Abstract
Ankaferd Blood Stopper (ABS) comprises a mixture of plants and stops bleeding via forming a protein network by erythroid aggregation. Bleeding causes reduction of iron levels in body. It has been indicated that ABS contains significant amount of iron. Thus, we investigated the biological activity of ABS-derived iron on iron-regulated genes during iron-deficiency anemia (IDA). IDA We selected Caco-2 and HepG2 cell lines as in vitro models of human intestine and liver, respectively. Iron deficiency anemia was induced by deferoxamine. The cells were treated with ferric ammonium citrate (FAC) and ABS. Messenger RNA levels of iron-regulated genes were analyzed by quantitative reverse transcription polymerase chain reaction to elucidate whether iron in ABS behaved similar to inorganic iron (FAC) during IDA. The results showed that ABS-derived iron influenced transcriptions of iron-regulated marker genes, including divalent metal transporter (Dmt1), transferrin receptor (TfR), ankyrin repeat domain 37 (Ankrd37), and hepcidin (Hamp) in IDA-induced Caco-2 and HepG2 cells. Our results suggest that when ABS is used to stop tissue bleeding, it might have an ability to reduce levels of IDA.
Keywords: Ankaferd Blood Stopper, iron, iron-deficiency anemia, Caco-2, HepG2, gene regulation
Introduction
Ankaferd Blood Stopper (ABS) is commonly used to stop bleeding of tissues.1,2 It has been shown that ABS reduces the growth rate of pathogens indicating its protective effect against microorganisms.3 Furthermore, ABS enhances bone cell growth and regeneration in vivo.4,5 It also affects the levels of transcription factors in human vessel endothelial cell model.6 These studies indicate that ABS has effect on the physiology of cells by influencing molecular and genetic mechanisms. It has been recently reported that ABS contains iron.7 Iron is one of the important transition metals in human and involves in variety of physiological functions including oxygen carrying, cell division, inflammation, and energy production.8 Consuming low levels of dietary iron or bleeding lead to iron-deficiency anemia (IDA) in human, whereas high iron in human body causes iron toxicity in tissues (hemochromatosis).8,9 Dietary iron absorption mainly occurs via enterocyte cells on duodenum and upper jejunum of small intestine. Intestinal iron absorption is essential to maintain iron balance in human body because human does not have active excretion mechanism for iron.10 Iron can be stored in liver or used by bone marrow to make more red blood cells. Mature red blood cells can be broken down by macrophages of spleen and iron is released into blood circulation.8 Iron metabolism is tightly controlled by the molecular and genetic mechanisms. Low iron (iron deficiency) and hypoxia are 2 main physiological signals for intestinal iron absorption and iron metabolism.11 Iron deficiency leads to induction of iron transporter genes and this is an important adaptation of cell to absorb more dietary iron into the body.12,13 When iron demand is low, induction of iron-regulated genes can reach normal physiological levels. Systemic iron level is controlled by hepcidin protein (HAMP) that is secreted from the liver. The HAMP leads to ferroportin 1 protein degradation during elevated iron level in human body.14
The recent investigation has shown that ABS has significant amount of iron.7 This observation let us hypothesize that iron in ABS solution might have ability to reduce IDA. We tested this idea in iron-deficiency-induced Caco-2 and HepG2 cell lines by analyzing expression of iron-regulating genes. We showed that iron in ABS solution was able to reduce IDA in vitro.
Material and Methods
Cell Culture
Caco-2 cells were maintained in Eagle’s minimum essential medium supplemented with 20% fetal bovine serum (FBS), 1% nonessential amino acids, 1% sodium pyruvate, and 1% penicillin and streptomycin. Same medium mix was used for HepG2 cells with 10% FBS. Cells were maintained and grown in 5% CO2 incubator at 37°C. Passage numbers of the cells were kept between 20 and 35. These cells have been commonly used as an in vitro model in human intestine and liver iron physiology.15,16 Experimental Caco-2 and HepG2 cells were plated in 12 wells and grown for 21 and 5 days postconfluent, respectively. Cell culture medium was changed every 2 days until deferoxamine mesylate (DFO), ABS, and ferric ammonium citrate (FAC) treatments were performed.
Iron Measurement From ABS
The ABS was provided by Dr Haznedaroglu (Department of Hematology, Faculty of Medicine, Hacettepe University, Ankara, Turkey). About 10 mL ABS was centrifuged at 100g for 5 minutes. The ABS samples were utilized for acid digestion, and atomic absorption spectroscopy was used to measure iron levels in ABS solution.17
Induction of Iron Deficiency and Treatments of FAC and ABS
Deferoxamine mesylate 100 µM was used for 24 hours to induce IDA in both cell types. Cells were treated by 100 µg/mL FAC, and FAC was used as control group for experimental conditions to compare ABS-derived iron on genetic regulation of cells. Both cell types were exposed to same amount of iron-containing FAC and ABS solutions with same volume.
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction
RNA isolation was performed by RNAzol reagent (MRC Inc., OH, USA) according to the company’s instruction. Total RNA sample 1 µg was used to make complementary DNA synthesis (Life Technologies, California), and quantitative reverse transcription polymerase chain reaction (RT-qPCR; Life Technologies) was performed by following company protocols. Gene-specific primers were used for iron- and hypoxia-regulated genes (Table 1). Cyclophilin A1 (CypA1) was chosen as a housekeeping gene to normalize the data. The RT-qPCR data were converted to gene expression levels via 2ΔCt calculation methods.16
Table 1.
Primer List
| Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) |
|---|---|---|
| CypA1 | TACGGGTCCTGGC ATCTTG | CGAGTTGTCCACA GTCAGCA |
| Dmt1 | TGCATCTTGCTGA AGTATGTCACC | CTCCACCATCAGC CACAGGAT |
| TfR | TCAGAGCGTCGG GATGATATCGG | CTTGATCCATCATC ATTCTGAACTGCC |
| Ankrd37 | AGCAGTCGCCTGT CCACTTAGC | AGCAGGCTTAGGC ACTCCAGG |
| Hamp | AGTGGGACAGCCA GACAGACG | CAGCTCTGCAAGTT GTCCCGT |
Statistical Analyses
All results were expressed as mean (standard deviation). One-way analysis of variance with post hoc Tukey test was performed for relative gene expression to compare experimental groups. GraphPad Prism (version 6.0 for Windows; GraphPad. CA,USA) was used to create all the figures and for statistical analysis.
Results
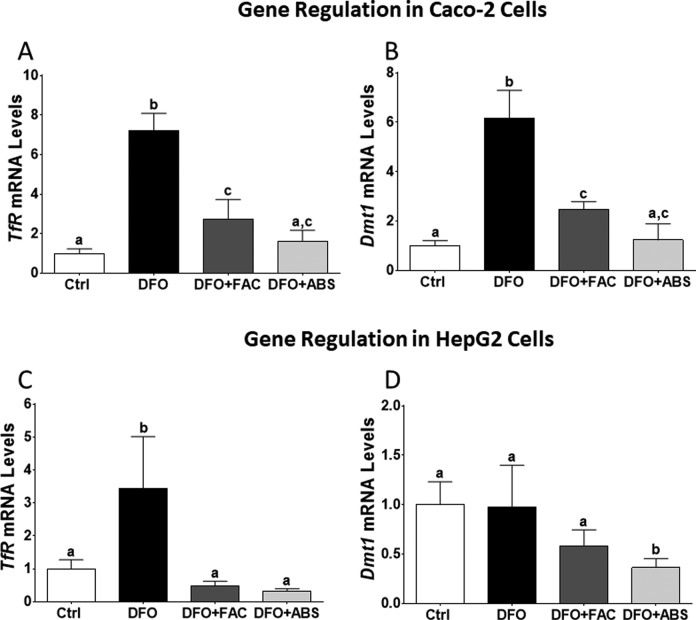
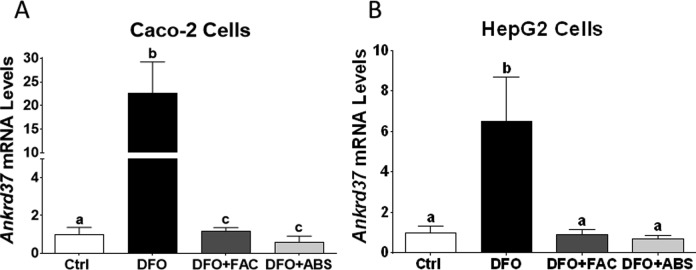
Iron level was measured 7.7 µg/mL in ABS solution. We did not observe any dead cells due to ABS, FAC, and DFO treatments in our experimental models. The DFO treatment significantly induced TfR and Dmt1 messenger RNA (mRNA) expression levels in Caco-2 cells (Figure 1A and B). The DFO increased TfR mRNA level in HepG2 cells; however, Dmt1 mRNA level was not affected by DFO treatment (Figure 1C and D). Both cells were treated with ABS in order to understand whether the effect of iron in ABS solution decreased mRNA expression levels of these genes. We found that ABS significantly reduced TfR and Dmt1 mRNA induction in Caco-2 cells (Figure 1A and B). Moreover, ABS also reduced TfR mRNA induction and the basal level of Dmt1 mRNA level in HepG2 cells (Figure 1C and D). Ferric ammonium citrate treatment showed very similar results that we observed from ABS treatment in terms of TfR and Dmt1 mRNA regulation in both cell lines under DFO-induced IDA (Figure 1). However, ABS significantly reduced basal level of Dmt1 mRNA relative to FAC treatment in HepG2 cells (Figure 1D). Next, possible role of ABS on iron-deficiency-induced hypoxia was studied by measuring mRNA level of hypoxia-regulating Ankrd37 gene.16,18 The DFO treatment significantly increased Ankrd37 mRNA expression in Caco-2 and HepG2 cells. Both FAC and ABS decreased induction of Ankrd37 transcription (Figure 2A and B). Iron is an important nutrient for Hamp gene regulation in liver, and HAMP protein is a key systemic regulator for iron metabolism in human.14 Thus, we tested whether FAC and ABS had effect on Hamp gene regulation with or without DFO-induced IDA in HepG2 cell. Results indicated that FAC and ABS treatments significantly reduced Hamp gene mRNA expression, and this reduction in Hamp mRNA was reversed by DFO (Figure 3). Moreover, DFO treatment did not affect Hamp mRNA regulation in HepG2 cells.
Figure 1.
Messenger RNA levels of iron-regulated genes in Caco-2 and HepG2 cells. Total RNA was isolated from experimental groups following quantitative reverse transcription polymerase chain reaction (RT-qPCR) which was performed to analyze iron-regulated gene expression (Caco-2 cells: A and B; HepG2 cells: C and D). Different letters show statistical significance between groups within each panel. Letters depict mean (standard deviation); n = 4 independent experiments, P ≤ .05. TfR indicates transferrin receptor; Dmt1, divalent mineral transporter 1.
Figure 2.
Effect of ABS on hypoxia-regulated Ankrd37 gene in Caco-2 and HepG2 cells. Ankrd37 is an iron-regulated hypoxic gene and mRNA levels of Ankrd37 were analyzed by RT-qPCR (Caco-2 cells: A; HepG2 cells: B). Different letters show statistical significance between groups within each panel. Letters depict mean (standard deviation); n = 4 independent experiments, P ≤ .05. Ankrd37 indicates ankyrin repeat domain 37; ABS, Ankaferd Blood Stopper; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
Figure 3.

Regulation of Hamp gene by ABS with or without iron-deficiency anemia. Hepcidin is small peptide that controls intestinal iron absorption and is produced by the liver. Hamp mRNA levels were determined by RT-qPCR. Different letters show statistical significance between groups within each panel. Letters depict mean (standard deviation); n = 4 independent experiments, P ≤ .05. Hamp indicates hepcidin; ABS, Ankaferd Blood Stopper; RT-qPCR, quantitative reverse transcription polymerase chain reaction; HAMP, hepcidin protein.
Discussion
In vitro and in vivo ABS-related studies including bleeding,19 different types of cancer,20 wound healing,21 and inflammation are of great interest.22 The ABS has ability to form impermeable contact between red blood cells via modulation of ankyrin, spectrin, and actin proteins with fibrinogen gamma.23 It has been purposed that iron can bind to fibrinogen and this interaction might play a role in the function of ABS on tissue bleeding.24 Recently, Akar et al indicated that ABS had significant amount of iron and very low concentration of copper and zinc.7 Thus, we investigated whether ABS-derived iron can ameliorate DFO-induced iron deficiency in vitro. We used human Caco-2 and HepG2 cell lines as models of this study. After Caco-2 cells are grown at 21 days postconfluent, they show physiological similarities with human enterocyte cells.16 Enterocyte cells of intestine are main contributor to arrange iron balance in human. Moreover, liver is also important for iron homeostasis in terms of iron storage.10 TfR and Dmt1 are used as marker genes to evaluate whether ABS-derived iron reduced effect of IDA. TfR and Dmt1 are very responsive to iron deficiency in vitro and in vivo.13,16 Results indicated that DFO significantly increased mRNA levels of TfR and Dmt1 genes, and this induction was reversed by FAC and ABS. However, DFO did not have an effect on Dmt1 mRNA levels in HepG2 cells. This result was consistent with the literature.25 This might be related to liver iron metabolism during anemia, because transferrin-transferrin receptor (TFR)-dependent endocytosis is one of the main molecular mechanisms for iron absorption during anemia in liver.26 Induction of TfR genes in this study might explain TFR-dependent regulation of iron uptake in HepG2 cells.
It has been recently shown that hypoxia is a key molecular signal for iron metabolism.11 Iron deficiency causes cellular hypoxia via increasing protein stability of hypoxia inducible factor 1α (HIF1α) and HIF2α transcription factors due to inactivation of HIF prolyl-hydroxylases (PHDs), but available iron can reduce stability of HIF1α and HIF2α by activation of PHD enzymes.27 Ankrd37 is one of the most sensitive genes that is regulated by hypoxia.16,18 Thus, Ankrd37 was used as a genetic marker for hypoxia in the current study. Data showed that DFO induced transcription of Ankrd37 gene, whereas FAC and ABS decreased induction of this gene. These results suggest that ABS-derived iron has ability to reduce iron-deficiency-induced hypoxia in both cell lines. Next, the effect of ABS-derived iron was investigated on transcriptional regulation of Hamp gene in HepG2 cells. It has been indicated that increased levels of iron in blood leads to induction of Hamp gene.28 Surprisingly, both FAC and ABS reduced Hamp mRNA expression without anemia. It has been indicated that Hamp mRNA expression was suppressed by iron loading in HepG2 cells,29,30 and this is consistent with the current data. It was suggested that systemic factor(s) was required for in vitro regulation of Hamp gene.30,31 The ABS-related studies have been dramatically increased in different scientific areas. Possible effects of iron in ABS should consider non-iron-related studies because iron has a significant effect on gene regulation in vitro and in vivo.13,16,32 Thus, ABS-derived iron might be a contributor to molecular and genetic studies.
Conclusion
Our results showed that FAC and ABS had very similar effects on gene regulation of anemic Caco-2 and HepG2 cells. This suggests that ABS-derived iron is biologically active to reduce IDA. It has been indicated that plant-based molecules can reduce bioavailability and biofunctionality of iron.9 Our results showed that plant-based molecules in ABS did not interfere with the function of iron in cells. ABS-derived iron might help to decrease anemia by providing iron when ABS stops tissue bleeding. This is the first in vitro study to investigate the effect of ABS on anemia. However, animal and human studies are necessary to clarify the effect of ABS-derived iron on IDA.
Footnotes
Authors’ Note: A.G. and S.G. designed the investigation and performed the experiments, and wrote the manuscript. I.C.H. provided ankaferd solution for this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Beyazit Y, Kurt M, Sayilir A, Suvak B, Ozderin YO. Successful application of Ankaferd Blood Stopper in a patient with lower gastrointestinal bleeding. Saudi J Gastroenterol. 2011;17(6):424–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akpinar MB, Atalay A, Atalay H, Dogan OF. Ankaferd Blood Stopper decreases postoperative bleeding and number of transfusions in patients treated with clopidogrel: a double-blind, placebo-controlled, randomized clinical trial. Heart Surg Forum. 2015;18(3):E118–E123. [DOI] [PubMed] [Google Scholar]
- 3. Cinar C, Odabas ME, Akca G, Isik B. Antibacterial effect of a new haemostatic agent on oral microorganisms. J Clin Exp Dent. 2012;4(3):e151–e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pamuk F, Cetinkaya BO, Keles GC, et al. Ankaferd Blood Stopper enhances healing after osseous grafting in patients with intrabony periodontal defects. J Periodontal Res. 2016;51(4):540–547. [DOI] [PubMed] [Google Scholar]
- 5. Servet E, Bekler H, Kilincoglu V, Ozler T, Ozkut A. Effect of bleeding on nerve regeneration and epineural scar formation in rat sciatic nerves: an experimental study. Acta Orthop Traumatol Turc. 2016;50(2):234–241. [DOI] [PubMed] [Google Scholar]
- 6. Yilmaz E, Gulec S, Torun D, Haznedaroglu IC, Akar N. The effects of Ankaferd(R) blood stopper on transcription factors in HUVEC and the erythrocyte protein profile. Turk J Haematol. 2011;28(4):276–285. [DOI] [PubMed] [Google Scholar]
- 7. Akar N, Ardcoglu Y, Oktem Z, Erduran N, Haznedaroglu IC. High iron content of Ankaferd hemostat as a clue for its hemostatic action of red blood cell origin. Blood Coagul Fibrinolysis. 2015;26(2):233–234. [DOI] [PubMed] [Google Scholar]
- 8. Knutson M, Wessling-Resnick M. Iron metabolism in the reticuloendothelial system. Crit Rev Biochem Mol Biol. 2003;38(1):61–88. [DOI] [PubMed] [Google Scholar]
- 9. Hurrell RF. Preventing iron deficiency through food fortification. Nutr Rev. 1997;55(6):210–222. [DOI] [PubMed] [Google Scholar]
- 10. Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307(4):G397–G409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9(2):152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravia JJ, Stephen RM, Ghishan FK, Collins JF. Menkes copper ATPase (Atp7a) is a novel metal-responsive gene in rat duodenum, and immunoreactive protein is present on brush-border and basolateral membrane domains. J Biol Chem. 2005;280(43):36221–36227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins JF. Gene chip analyses reveal differential genetic responses to iron deficiency in rat duodenum and jejunum. Biol Res. 2006;39(1):25–37. [PMC free article] [PubMed] [Google Scholar]
- 14. Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823(9):1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao N, Gao J, Enns CA, Knutson MD. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J Biol Chem. 2010;285(42):32141–32150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu Z, Gulec S, Collins JF. Cross-species comparison of genomewide gene expression profiles reveals induction of hypoxia-inducible factor-responsive genes in iron-deprived intestinal epithelial cells. Am J Physiol Cell Physiol. 2010;299(5):C930–C938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gulec S, Collins JF. Investigation of iron metabolism in mice expressing a mutant Menke’s copper transporting ATPase (Atp7a) protein with diminished activity (Brindled; Mo (Br) (/y)). PLoS One. 2013;8(6):e66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benita Y, Kikuchi H, Smith AD, Zhang MQ, Chung DC, Xavier RJ. An integrative genomics approach identifies hypoxia inducible factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res. 2009;37(14):4587–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baykul T, Alanoglu EG, Kocer G. Use of Ankaferd Blood Stopper as a hemostatic agent: a clinical experience. J Contemp Dent Pract. 2010;11(1):E088–E094. [PubMed] [Google Scholar]
- 20. Mumcuoglu M, Akin DF, Ezer U, Akar N. Ankaferd Blood Stopper induces apoptosis and regulates PAR1 and EPCR expression in human leukemia cells. EJMHG. 2015;16(1):19–27. [Google Scholar]
- 21. Yuce S, Candirli C, Yenidunya S, Muslu B. New hemostatic agent: the effect of Ankaferd Blood Stopper on healing wounds in experimental skin incision model. Turk J Med Sci. 2014;44(2):288–294. [DOI] [PubMed] [Google Scholar]
- 22. Karabiyik A, Yilmaz E, Gulec S, Haznedaroglu I, Akar N. The dual diverse dynamic reversible effects of Ankaferd Blood Stopper on EPCR and PAI-1 inside vascular endothelial cells with and without LPS challenge. Turk J Haematol. 2012;29(4):361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozel-Demiralp D, Igci N, Ayhan B, Egin Y, Haznedaroglu IC, Akar N. Prohemostatic and antithrombin activities of Ankaferd hemostat are linked to fibrinogen gamma chain and prothrombin by functional proteomic analyses. Clin Appl Thromb Hemost. 2012;18(6):604–610. [DOI] [PubMed] [Google Scholar]
- 24. Sacak B, Akdeniz ZD, Sirinoglu H, et al. Microvascular anastomosis using Ankaferd Blood Stopper: demonstration of long-term histopathologic effects on vascular tissue. Blood Coagul Fibrinolysis. 2014;25(7):721–725. [DOI] [PubMed] [Google Scholar]
- 25. Ba Q, Hao M, Huang H, et al. Iron deprivation suppresses hepatocellular carcinoma growth in experimental studies. Clin Cancer Res. 2011;17(24):7625–7633. [DOI] [PubMed] [Google Scholar]
- 26. Anderson ER, Shah YM. Iron homeostasis in the liver. Compr Physiol. 2013;3(1):315–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peyssonnaux C, Nizet V, Johnson RS. Role of the hypoxia inducible factors HIF in iron metabolism. Cell Cycle. 2008;7(1):28–32. [DOI] [PubMed] [Google Scholar]
- 28. Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. J Nutr. 2008;138(11):2284–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gehrke SG, Kulaksiz H, Herrmann T, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102(1):371–376. [DOI] [PubMed] [Google Scholar]
- 30. Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood. 2008;112(10):4292–4297. [DOI] [PubMed] [Google Scholar]
- 32. Coffey R, Nam H, Knutson MD. Microarray analysis of rat pancreas reveals altered expression of Alox15 and regenerating islet-derived genes in response to iron deficiency and overload. PLoS One. 2014;9(1):e86019. [DOI] [PMC free article] [PubMed] [Google Scholar]