google Scholar]
- 9. Lillo-Le Louët A, Boutouyrie P, Alhenc-Gelas M, et al. Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J Thromb Haemost. 2004;2(11):1882–1888. [DOI] [PubMed] [Google Scholar]
- 10. Ganzel C, Rowe J, Raveh D. Platelet factor 4/heparin-particle gel immunoassay (PaGIA) is a weak method for heparin-induced thrombocytopenia (HIT) evaluation of post cardio-pulmonary bypass surgery patients. J Thromb Thrombolysis. 2014;38(3):314–320. [DOI] [PubMed] [Google Scholar]
- 11. Trossaërt M, Gaillard A, Commin PL, Amiral J, Vissac AM, Fressinaud E. High incidence of anti-heparin/platelet factor 4 antibodies after cardiopulmonary bypass surgery. Br J Haematol. 1998;101(4):652–655. [DOI] [PubMed] [Google Scholar]
- 12. Bakchoul T, Zöllner H, Greinacher A. Current insights into the laboratory diagnosis of HIT. Int J Lab Hem. 2014;36(3):296–305. [DOI] [PubMed] [Google Scholar]
- 13. Lee GM, Arepally GM. Heparin-induced thrombocytopenia. Hematology. 2013;2013:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pouplard C, Gueret P, Fouassier M, et al. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific of heparin/P: F4 for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2007;5(7):1373–1379. [DOI] [PubMed] [Google Scholar]
- 15. Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative -interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304–1312. [DOI] [PubMed] [Google Scholar]
- 16. Selleng S, Malowsky B, Strobel U, et al. Early-inset and persisting thrombocytopenia in post-cardiac surgery patients is rarely due to heparin-induced thrombocytopeinia, even when antibody tests are positive. J Thromb Haemost. 2010;8(1):30–36. [DOI] [PubMed] [Google Scholar]
- 17. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159(5):528–540. [DOI] [PubMed] [Google Scholar]
- 18. Warkentin TE. Heparin-induced thrombocytopenia. Curr Opin Crit Care. 2015;21(6):576–585. [DOI] [PubMed] [Google Scholar]