Abstract
Lubricin, also known as proteoglycan 4, acts as an antiadhesive and boundary lubricant to prevent cartilage damage in healthy joints. Following injury, a decrease in synovial fluid (SF) lubricin may lead to secondary osteoarthritis (OA). Inflammatory biomarkers, such as IL-1β and TNF-α, are also implicated in the pathophysiology of OA. Interestingly, they have been shown to suppress the expression and secretion of lubricin in SF. This study aims to compare circulating levels of inflammatory biomarkers and lubricin between total joint arthroplasty (TJA) patients and healthy individuals. Doing so may better elucidate their roles in OA and extend the understanding of inflammation as a regulator of lubricin. Deidentified plasma samples were obtained 1 day preoperatively and 1 day postoperatively from patients undergoing TJA. Utilizing biochip array technology, they were profiled for IL-2, IL-4, IL-6, IL-8, IL-10, VEGF, IFN-γ, IL-1α, IL-1β, MCP-1, EGF, and TNF-α. Circulating lubricin levels were also measured using enzyme-linked immunosorbent assay. Compared to healthy controls, IL-6, IL-8, VEGF, IL-1β, MCP-1, EGF, and TNF-α were significantly increased pre- and postoperatively. Lubricin was significantly decreased. This may indicate that elevations in inflammatory cytokines initiate a cascade of events, leading to decreased lubricin, which places the joint at increased risk of developing OA.
Keywords: arthroplasty, lubricin, inflammation, osteoarthritis
Introduction
Osteoarthritis (OA) is a slow progressive degenerative joint disease of multifactorial etiology associated with cartilage destruction, subchondral bone remodeling, and inflammation of the synovium.1 Due to its predilection for lower extremity joints, such as the knee and hip, OA is the leading cause of lower extremity disability in older adults, and its incidence is rising due to an aging population and increasing obesity.2 While lifestyle modifications, nutritional supplements, oral drug therapies, intra-articular injections, and several surgical interventions have demonstrated favorable outcomes on symptomatology, there are currently no approved OA treatments capable of slowing disease progression or delaying the need for total joint arthroplasty (TJA).3,4 By 2030, the demand for total hip arthroplasty (THA) is estimated to rise 174% to 572 000, while the demand for total knee arthroplasty (TKA) is projected to rise 673% to 3.48 million.5
It has been hypothesized that OA begins, in part, following an increase in coefficient of friction (COF) due to loss of boundary lubricant in synovial fluid (SF).6 According to this hypothesis, the absence of lubrication causes elevated shear stress at the articular cartilage surface, which in turn disturbs chondrocyte metabolic function and survival. Eventually, the combination of increased friction, proteoglycan loss within the cartilage matrix, and chondrocyte apoptosis results in the full-thickness cartilage damage characteristic of end-stage OA.7–9
Lubricin, a mucinous glycoprotein encoded by the proteoglycan 4 (PRG4) gene, acts as an antiadhesive and boundary lubricant to prevent cartilage damage in healthy joints. Produced by synoviocytes and superficial zone chondrocytes, it is present in SF and on the surface of articular cartilage.10 Patients with camptodactyly-arthropathy-coxavara-pericarditis (CACP) syndrome, an autosomal recessive condition due to loss-of-function mutations in lubricin, develop early cartilage failure. Recapitulation of CACP syndrome in PRG4 gene knockout mice has demonstrated precocious cartilage changes, including surface cartilage fibrillation and the disappearance of underlying superficial zone chondrocytes.11,12
Following injury, a decrease in SF lubricin concentration may place the joint at an increased risk of wear-induced damage, leading to secondary OA.13 Common traumatic knee injuries, such as anterior cruciate ligament (ACL) tears and meniscal injuries, are strong risk factors for the development of OA.14 Several studies have shown that native and recombinant lubricin, when injected intra-articularly, has a disease-modifying effect in rodent models of post-traumatic OA.15–18 Given the ability of lubricin to reestablish boundary lubrication, provide chondroprotection, and restore low COF, intra-articular injection of lubricin may be a potential treatment for OA.10
Inflammatory cytokines, such as IL-1β and TNF-α, have also been implicated in the pathophysiology of OA. Several studies have identified elevated IL-1β levels in the SF, synovium, cartilage, and the subchondral bone layer of patients with OA.19 At the cellular level, IL-1β activates matrix degrading enzymes, induces chondrocyte apoptosis, and downregulates expression of matrix components like type II collagen and aggrecan.20 In addition, IL-1β stimulates the production of reactive oxygen species that directly damage the articular cartilage.19 TNF-α has also been observed at increased concentrations in the SF, synovium, cartilage, and the subchondral bone layer of patients with OA.19 Its action is similar to and synergistic with IL-1β, activating matrix metalloproteinases (MMPs), inducing chondrocyte apoptosis, and blocking chondrocyte synthesis of type II collagen and proteoglycans.19,21 Interestingly, IL-1β and TNF-α have both been shown to suppress the expression and secretion of lubricin. Treatment of bovine articular cartilage explants with IL-1β and TNF-α decreased the levels of both cartilage surface-associated and soluble lubricin.16,22 To date, a study comparing plasma levels of inflammatory biomarkers and lubricin between normal individuals and TJA patients pre- and postoperatively has yet to be reported.
Materials and Methods
Methods
In accordance with the institutional review board of Loyola University Chicago Health Sciences Division, deidentified samples of plasma (n = 105, age 36-86 years, with a mean age of 66 years ± 10.67) were obtained from patients undergoing THA and TKA at Loyola University Medical Center and the University of Utah Orthopedics Clinic. Plasma was isolated following blood sample collection into tubes containing 3.2% sodium citrate during routine preoperative consultations and 1 day postoperatively. They were placed into multiple aliquots to reduce the number of freeze–thaw cycles and stored at −80°C. Patients undergoing TJA revision were excluded from this study.
Utilizing Cytokine Biochip Immunoassays from Randox Laboratories Ltd (Crumlin, UK), samples were profiled for 12 cytokines and growth factors (IL-2, IL-4, IL-6, IL-8, IL-10, VEGF, IFN-γ, IL-1α, IL-1β, MCP-1, EGF, and TNF-α). Circulating lubricin levels were measured using a PRG4 enzyme-linked immunosorbent assay kit obtained from MyBioSource.com.
Controls
Samples of normal, citrated plasma were obtained from healthy individuals from the University of Utah Orthopedics Clinic (n = 35, age 50-78 years, with a mean age of 64 years ± 9.2, nonsmokers) and George King Biomedical Inc (n = 48, age 18-35 years, with a mean age of 32.5 years ± 9.7, nonsmokers). They were placed into multiple aliquots to reduce the number of freeze–thaw cycles and stored at −80°C. The University of Utah samples served as age-matched controls, while the combination of University of Utah and George King Biomedical samples served as non-age-matched, aggregate controls. A pool of normal human plasma (NHP) composed of non-age-matched pooled plasma served as a control as well.
Statistical Analysis
Statistical analysis was performed using Microsoft Excel and GraphPad Prism Software version 7. Inflammatory biomarker analysis compared TJA patient plasma values with age-matched normal plasma values from the University of Utah samples. Inflammatory biomarker analysis also compared TJA patient plasma values with non-age-matched, aggregate normal plasma values from the University of Utah and the George King Biomedical samples. Lubricin analysis compared TJA patient plasma values with pooled NHP values. Results were expressed as means ± standard error of the mean as well as percentage changes ± standard error of the mean from normal mean. Analysis of variance tests were used to identify significant differences between the control and experimental groups. A P value less than .05 was considered significant. Nonparametric Spearmen correlation tests were also used to determine correlations between 2 variables.
Results
Percentage Changes of Inflammatory Biomarkers in TJA Patients From Normal Means
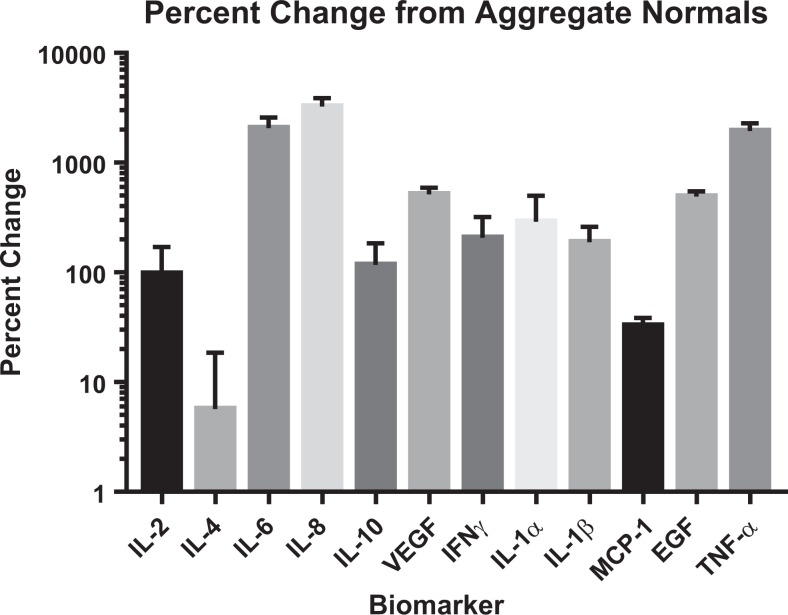
Preoperative levels of IL-2, IL-4, IL-6, IL-8, IL-10, VEGF, IFN-γ, IL-1α, IL-1β, MCP-1, EGF, and TNF-α all demonstrated positive percentage changes from their respective aggregate normal means. IL-8, IL-6, and TNF-α showed the largest changes (3241% ± 6.36, 2066% ± 5.09, and 1939% ± 3.39), followed by VEGF, EGF, IL-1α, and IFN-γ (513% ± 0.76, 491% ± 0.56, 290% ± 2.11, 207% ± 1.12). IL-1β, IL-10, IL-2, and MCP1 followed with smaller changes from their respective normal means (189% ± 0.71, 117% ± 0.67, 97% ± 0.73, and 33% ± 0.06). The smallest change was demonstrated by IL-4 (6% ± 0.13; Figure 1).
Figure 1.
Percent changes in TJA patients pre-operatively from (A) aggregate normal means and (B) age-matched normal means.
When compared to their respective age-matched normal means, all preoperative levels of inflammatory biomarkers again demonstrated positive percentage changes, with the exception of IL-4. IL-8, IL-6, and TNF-α showed the largest changes (2852% ± 5.62, 2321% ± 5.69, and 1997% ± 3.49), followed by VEGF, EGF, IL-1α, and IL-1β (323% ± 0.53, 225% ± 0.31, 192% ± 1.58, and 166% ± 0.65). IFN-γ, IL-10, IL-2, and MCP-1 followed with smaller changes from their respective normal means (147% ± 0.91, 122% ± 0.68, 58% ± 0.58, and 7% ± 0.05). The only negative change was demonstrated by IL-4 (−19% ± 0.10).
Comparison of Inflammatory Biomarkers in TJA Patients Versus Aggregate Normal Controls
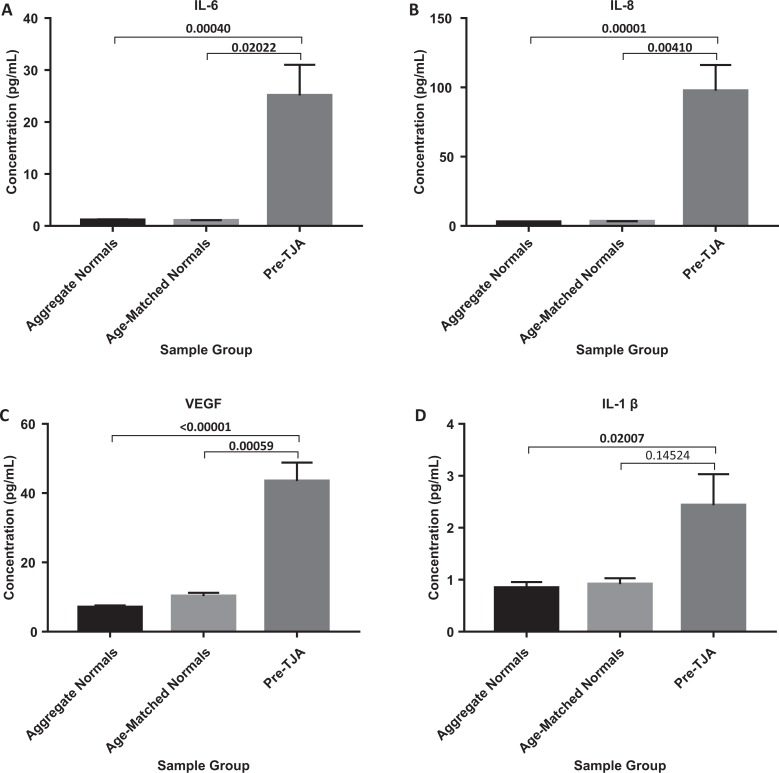
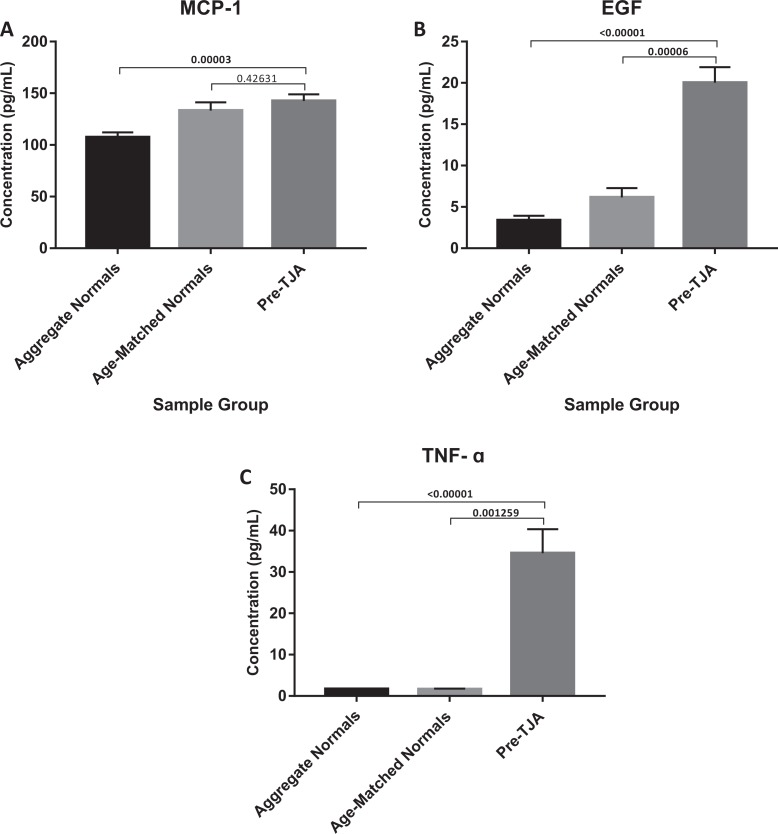
Preoperative levels of IL-6, IL-8, VEGF, IL-1β, MCP-1, EGF, and TNF-α all demonstrated significant increases when compared to aggregate normal controls (P = .00040, P = .00001, P < .00001, P = .02007, P = .00003, P < .0001, and P < .0001; Figures 2 and 3). Although not significant, preoperative levels of IL-2, IL-4, IL-10, IFN-γ, and IL-1α all appeared to trend toward an increase (Table 1).
Figure 2.
Comparison of IL-6, IL-8, VEGF, and IL-1ß in TJA patients pre-operatively vs aggregate normals and age-matched normals.
Figure 3.
Comparison of MCP-1, EGF, and TNFα in TJA patients pre-operatively vs aggregate normals and age-matched normals.
Table 1.
Aggregate Normal, Age-Matched Normal, Preoperative, and Postoperative Means ± SEM for All Measured Inflammatory Biomarkers.a
| IL-2 (pg/mL) | Aggregate normal controls | 1.36 ± 0.17 | IFN-γ (pg/mL) | Aggregate normal controls | 0.21 ± 0.03 |
| Age-matched normal controls | 1.70 ± 0.14 | Age-matched normal controls | 0.26 ± 0.02 | ||
| Preoperative | 2.69 ± 3.18 (P = .23933, P = .56900) | Preoperative | 0.64 ± 1.55 (P = .10567, P = .35198) | ||
| Postoperative | 3.32 ± 4.30 (P = .33732, P = .69712) | Postoperative | 0.73 ± 2.03 (P = .25300, P = .50237) | ||
| IL-4 (pg/mL) | Aggregate normal controls | 1.64 ± 0.12 | IL-1α (pg/mL) | Aggregate normal controls | 0.14 ± 0.01 |
| Age-matched normal controls | 2.14 0.10 | Age-matched normal controls | 0.18 ± 0.01 | ||
| Preoperative | 1.73 ± 1.47 (P = .72204, P = .28455) | Preoperative | 0.53 ± 1.71 (P = .22264, P = .48392) | ||
| Postoperative | 2.09 ± 2.02 (P = .33021, P = .94392) | Postoperative | 0.72 ± 1.97 (P = .17438, P = .41636) | ||
| IL-6 (pg/mL) | Aggregate normal controls | 1.16 ± 0.10 | IL-1β (pg/mL) | Aggregate normal controls | 0.84 ± 0.11 |
| Age-matched normal controls | 1.04 ± 0.06 | Age-matched normal controls | 0.92 ± 0.07 | ||
| Preoperative | 25.11 ± 7.78 (P = .00040, P = .02022) | Preoperative | 2.44 ± 2.47 (P = .02007, P = .14524) | ||
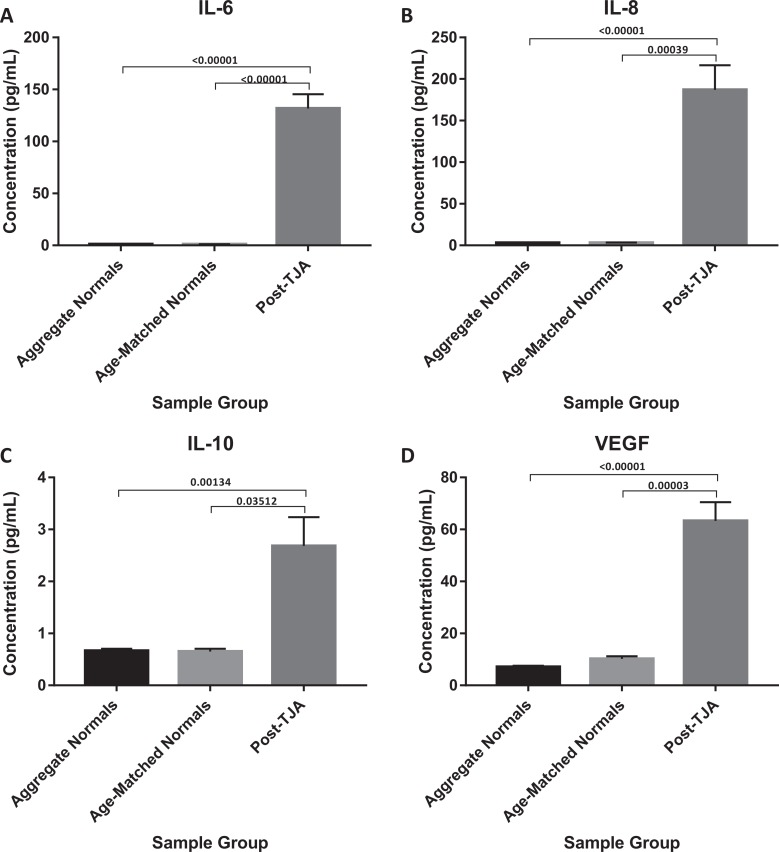
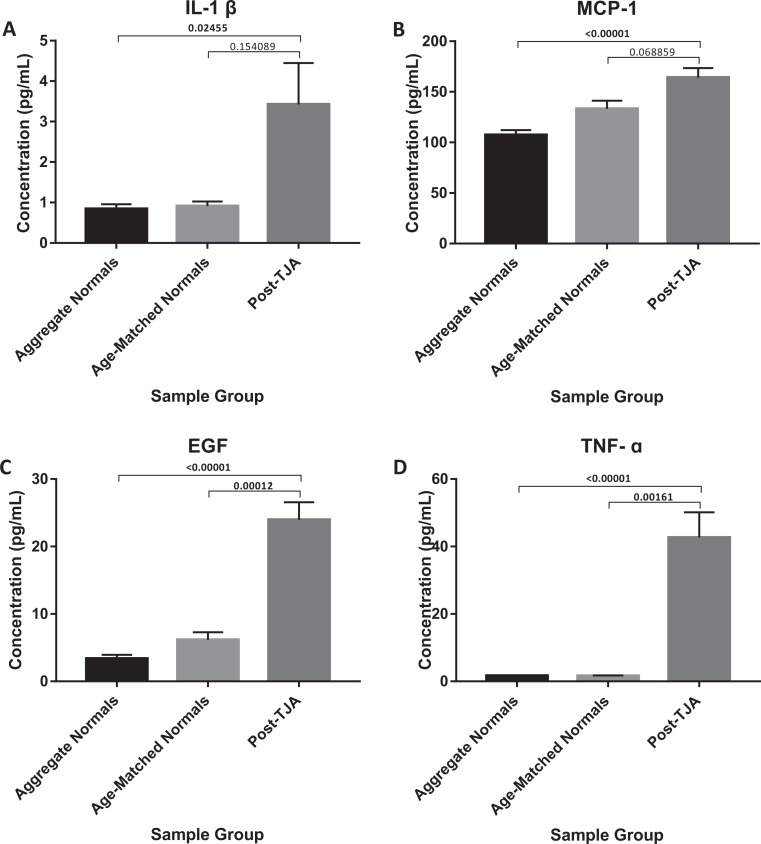
| Postoperative | 131.82 ± 11.73 (P < .00001, P < .00001) | Postoperative | 3.43 ± 3.21 (P = .02455, P = .15409) | ||
| IL-8 (pg/mL) | Aggregate normal controls | 2.92 ± 0.10 | MCP-1 (pg/mL) | Aggregate normal controls | 107.43 ± 4.37 |
| Age-matched normal controls | 3.31 ± 0.11 | Age-matched normal controls | 133.24 ± 4.80 | ||
| Preoperative | 97.60 ± 13.80 (P = .00001, P = .00410) | Preoperative | 142.61 ± 7.99 (P = .00003, P = .42631) | ||
| Postoperative | 187.07 ± 17.26 (P < .00001, P = .00039) | Postoperative | 164.04 ± 9.76 (P < .00001, P = .06886) | ||
| IL-10 (pg/mL) | Aggregate normal controls | 0.67 ± 0.03 | EGF (pg/mL) | Aggregate normal controls | 3.39 ± 0.50 |
| Age-matched normal controls | 0.65 ± 0.03 | Age-matched normal controls | 6.16 ± 0.67 | ||
| Preoperative | 1.45 ± 2.14 (P = .30792, P = .12343) | Preoperative | 20.02 ± 4.39 (P < .00001, P = .00006) | ||
| Postoperative | 2.68 ± 2.37 (P = .00134, P = .03512) | Postoperative | 23.97 ± 5.12 (P < .00001, P = .00012) | ||
| VEGF (pg/mL) | Aggregate normal controls | 7.09 ± 0.47 | TNF-α (pg/mL) | Aggregate normal controls | 1.70 ± 0.09 |
| Age-matched normal controls | 10.26 ± 0.57 | Age-matched normal controls | 1.65 ± 0.07 | ||
| Preoperative | 43.46 ± 7.44 (P < .00001, P = .00059) | Preoperative | 34.58 ± 7.68 (P < .00001, P = .00126) | ||
| Postoperative | 63.33 ± 8.53 (P < .00001, P = .00003) | Postoperative | 42.69 ± 8.67 (P < 0.00001, P = .00161) |
Abbreviation: SEM, standard error of the mean.
a P values obtained by analysis of variance (ANOVA) are displayed as (P, P′), where P refers to the comparison of total joint arthroplasty (TJA) patients versus aggregate normal controls and P′ refers to the comparison of TJA patients versus age-matched normal controls.
Note: Statistically significant P values are bolded.
Postoperative levels of IL-6, IL-8, IL-10, VEGF, IL-1β, MCP-1, EGF, and TNF-α all demonstrated significant increases when compared to aggregate normal controls (P < .0001, P < .00001, P = .00134, P < .00001, P = .02455, P < .00001, P < .00001, and P < .00001; Figures 4 and 5). Although not significant, postoperative levels of IL-2, IL-4, IFN-γ, and IL-1α all appeared to trend toward an increase (Table 1). When compared to their respective preoperative plasma concentrations, only postoperative concentrations of IL-6, IL-8, and VEGF demonstrated a significant difference (P < .00001, P = .010548, and P = 0.027726).
Figure 4.
Comparison of IL-6, IL-8, IL-10, and VEGF in TJA patients post-operatively vs aggregate normals and age-matched normal.
Figure 5.
Comparison of IL-1ß, MCP-1, EGF, and TNF-α in TJA patients post-operatively vs aggregate normals and age-matched normals.
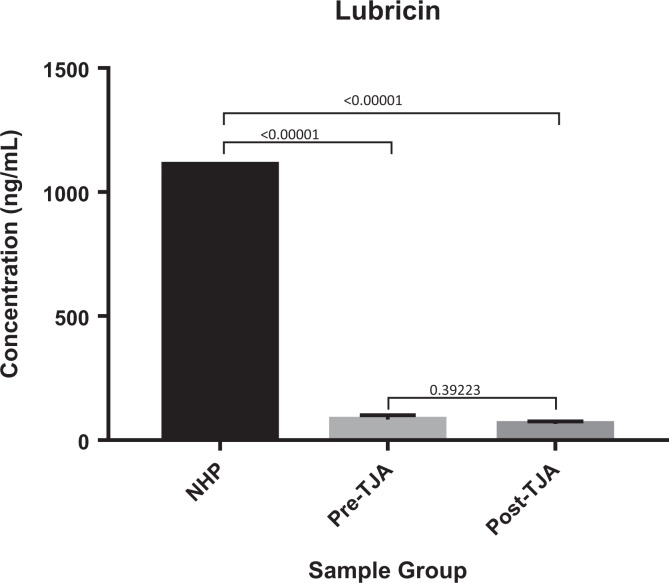
Comparison of Lubricin in TJA Patients Versus Pooled NHP
Pre- and postoperative levels of lubricin showed significant decreases when compared to normal controls (P < .00001, P < .00001). Postoperative levels of lubricin were not statistically changed when compared to preoperative levels (P = .39223; Figure 6).
Figure 6.
Comparison of Lubricin in TJA patients pre- and post-operatively vs normal human pooled plasma.
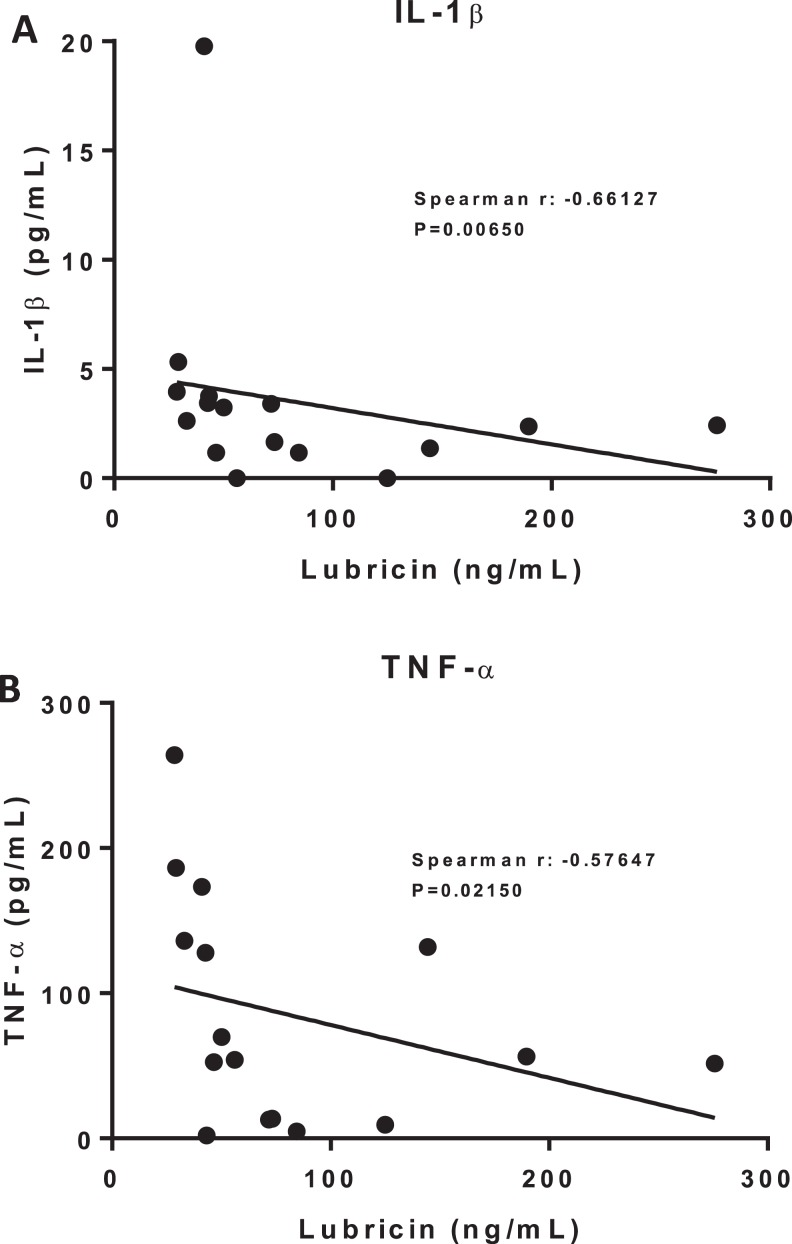
Correlations Between Inflammatory Biomarkers and Lubricin
IL-1β levels showed a significant negative correlation with lubricin levels (Spearman r = −0.66127, P = .00650; Figure 7A). TNF-α levels also showed a significant negative correlation with lubricin levels (Spearman r = −0.57647, P = .02150; Figure 7B).
Figure 7.
Correlations with Lubricin. (A) IL-1ß vs. Lubricin. (B) TNF-α vs. Lubricin.
Discussion
The development of disease-modifying treatments of OA has not progressed for decades. Unlike rheumatoid arthritis, for which synthetic and biological disease-modifying antirheumatic drugs have emerged to reverse or reduce the progression of joint damage, OA remains ever elusive with its current gamut of therapeutics limited to symptomatic relief.3,4,23,24 Identifying pharmacologic targets, local or systemic, that can slow OA or delay the need for TJA would be a major medical advance.
The objective of this study was to investigate circulating levels of inflammatory biomarkers and lubricin in TJA patients. Doing so further elucidated their individual roles in the pathogenesis of OA and extended the understanding of inflammation as a regulator of lubricin. In addition, further support is provided for lubricin and anticytokine therapies as potential disease-modifying treatments of OA.
In measuring plasma concentrations of inflammatory biomarkers, IL-6, IL-8, VEGF, IL-1β, MCP-1, EGF, and TNF-α were found to be significantly elevated preoperatively compared to normal controls. This observation is consistent with the current literature, which implicates each of these circulating biomarkers in various pathophysiologic processes observed in OA. IL-6 promotes bone resorption and shows synergy with IL-1β and TNF-α,25,26 while IL-8 has been shown to induce cartilage degeneration.27 VEGF, more commonly known as a potent angiogenic factor, can also act as a destructive factor to cartilage in OA. In vitro, it was shown to be induced in chondrocytes by high-intensity impact stress and to act as an autocrine inducer of MMPs.28 IL-1β, as mentioned before, activates matrix degrading enzymes, downregulates matrix components, induces chondrocyte apoptosis, and causes oxidative damage to articular cartilage.19,20 MCP-1, a monocyte/macrophage chemokine, is thought to expedite cartilage damage by enhancing chondrocyte apoptosis while inhibiting their proliferation. Additionally, stimulating its expression in wild-type chondrocytes was shown to increase MMPs.29 High concentrations of EGF have been found in the SF and synovium of patients having joint disease.30,31 And finally, TNF-α, as previously discussed, induces the synthesis of MMPs and inhibits the synthesis of proteoglycans and type II collagen within the synovium.19,21
Plasma concentrations of IL-2, IL-4, IL-10, IFN-γ, and IL-1α were not significantly different between preoperative TJA patients and normal controls. Nonetheless, these inflammatory biomarkers all appeared to trend toward an increase. This suggests that they too may demonstrate significant elevations if a similar study were conducted with a larger TJA patient population and greater statistical power. Elevations would be consistent with previous studies showing increased SF levels of IL-2, IL-4, IL-10, IFN-γ, and IL-1α in patients with OA.32–35 In the case of IL-4 and IL-10, it is also possible that the anti-inflammatory response was insufficient or lacking in these patients, especially given the fact that they had OA extensive enough to require TJA. Recent work has demonstrated the strong chondroprotective effects of these cytokines. IL-4 was found to inhibit proteoglycan degradation and MMP secretion,36,37 while IL-10 was shown to reduce MMP production, inhibit chondrocyte apoptosis, and increase proteoglycan synthesis.19,38,39 Thus, in their paucity or absence, it is plausible that patients progress more rapidly toward end-stage OA and ultimately TJA. It may be of future interest to examine the plasma of patients with early-stage OA to determine if more of an equilibrium is maintained between anti- and pro-inflammatory cytokines.
Altogether, these preoperative inflammatory biomarker findings provide further evidence in support of the inflammatory theory of OA. Traditionally classified as a noninflammatory arthritis or disease of “wear and tear,” it is becoming more apparent that inflammatory mediators also play a role in the initiation and perpetuation of the OA process.40
Postoperatively, plasma concentrations of IL-6, IL-8, IL-10, VEGF, IL-1β, MCP-1, EGF, and TNF-α were all found to be significantly elevated when compared to normal controls. IL-2, IL-4, IFN-γ, and IL-1α, on the other hand, were not found to be significantly different. This exposes a major limitation of the current study; it lacks the ability to distinguish between changes due to postsurgical trauma and changes due to other sources of inflammation, like OA. However, in comparing pre- and postoperative levels of inflammatory biomarkers, it may be possible to isolate surgically induced changes. In this study, only IL-6, IL-8, and VEGF demonstrated significant increases between pre- and postoperative concentrations. This may indicate that postoperative elevations of IL-6, IL-8, and VEGF from normal samples were surgically induced, while the other postoperative increases from normal samples were already present in the circulation due to OA. Such a conclusion is supported by research demonstrating that both IL-6 and IL-8 peak in the first 6 to 12 hours after TJA, then decrease to their respective baselines by 48 to 72 hours postoperatively.41,42 Plasma levels of VEGF have also been shown to increase after major surgery,43 though additional investigation is needed to observe this following TJA specifically. Postoperative increases in IL-10, IL-1β, MCP-1, EGF, and TNF-α are again consistent with various pathophysiologic processes observed in OA, as cited above.
In regard to lubricin, both pre- and postoperative circulating levels were found to be significantly reduced when compared to normal controls. This decrease was unexpected, as previous work done in this laboratory has shown significant elevations in pre- and postoperative lubricin levels when compared to normal controls.44 In both studies, lubricin antigen levels were measured using a commercially available assay. It is of future interest to validate this laboratory’s findings, using additional methods of plasma lubricin measurement. This observed decrease in plasma lubricin does draw comparisons to declines in SF lubricin following ACL injury in animal models.13 This may indicate that acute or chronic insults to the joint are accompanied by local and systemic reductions in lubricin that may initiate or perpetuate OA. Moving forward, the study of plasma lubricin levels in relation to SF lubricin levels in patients undergoing TJA could be a valuable area of investigation.
Interestingly, the current study also found that decreases in plasma lubricin correlated with increases in plasma IL-1β and TNF-α. This parallels previous investigation of lubricin regulation in which exposure of synoviocytes, chondrocytes, and cartilage explants to IL-1β and TNF-α resulted in marked reductions in secreted and cartilage-bound lubricin.22 Together, these findings indicate that inflammatory cytokines may initiate a cascade of events that leads to the decreased production of lubricin, placing the joint at an increased risk of the cartilage wear characteristic of OA.
Conclusion
Herein, it is demonstrated that IL-6, IL-8, VEGF, IL-1β, MCP-1, EGF, and TNF-α are found at elevated circulating levels in TJA patients. Combined with existing literature implicating each in the pathogenesis of OA, the data presented here suggest a molecular basis of disease progression, with elevated levels of inflammatory biomarkers potentially indicative of greater degrees of OA. Furthermore, this study confirms that plasma levels of IL-6 and IL-8 are increased following TJA. A postsurgical increase in plasma VEGF was also identified. As far as lubricin, decreased concentrations in pre- and postoperative samples indicate that its decline may also play a role in the pathogenesis of OA. Coupled with correlational analysis revealing a link between lubricin and inflammatory biomarkers IL-1β and TNF-α, the data presented here extend the understanding of inflammation as a downregulator of lubricin in the setting of OA. This current study underscores the multifactorial nature of OA and further supports the potential of lubricin and anticytokine therapies as effective disease-modifying treatments of OA.
Acknowledgments
The authors gratefully acknowledge the staffs of the Department of Pathology at the Loyola University Medical Center and the University of Utah Orthopedics Clinic for the expert collection of blood samples. The authors would also like to thank members of the Hemostasis and Thrombosis Research Laboratory for their continued guidance and collaboration and Dr Ghanayam, Chair of the Department of Orthopedics, and Dr Wojick, Chair of the Department of Pathology, for their support. A special thanks to Dr Gail Hecht and Dr Brubaker for their leadership and continual support of the STAR program.
Authors’ Note: The primary author is a recipient of the Callahan Student Fellowship award from the Department of Orthopedics.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2010;7(1):33–42. doi:10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 2. Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol. 2014;28(1):5–15. doi:10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 3. Auw Yang KG, Saris DBF, Dhert WJA, Verbout AJ. Osteoarthritis of the knee: current treatment options and future directions. Current Orthopaedics. 2004;18(4):311–320. doi:10.1016/j.cuor.2004.04.005. [Google Scholar]
- 4. Losina E, Daigle ME, Suter LG, et al. Disease-modifying drugs for knee osteoarthritis: can they be cost-effective? Osteoarthritis Cartilage. 2013;21(5):655–667. doi:10.1016/j.joca.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone and Joint Surg Am. 2007;89(4):780 doi:10.2106/jbjs.f.00222. [DOI] [PubMed] [Google Scholar]
- 6. Jay GD. Lubricin and surfacing of articular joints. Current Opinion in Orthopaedics. 2004;15(5):355–359. doi:10.1097/01.bco.0000136127.00043.a8. [Google Scholar]
- 7. Lorenz H, Richter W. Osteoarthritis: cellular and molecular changes in degenerating cartilage. Prog Histochem Cytochem. 2006;40(3):135–163. doi:10.1016/j.proghi.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 8. Goggs R, Carter SD, Schulze-Tanzil G, Shakibaei M, Mobasheri A. Apoptosis and the loss of chondrocyte survival signals contribute to articular cartilage degradation in osteoarthritis. Vet J. 2003;166(2):140–158. doi:10.1016/s1090-0233(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 9. Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2000;43(9):1916–1926. doi:10.1002/1529-0131(200009)43:9<1916:: aid-anr2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 10. Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A. 2013;110(15):5852–5857. doi:10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–631. doi:10.1172/jci200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marcelino J, Carpten JD, Suwairi WM, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23(3):319–322. [DOI] [PubMed] [Google Scholar]
- 13. Elsaid KA, Fleming BC, Oksendahl HL, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–1715. doi:10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomas AC, Hubbard-Turner T, Wikstrom EA, Palmieri-Smith RM. Epidemiology of posttraumatic osteoarthritis. J Athl Train. 2017;52(6):491–496. doi:10.4085/1062-6050-51.5.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elsaid KA, Zhang L, Waller K, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis Cartilage. 2012;20(8):940–948. doi:10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 16. Flannery CR, Hughes CE, Schumacher BL, et al. Articular cartilage superficial zone protein (szp) is homologous to megakaryocyte stimulating factor precursor and is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophy Res Commun. 1999;254(3):535–541. doi:10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 17. Jay GD, Fleming BC, Watkins BA, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2010;62(8):2382–2391. doi:10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jay GD, Elsaid KA, Kelly KA, et al. Prevention of cartilage degeneration and gait asymmetry by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 2012;64(4):1162–1171. doi:10.1002/art.33461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:1–19. doi:10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shakibaei M, Schulze-Tanzil G, John T, Mobasheri A. Curcumin protects human chondrocytes from IL-1beta-induced inhibition of collagen type ii and î21-integrin expression and activation of caspase-3: an immunomorphological study. Ann Anat. 2005;187(5-6):487–497. doi:10.1016/j.aanat.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 21. Lee SW, Song YS, Lee SY, et al. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6(4):e19163 doi:10.1371/journal.pone.0019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones AR, Flannery CR. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 2007;13:40–45. doi:10.22203/ecm.v013a04. [DOI] [PubMed] [Google Scholar]
- 23. Karsdal MA, Michaelis M, Ladel C, et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24(1):2013–2021. doi:10.1016/j.joca.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 24. Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69(6):964–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kwan Tat S, Padrines M, Théoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-Alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15(1):49–60. doi:10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26. Chenoufi HL, Diamant M, Rieneck K, Lund B, Stein GS, Lian JB. Increased MRNA expression and protein secretion of interleukin-6 in primary human osteoblasts differentiated in vitro from rheumatoid and osteoarthritic bone. J Cell Biochem. 2001;81(4):666–678. doi:10.1002/jcb.1104. [DOI] [PubMed] [Google Scholar]
- 27. Matsukawa A, Yoshimura T, Maeda T, Ohkawara S, Takagi K, Yoshinaga M. Neutrophil accumulation and activation by homologous IL-8 in rabbits. IL-8 induces destruction of cartilage and production of IL-1 and IL-1 receptor antagonist in vivo. J Immunol. 1995;154(10):5418–5425. [PubMed] [Google Scholar]
- 28. Pufe T, Lemke A, Kurz B, et al. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol. 2004;164(1):185–192. doi:10.1016/s0002-9440(10)63109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu YK, Ke Y, Wang B, Lin JH. The role of MCP-1-CCR2 ligand-receptor axis in chondrocyte degradation and disease progress in knee osteoarthritis. Biol Res. 2015;48:64 doi:10.1186/s40659-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kusada J, Otsuka T, Matsui N, Hirano T, Asai K, Kato T. Immuno-reactive human epidermal growth factor (h-EGF) in rheumatoid synovial fluids. Nihon Seikeigeka Gakkai Zasshi. 1993;67(9):859–865. [PubMed] [Google Scholar]
- 31. Farahat MN, Yanni G, Poston R, Panayi GS. Cytokine expression in synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 1993;52(12):870–875. doi:10.1136/ard.52.12.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas Vangsness C, Narvy S, Burke W, Fedenko A, MacPhee R . Human knee cytokine synovial fluid analysis correlated with grade of knee arthritis (SS-47). Arthroscopy. 2007;23(6). doi:10.1016/j.arthro.2007.03.059. [Google Scholar]
- 33. Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D694–D703. [DOI] [PubMed] [Google Scholar]
- 34. Tsuchida Al, Beekhuizen M, ‘t Hart MC, et al. Cytokine profiles in the joint depend on pathology, but are different between synovial fluid, cartilage tissue and cultured chondrocytes. Arthritis Res Ther. 2014;16(5):441 doi:10.1186/s13075-014-0441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res. 2013;31(7):1039–1045. doi:10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Meegeren MER, Roosendaal G, Jansen NWD. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthritis Cartilage. 2012;20(7):764–772. doi:10.1016/j.joca.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 37. Doi H, Nishida K, Yorimitsu M, et al. Interleukin-4 downregulates the cyclic tensile stress-induced matrix metalloproteinases-13 and cathepsin b expression by rat normal chondrocytes. Acta Medica Okayama. 2008;62(2):119–126. [DOI] [PubMed] [Google Scholar]
- 38. Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 Inhibits metalloproteinase and stimulates timp-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96(5):2304–2310. doi:10.1172/jci118286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. John T, Müller RD, Oberholzer A, et al. Interleukin-10 modulates pro-apoptotic effects of TNF-α in human articular chondrocytes in vitro. Cytokine. 2007;40(3):226–234. doi:10.1016/j.cyto.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 40. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. doi:10.1177/1759720x12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah K, Mohammed A, Patil S, McFadyen A, Meek RM. Circulating cytokines after hip and knee arthroplasty: a preliminary study. Clin Orthop Relat Res. 2008;467(4):946–951. doi:10.1007/s11999-008-0562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kwak S, Chun Y, Rhyu K, Cha J, Cho Y. Quantitative analysis of tissue injury after minimally invasive total hip arthroplasty. Clin Orthop Surg. 2014;6(3):279–284. doi:10.4055/cios.2014.6.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belizon A, Balik E, Feingold DL, et al. Major abdominal surgery increases plasma levels of vascular endothelial growth factor. Ann Surg. 2006;244(5):792–798. doi:10.1097/01.sla.0000225272.52313.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wanderling C, Liles J, Davis E, et al. Levels of matrix-degrading enzymes and lubricin in patients with degenerative joint disease requiring arthroplasty. Clin Appl Thromb Hemost. 2017;24(1):41–46. doi:10.1177/1076029617724231. [DOI] [PMC free article] [PubMed] [Google Scholar]