Abstract
Hemostasis is a complex dynamic process involving bleeding and thrombosis as two end-points. Conventional coagulation tests which are measured in plasma examine only isolated portions of the coagulation cascade, thereby giving no information on important interactions essential to the clinical evaluation of hemostatic function. Thromboelastography (TEG), originally described in 1948 has improved over the decades and become a valuable tool of coagulation testing because of the limitations of standard coagulation tests. TEG is a technique that provides data about the entire coagulation system, from the beginning of clot formation to fibrinolysis, involving both cellular and plasma components of hemostasis. Rotational thromboelastometry (ROTEM) which evolved from TEG technology overcome several limitations of classical TEG while maintaining a good correlation with conventional TEG determination. ROTEM analyses are useful for rapid assessment of global clotting function in various clinical situations including liver transplantation, cardiac surgery, obstetrics, trauma, hemophilia and idiopathic thrombocytopenic purpura. ROTEM has been also reported to be useful in identifying various hypercoagulable conditions including major surgery, malignancy, Behcet’s disease and apheresis. Further developments in ROTEM based transfusion strategies may also reduce transfusion requirements and improve clinical outcomes by optimizing the administration of blood components. This is a literature review of ROTEM including its technique, interpretation and specially clinical applications in different scenarios of bleeding and thrombotic disorders.
Keywords: thromboelastometry, hemostasis, bleeding, thrombosis
Introduction
Hemostasis is a well-defined dynamic process that involves bleeding and thrombosis as 2 end points. Traditional laboratory tests utilized to assess hemostasis generally represent isolated portions of the coagulation cascade and do not provide information on important interactions which are essential for the clinical evaluation of hemostatic disorders.1 Because of the limitations of standard coagulation tests, better techniques of coagulation monitoring such as thromboelastography (TEG) have been reexamined.
Thromboelastography, first described by Hartert in 1948,2 is a method for the evaluation of the entire process of blood coagulation as a graph from the beginning of clot formation to fibrinolysis.3 Unlike standard laboratory tests performed on centrifuged plasma fractions; TEG, which is performed on whole blood, has the advantage of providing a better evaluation of the interaction between the cellular and plasma components of the hemostatic sytem.4 Thromboelastography measures both the quantity of clotting and most importantly the quality of clotting, which is not recorded by routine coagulation profile.
Rotational thromboelastometry (ROTEM), which was developed from the same technology, has avoided several limitations of classical TEG while maintaining a good correlation with conventional TEG determination. The most important benefits of ROTEM technology include the rapid availability of test results, less susceptibility to mechanical stress, movement and vibration, as well as providing enhanced reproducibility.5 The data are also continuous, digital, and retrievable for further calculations.6
This review article specifically focuses on the clinical applications of ROTEM in bleeding and thrombotic disorders, including the author’s own experience in different clinical scenarios and also briefly addresses the technique and interpretation of ROTEM.
Technique of ROTEM
Thromboelastography and ROTEM are viscoelastic assays which provide a graphical evaluation of the entire coagulation process in whole blood. Classical TEG is performed by filling a cuvette with native whole blood, and a pin is immersed in the sample by a torsion wire. The cup is rotated through 4° 45’ over 5 seconds with a 1-second rest period at each end. As long as the blood is liquid, this movement is unrestricted. As the blood clots and fibrin strands begin to form between the cup and the pin, the rotational movement of the pin is restricted which is detected optically and displayed as a TEG tracing.7
In ROTEM, which works slightly differently from classical TEG, the pin is rotated. The movement of the pin is detected by an optical detection system which is transmitted to and processed by a computer with specific software. Rotational thromboelastometry is an enhancement of TEG that includes 4 channels allowing more samples or diagnostics to be run simultaneously, while TEG has 2 channels. Thromboelastography requires manual pipetting of blood samples into the cups, whereas ROTEM is technically easy to use via an electronic pipette for interactive test operation and an integrated computer for automatic analysis. Unlike TEG that is very sensitive to vibration, ROTEM is less susceptible to mechanical stress, movement, and vibration. In general, ROTEM as a digitized point-of-care system, provides a number of advantages that render this technology much more practicable for routine clinical use at the bedside or in the operating room including standardized measuring technique, pathway subanalysis, and rapid, digitized, replicable signatures.5–7
Parameters of ROTEM
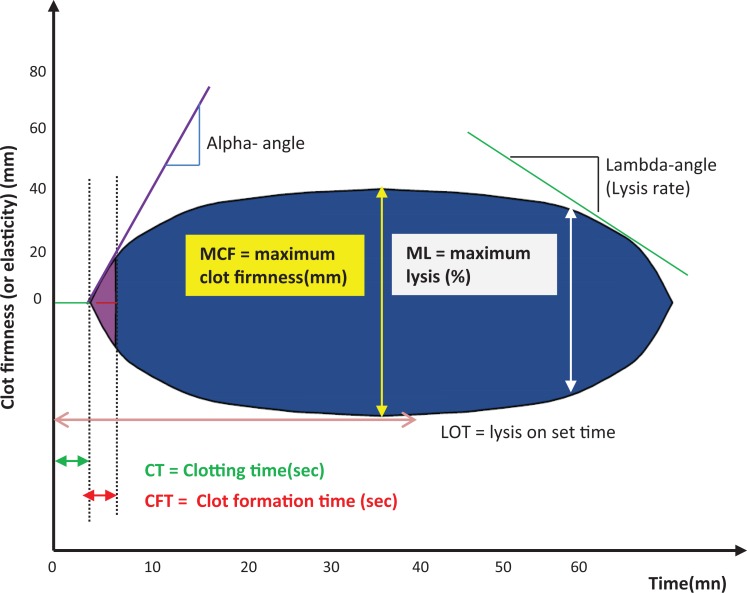
Important parameters of ROTEM tracing include clotting time (CT), clot formation time (CFT), and maximum clot formation (MCF) which measure clot formation and maximum lysis (ML), which measures clot lysis as shown in Figure 1.
Figure 1.
ROTEM tracing and parameters.
Clotting time is the time from start of the measurement until initiation of clotting. Clotting time is influenced by the activities of coagulation factors.
Clot formation time is the time from initiation of clotting until a clot firmness of 20 mm is detected. Clot formation time is influenced by the activities of coagulation factors, platelet count/function, thrombin formation, fibrin precipitation, fibrinogen, and hematocrit.
Maximum clot formation (MCF) represents the firmness of the clot. It is affected by fibrin and fibrinogen concentration, platelet count/function, thrombin concentration, factor XIII, and hematocrit.
Maximum lysis is the reduction of the clot firmness after MCF in relation to MCF. It shows stability of the clot (ML < 15%) or fibrinolysis (ML > 15% within 1 hour).8
Tests of ROTEM
Rotational thromboelastometry has 4 channels with different reagents to detect abnormalities in different components involved in coagulation. For the analysis, citrated blood is usually used. As in the laboratory coagulation analysis, various activators or inhibitors are added to the sample in order to represent the different processes of hemostasis.
INTEM: contact pathway activation of coagulation with phospholipid and ellagic acid and provides information similar to that of the activated partial thromboplastin time (APTT).
EXTEM: tissue factor pathway activation of coagulation with tissue factor and provides information similar to that of the prothrombin time (PT).
APTEM: contains aprotinin for inhibiting fibrinolysis.
FIBTEM: utilizes cytochalasin D, a platelet inhibitor that blocks the platelet contribution to clot formation, allowing qualitative analysis of the functional fibrinogen component.8
Normal values of ROTEM parameters provided by the manufacturer have been shown in Table 1.
Table 1.
Normal Values of ROTEM Parameters.
| Test | Clotting Time (sec) | Clot Formation Time (sec) | MCF (mm) | Maximum Lysis (%)a |
|---|---|---|---|---|
| EXTEM | 38-79 | 34-159 | 50-72 | <15 |
| INTEM | 100-240 | 30-130 | 50-72 | <15 |
| FIBTEM | – | – | 9-25 | – |
| APTEM | A better clot formation when compared to EXTEM is an early sign of hyperfibrinolysis | |||
| HEPTEM | A better clot quality when compared to INTEM indicates the presence of heparin or heparin-like anticoagulants in the sample | |||
aWithin 1 hour of measurement
Bleeding and ROTEM
Rotational thromboelastometry analyses are increasingly included in evaluation of global clotting function and monitoring of hemostatic treatment in various clinical situations including liver transplantation (LT), cardiac surgery, obstetrics, trauma, and hemophilia that are universally recommended in addition to idiopathic thrombocytopenic purpura (ITP) according to our personal experience.
Liver Transplantation
Extensive bleeding associated with LT is a major challenge due to the underlying hepatic cirrhosis, replacement of massive blood loss during the operation and changes in the production and clearance of clotting factors at prehepatic, anhepatic, and neohepatic stages during surgery.7 Liver transplant represents an ideal application for viscoelastic tests because conventional coagulation tests offer limited and potentially misleading information in the context of liver disease.9
Early detection of coagulopathy is important in the prevention of bleeding during LT. Since traditional laboratory assays have long turnaround times for results, early variables detected by ROTEM enable individualized goal-directed therapy and reduced unrationalized product usage in LT. Five-minute parameter of ROTEM (A5: clot amplitude at 5 min) was shown to be effective in detecting critically low platelet and fibrinogen levels in LT since A5-EXTEM and A5-INTEM were highly correlated with platelet (r = .76 and r = .77, respectively) and fibrinogen (r = .63 and r = .64, respectively) levels.10 Rotational thromboelastometry–guided bleeding management in visceral and liver surgery decreased red blood cell (RBC) transfusions by 62%, fresh frozen plasma (FFP) by 95%, and platelets by 66%, while the incidence of massive transfusion was reduced by 66%.11 Moreover, measurement of FIBTEM-ROTEM to quantify the fibrin contribution to clot strength can guide fibrinogen replacement. By implementation of ROTEM-guided management, nearly 90% of FFP transfusion can be avoided, and patients may preferably be treated by a more targeted administration of purified fibrinogen concentrate and 4-factor prothrombin complex concentrate (PCC) while being more cost-effective.12
Hyperfibrinolysis, which is a serious and common complication during LT, can also be detected by ROTEM. By using APTEM-ROTEM test, Trzebicki et al demonstrated that ROTEM provides an immediate diagnosis of fibrinolysis, justifies the implementation of targeted treatment, and confirms the effectiveness of the therapy.13 In a recent study by Shimauchi et al, which aimed to determine the incidence of fibrinolysis in LT recipients and assess its relevance to mortality using ROTEM, fibrinolysis that developed in the pre–anhepatic phase was associated with increased 30-day and 6-month mortalities (P = .0003 and .0026, respectively).14
Cardiac Surgery
Cardiopulmonary bypass is frequently followed by a bleeding diathesis which has been variously attributed to the actions of heparin, excess or inadequate protamine administration, hypothermia, hemodilution, excessive activation of fibrinolysis, depletion of coagulation factors and a reduction in platelet number and function.15–17 Both bleeding and allogeneic blood transfusion is associated with increased morbidity, mortality, and hospital costs in cardiac and aortic surgery.
Potential value of TEG-guided therapy in cardiac surgery has previously been described in a large number of clinical trials and several systematic reviews. In the latest review published by Görlinger et al, which included 16 prospective and retrospective studies with 8507 patients, the utility of point-of-care testing including TEG/ROTEM was associated with reduced transfusions, less use of RBC and FFP, a change in transfusion patterns of fibrinogen and platelet concentrates, and improved patient outcome including 6-month mortality.18
A fast differential diagnosis of the intra- and postoperative bleeding situations is the basis of targeted therapy in cardiac surgery. In a study by Ogawa et al, ROTEM variables demonstrated clinically relevant correlations with platelet counts and fibrinogen levels, and the authors stated that decreasing levels of fibrinogen can quickly be determined (<15-20 minutes) using FIBTEM.19 The FIBTEM-MCF serves as an appropriate ROTEM parameter for dosing fibrinogen concentrate in these patients.20 Moreover, the effects of protamine and heparin can also be detected and easily differentiated by ROTEM.21
Finally, ROTEM has been used in pediatric cardiac surgery. Hemostatic changes in congenital heart surgery employing deep hypothermic circulatory arrest was monitored by ROTEM.22 Rotational thromboelastometry detected clotting factor depletion and platelet dysfunction, induced by cardiopulmonary bypass surgery within 15 minutes, which is much faster than standard tests, and guided the quick and specific treatment with blood products. Routine use of intraoperative ROTEM to guide transfusions in pediatric cardiac surgery has also reduced the overall proportion of patients receiving transfusions.23
In view of these findings, European Association for Cardio-Thoracic Surgery guidelines have included an evaluation of the utility of TEG/ROTEM during cardiac surgery to decrease blood transfusion and optimize the administration of blood products.24
Obstetrics
Postpartum hemorrhage (PPH) is still the leading cause of maternal morbidity and mortality worldwide and accounts for approximately 143 000 deaths per year.25
Recent studies suggest that fibrinogen seems to play an important role in the course of PPH, and it could be an early predictor on the severity of PPH. The FIBTEM values decline even more rapidly than fibrinogen levels and can be useful for early guidance of interventions.26 Since standard laboratory coagulation tests correlate poorly with blood loss in PPH, ROTEM/TEG-based algorithms for diagnosis and treatment of specific coagulation deficiencies in patients with PPH have been recommended.27 The use of early values of clot firmness—A5 (clot amplitude at 5 min) and A10 (clot amplitude at 10 min)—allows for fast and reliable prediction of MCF values and therefore can be used to guide hemostatic therapy in severe bleeding.28 Since there is a strong correlation with FIBTEM and fibrinogen levels in PPH, it is possible to define cutoff values for ROTEM parameters to detect fibrinogen levels <1.5 g/l in postpartum haemorrhage.29 In addition to the advantage of FIBTEM test that enables quick diagnosis of fibrinogen deficiency, ROTEM technology can identify hyperfibrinolysis that may be a contributor to maternal hemorrhage in women with suspected amniotic fluid embolism.30
Therefore, European Society of Anaesthesiology guideline for the management of severe perioperative bleeding has recommended viscoelastic hemostatic assays for the monitoring and management of coagulation in patients with PPH.31
Traumatic Injury
Severe trauma can lead to acute traumatic coagulopathy (ATC) by the activation of protein C, endothelial glycocalyx disruption, the consumption of fibrinogen, and platelet dysfunction.32 Patients presenting with ATC have higher mortality and morbidity. Since the outcome of ATC is influenced by the delay in the detection of this multifactorial condition, complete and rapid monitoring of blood coagulation and fibrinolysis using viscoelastic methods may facilitate a more accurate targeting in the therapy of ATC.
It is often assumed that the conventional coagulation screens monitor only the initiation phase of blood coagulation and represent only the first 4% of thrombin production.33 It is, therefore, possible that the conventional coagulation screen appears normal, while the overall state of blood coagulation is abnormal.34–39 Diagnosis and Treatment of Trauma-Induced Coagulopathy study has shown that ROTEM parameters were more appropriate than standard coagulation assays for the diagnosis and treatment of trauma-induced coagulopathy in 334 blunt trauma patients.38 Furthermore, the turnaround times of ROTEM have been shown to be significantly shorter compared to conventional laboratory testing, with a reduction in time of about 30 to 60 minutes.36,37,40 With a threshold of clot amplitude at 5 minutes < 35 mm, ROTEM can identify ATC at 5 minutes and predict the need for massive transfusion.36 Additionally, a recent strong evidence for ROTEM in traumatic coagulopathy was provided by multimodal program of work which determined that ROTEM could identify early coagulopathy within 5 minutes, as a large improvement on laboratory tests in trauma patients.41 In addition to achievement of quick results with ROTEM analysis compared with conventional laboratory tests, ROTEM results show a significant correlation with fibrinogen levels and platelet counts.40 Retrospective reviews of single-center experiences managing massive blood loss in trauma patients have suggested that the use of thromboelastometry-guided fibrinogen concentrate and prothrombin complex concentrate reduced mortality when compared to expected mortality,42 reduced the exposure to allogeneic blood products,43 and increased 30-day survival.44
Hyperfibrinolysis is significantly and independently associated with higher mortality in trauma patients. Rotational thromboelastometry provides real-time recognition of hyperfibrinolysis, may guide therapy with antifibrinolytic agents, and contribute to a reduction in the extremely high mortality of traumatized patients with fulminant hyperfibrinolysis.34,39
Finally, an updated European guideline has recommended viscoelastic methods to be performed in order to assist in characterizing the coagulopathy in addition to the measurement of PT, APTT, fibrinogen, and platelets for coagulation monitoring and guiding hemostatic therapy.45
Hemophilia
Hemophilia A (factor VIII deficiency) and hemophilia B (factor IX deficiency) are the most common severe inherited bleeding disorders. Acute bleeding in a patient with hemophilia requires prompt evaluation and immediate treatment. Monitoring of the therapeutic efficiency of bypassing agents such as recombinant-activated FVII and activated prothrombin complex concentrates is desired in hemophilia A patients with inhibitors. The optimal use of bypassing agents, however, is currently hampered by a lack of laboratory assays to determine the adequate dosage and to monitor therapeutic response.46 Recent studies have shown that ROTEM has a great potential in monitoring the clinical response of bypassing agents in the management of bleeding episodes.47,48 A systematic monitoring protocol using ROTEM was also described for the management of hemophilia patients during the perioperative period. Furukawa et al demonstrated that ROTEM profile provided a useful guide for establishing an effective dose of bypassing agent and facilitated good hemostatic control during and after surgery.49
Rotational thromboelastometry appears to be useful for the monitoring of novel therapeutic products in hemophilia, as the major potential limitation of these new products is the inability to measure their hemostatic effects accurately and in a simple way.50 A recent study of a FVIII- mimetic bispecific antibody, ACE910, a FVIII -mimetic bispecific antibody demonstrated that ROTEM could be useful for the hemostatic monitoring of this new treatment agent.51 The potential role of ROTEM in the monitoring of treatment response and inhibitor eradication in an acquired hemophilia A patient with life-threatening bleeding was also documented.52
Idiopathic Thrombocytopenic Purpura
Adult patients with ITP typically present with petechiae, purpura, and easy bruising. Mucosal bleeding tends to be more frequent when platelet count is less than 20 × 109/L.53 The incidence of severe bleeding increases when platelet count is below 10 × 109/L.54–57 However, Wang and colleagues have shown that the platelet count may not predict the risk of bleeding since the platelet count is not an indicator of platelet function.55 In our study aiming to investigate the changes in TEG parameters in patients with ITP and to find a parameter in predicting bleeding risk, we documented that ROTEM determines the contribution of fibrinogen and platelets to clot strength in patients with ITP. MCF was found to be the most important TEG parameter in predicting bleeding in patients with ITP that makes TEG superior to other hemostatic tests.56 Recently, the hemostatic response of recombinant FVIIa and fibrinogen combination in patients with ITP with very low platelet counts was investigated by whole blood clot formation recorded by ROTEM. This combination revealed a significant synergistic effect, improving all ROTEM profiles in whole blood and represented an alternative to platelet transfusion.57 The maintenance of fibrinogen concentration is critical in the presence of thrombocytopenia. The effects of fibrinogen levels in the presence of thrombocytopenia have been shown quite well by ROTEM variables. In vitro and clinical data obtained from Lang et al’s study demonstrated that the clot strength increases in a fibrinogen concentration–dependent manner independent of platelet count when analyzed by ROTEM and showed that EXTEM and FIBTEM may be useful in guiding fibrinogen repletion therapy in the presence of thrombocytopenia.58
Hypercoagulation and ROTEM
ROTEM analyses enable to identify various hypercoagulable conditions not only in patients with major surgery as reported by others in the previous literature but also in patients with malignancy, Behcet disease, and apheresis donors that are our personal experiences.
Major Surgery
Thromboembolic complications of surgery such as deep vein thrombosis and pulmonary embolism induce substantial postoperative morbidity and mortality.59 Routine laboratory tests do not detect patients with acquired or congenital hypercoagulability who may be at an increased risk of perioperative thromboembolism. Hincker et al has pioneered the use of ROTEM as a tool to identify patients at an increased risk of thromboembolic complications after major surgery. In 313 patients undergoing major noncardiac surgery, ROTEM patterns in EXTEM and INTEM were significantly different in those patients who developed postoperative thromboembolic complications. Specifically, the patients with thromboembolic complications had significantly lower CFT, higher α angle, and larger maximum clot firmness (MCF). INTEM clot firmness at 10 minutes (A10) was the best predictor of thromboembolic complications, with an receiver operating characteristic (ROC) area under the curve of 0.75. These results support the conclusion that rotational TEG may be able to detect patients who are susceptible to postoperative thromboembolic complications.60
Malignancy
Thrombosis is one of the major complications of cancer.61 Hypercoagulability in patients with cancer is difficult to detect through standard coagulation tests unless the platelet count and fibrinogen concentration is markedly increased.1 The TEG is a sensitive method that is able to identify and measure hypercoagulability that is not detected by routine laboratory tests.62,63 The results of our study yield quite well the significant hypercoagulability detected by ROTEM in 78 patients with solid tumors including gastrointestinal system tumors, respiratory system tumors, and miscellaneous group of ovarian, renal, nasopharyngeal, mesothelioma, and unknown origin. This hypercoagulability was diagnosed readily by the presence of an accelerated clot formation which was shown by the shortening of CFT and by an increase of the clot strength that was evidenced through the increase in MCF on all assays (INTEM, EXTEM, FIBTEM, APTEM) when compared to the healthy controls. The ROTEM was also able to identify the contribution of fibrinogen and platelets to clot strength in this patient population.64
Na et al investigated the changes of coagulation in 22 patients with colorectal cancer using ROTEM following magnesium sulphate infusion. Their hypothesis was that magnesium would attenuate postoperative hypercoagulability. All maximum clot firmness values of ROTEM analysis were significantly lower on the third postoperative day in the magnesium group compared with the control group (P < .05), and the authors concluded that intraoperative administration of intravenous magnesium sulphate reduces blood hypercoagulability in patients undergoing laparoscopic colorectal cancer surgery.65 In another study including 67 patients with lung cancer, patients showed reduced CT, increased MCF, and increased α angle compared with 72 age-matched healthy controls. However, no differences were observed between patients who did or did not encounter a venous thromboembolism, highlighting the limitation of ROTEM in identifying venous thromboembolism (VTE) risk in patients with lung cancer.66
Additionally, preexisting hypercoagulability was detected in 22 of 72 patients with intra-abdominal malignancies commonly localized in the pancreas, esophagus, and liver who had undergone potentially curative cancer resection. Using ROTEM, 86% of hypercoagulable patients had EXTEM abnormalities, whereas 45% and 32% had abnormalities with FIBTEM and INTEM. The most common abnormalities were in the clot strength as evidenced by elevated MCF and clot kinetics as evidenced by low CFT.67 The same authors have documented the persistence of the hypercoagulable state after the resection of intra-abdominal malignancies. Their data showed that 40% were hypercoagulable before surgery. After surgical resection, an even higher proportion became hypercoagulable, as reflected by more rapid CFT (low CFT, high α) and higher MCF. By week 1, 86% (n = 30) had abnormal ROTEM values, including 17 (81%) of 21 who had normal coagulation profiles preoperatively. Most (n = 30, 86%) remained hypercoagulable at 3 to 4 weeks. These results support the conclusion that surgical resection does not immediately reverse tumor-induced hypercoagulability and support the use of postdischarge thromboprophylaxis regimens.68 ROTEM was also found to be useful in identifying patients with cholangiocarcinoma at risk of postoperative venous thromboembolism.69
Behcet Disease
Behcet disease is a multisystemic disorder characterized by recurrent oral and genital ulceration, skin eruptions, inflammatory ocular involvement, and neurological manifestations. Although thrombosis is the major clinical finding in patients with Behcet disease, the cause of the thrombosis is still unclear.70 We performed ROTEM analysis to assess the role of hemostatic mechanisms in the development of thrombosis in 30 patients with Behcet disease. Both in INTEM and EXTEM assays, there was a significant increase in MCF and a decrease in CFT in patients with Behcet disease compared with the controls. We concluded that the primary hemostatic mechanisms which can be detected by ROTEM may play a role in the development of thrombosis in Behcet disease.71
Apheresis Donors
Even though apheresis technology is considered generally safe for donors, the question of donor safety raised by the contact of donor blood with artificial surfaces still remains. During extracorporeal circulation, adsorption of clotting factors, platelet adhesion and aggregation, and adherence of white blood cells and RBCs to the surfaces of the tubes for blood circulation in the blood cell separator can lead to a hypercoagulable state in plateletpheresis donors.72–76
In our study investigating the effects of red cell apheresis on the coagulation system of 29 donors by ROTEM, CFT was significantly shortened while MCF was significantly prolonged immediately after apheresis and 24 hours after apheresis compared with baseline values both in INTEM and EXTEM assays. Authors advocated the use of TEG for the documentation of a hypercoagulable state in donors undergoing repeated apheresis procedures to identify those at risk of thrombotic episodes and increase their safety.77
Conclusion
Rotational thromboelastometry technology provides a rapid and dynamic assessment of hemostasis in vitro. It is a valuable analytic tool for clinicians in making an early diagnosis of a specific coagulopathy and a decision for the most appropriate treatment. Diagnosis and treatment algorithms incorporating ROTEM analysis for bleeding patients in varied clinical settings have been developed. Rotational thromboelastometry also measures hypercoagulability in various clinical scenarios where it is not detected by routine coagulation tests. Further developments in ROTEM-based transfusion strategies may also reduce transfusion requirements and improve clinical outcomes by optimizing the administration of blood components. Besides, ROTEM studies and treatment guidelines should be expanded to various fields such as neurosurgery, orthopedics, urology, gastrointestinal surgery, sepsis, renal transplantation, vasculitis, and many other hematological disorders as well as the assessment of the influence of antithrombotic, antiplatelet, and antifibrinolytic drugs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mallett SV, Cox DJA. Thromboelastography. Br J Anaesth. 1992;69:307–313. [DOI] [PubMed] [Google Scholar]
- 2. Hartert H. Blood coagulation studies using thromboelastography, a new evaluation technique. Kin Wochenschr. 1948;26:577–583. [Google Scholar]
- 3. Traverso CI, Caprini JA, Arcelus JI. The normal thromboelastogram and its interpretation. Semin Thromb Hemost. 1995;21(suppl 4):7–13. [DOI] [PubMed] [Google Scholar]
- 4. Spiel AO, Mayr FB, Firbas C, Quehenberger P, Jilma B. Validation of rotation thrombelastography in a model of systemic activation of fibrinolysis and coagulation in humans. J Thromb Haemost. 2005;4(2):411–416. [DOI] [PubMed] [Google Scholar]
- 5. Schobersberger W, Mittermayr M, Fries D, et al. Changes in blood coagulation of arm and leg veins during a simulated long-haul flight. Thromb Res. 2007;119(3):293–300. [DOI] [PubMed] [Google Scholar]
- 6. Sørensen B, Johansen P, Christiansen K, Woelke M, Ingerslev J. Whole blood coagulation thromboelastographic profiles employing minimal tissue factor activation. J Thromb Haemost 2002;1(3):551–558. [DOI] [PubMed] [Google Scholar]
- 7. Whiting D, DiNardo JA. TEG and ROTEM: Technology and clinical applications. Am J Hematol. 2014;89(2):228–232. [DOI] [PubMed] [Google Scholar]
- 8. Calatzis A, Görlinger K, Spannagl M, Vorweg M. ROTEM Analysis Targeted Treatment of Acute Haemostatic Disorders. https://www.rotem.de/en
- 9. Donohue C, Mallett SV. Reducing transfusion requirements in liver transplantation. World J Transplant. 2015;5(4):165–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Song JG, Jeong SM, Jun IG, et al. Five-minute parameter of thromboelastometry is sufficient to detect thrombocytopenia and hypofibrinogenaemia in patients undergoing liver transplantation. Br J Anaesth. 2004;112(2):290–297. [DOI] [PubMed] [Google Scholar]
- 11. Görlinger K, Fries D, Dirkmann D. Reduction of fresh frozen plasma requirements by perioperative point-of-care coagulation management with early calculated goal-directed therapy. Transfus Med Hemother. 2012;39(2):104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Görlinger K, Dirkmann D, Müller-BeiBenhirtz H, et al. Thromboelastometry-based perioperative coagulation management in visceral surgery and liver transplantation: experience of 10 years and 1105LTX. Liver Transpl. 2010;16:S86. [Google Scholar]
- 13. Trzebicki J, Flakiewicz E, Kosieradzki M, et al. The use of thromboelastography in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann Transplant. 2010;15(3):19–24. [PubMed] [Google Scholar]
- 14. Shimauchi T, Yamaura K, Higashi M, et al. Fibrinolysis in living donor liver transplantation recipients evaluated using thromboelastometry: Impact on mortality. Transplant Proc 2017;49(9):2117–2121. [DOI] [PubMed] [Google Scholar]
- 15. Hunt BJ, Parratt RN, Segal HC, et al. Activation of coagulation and fibrinolysis during cardiothoracic operations. Ann Thorac Surg. 1998;65(3):712–718. [DOI] [PubMed] [Google Scholar]
- 16. Ferraris VA, Ferraris SP, Singh A, et al. The platelet thrombin receptor and postoperative bleeding. Ann Thorac Surg. 1998;65(2):352–358. [DOI] [PubMed] [Google Scholar]
- 17. Woodman RC, Harker LA. Bleeding complications associated with cardiopulmonary bypass. Blood. 1990;76(9):1680–1697. [PubMed] [Google Scholar]
- 18. Görlinger K, Dirkmann D, Hanke AA. Potential value of transfusion protocols in cardiac surgery. Curr Opin Anaesthesiol. 2013;26(2):230–243. [DOI] [PubMed] [Google Scholar]
- 19. Ogawa S, Szlam F, Chen EP, et al. A comparative evaluation of rotation thromboelastometry and standart coagulation tests in hemodilution-induced coagulation changes after cardiac surgery. Transfusion. 2012;52(1):14–22. [DOI] [PubMed] [Google Scholar]
- 20. Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102(6):785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mittermayr M, Margreiter J, Velik-Salchner C, et al. Effects of protamine and heparin can be detected and easily differentiated by modified thromboelastography (ROTEM):an in vitro study. Br J Anaesth. 2005;95(3):310–316. [DOI] [PubMed] [Google Scholar]
- 22. Straub A, Schiebold D, Wendel HP, et al. Using reagent-supported thromboelastometry (ROTEM) to monitor haemostatic changes in congenital heart surgery employing deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. 2008;34(3):641–647. [DOI] [PubMed] [Google Scholar]
- 23. Romlin BS, Wahlander H, Berggren H, et al. Intraoperative thromboelastometry is associated with reduced transfusion prevalence in pediatric cardiac surgery. Anesth Analg. 2011;112(1):30–36. [DOI] [PubMed] [Google Scholar]
- 24. Dunning J, Versteegh M, Fabbri A, et al. Guideline on platelet and anticoagulation management in cardiac surgery. Eur J Cardiovasc Thorac Surg. 2008;7(4):690–697. [Google Scholar]
- 25. Dolea C, AbouZahr C, Stein C. Global burden of maternal haemorrhage in the year 2000. Geneva: Evidence and Information for Policy (EIP), World Health Organisation; 2003. http://www.who.int/healthinfostatistics/ [Google Scholar]
- 26. De Lange NM, Lance MD, de Groot R, et al. Obstetric hemorrhage and coagulation: an update. Thromboelastography, thromboelastometry, and conventional coagulation tests in the diagnosis and prediction of postpartum hemorrhage. Obstet Gynecol Surv 2012;67(7):426–435. [DOI] [PubMed] [Google Scholar]
- 27. Solomon C, Collis RE, Collins PW. Haemostatic monitoring during postpartum haemorrhage and implications for management. Br J Anaesth. 2012;109(6):851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Görlinger K, Dirkmann D, Solomon C, et al. Fast interpretation of thromboelastometry in non-cardiac surgery: reliability in patients with hypo-, normo-, and hypercoagulability. Br J Anaesth. 2013;110(2):222–230. [DOI] [PubMed] [Google Scholar]
- 29. Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thrombelastometry. BJOG. 2009;116(8):1097–1102. [DOI] [PubMed] [Google Scholar]
- 30. Collins NF, Bloor M, McDonnell NJ. Hyperfibrinolysis diagnosed by rotational thromboelastometry in a case of suspected amniotic fluid embolism. Int J Obstet Anesth 2013;22(1):71–76. [DOI] [PubMed] [Google Scholar]
- 31. Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: First update. Eur J Anaesthesiol. 2016;34(6):332–395. [DOI] [PubMed] [Google Scholar]
- 32. Simmons JW, Powell MF. Acute traumatic coagulopathy: pathophysiology and resuscitation. Br J Anaesth. 2016;117(suppl 3):iii31–iii43. [DOI] [PubMed] [Google Scholar]
- 33. Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23(1):17–25. [DOI] [PubMed] [Google Scholar]
- 34. Levrat A, Gros A, Rugeri L, et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br J Anaesth. 2008;100(6):792–797. [DOI] [PubMed] [Google Scholar]
- 35. Johansson PI, Stensballe J. Effect of Haemostatic Control Resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96(2):111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Davenport R, Manson J, De’Ath H, et al. Functional definition and characterization of acute traumatic coagulopathy. Crit Care Med. 2011;39(12):2652–2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schöchl H, Frietsch T, Pavelka M, et al. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J Trauma. 2009;67(1):125–131. [DOI] [PubMed] [Google Scholar]
- 38. Tauber H, Innerhofer P, Breitkopf R, et al. Prevalence and impact of abnormal ROTEM(R) assays in severe blunt trauma: results of the ‘Diagnosis and Treatment of Trauma-Induced Coagulopathy (DIA-TRE-TIC) study’. Br J Anaesth. 2011;107(3):378–387. [DOI] [PubMed] [Google Scholar]
- 39. Theusinger OM, Wanner GA, Emmert MY, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011;113(5):1003–1012. [DOI] [PubMed] [Google Scholar]
- 40. Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5(2):289–295. [DOI] [PubMed] [Google Scholar]
- 41. Brohi K, Eaglestone S. Traumatic Coagulopathy and Massive Transfusion: Improving Outcomes and Saving Blood. Southampton (UK: ):NIHR Journals Library; 2017. [PubMed] [Google Scholar]
- 42. Schöchl H, Nienaber U, Hofer G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15(2):R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shaz BH, Dente CJ, Nicholas J, et al. Increased number of coagulation products in relationship to red blood cell products transfused improves mortality in trauma patients. Transfusion. 2010;50(2):493–500. [DOI] [PubMed] [Google Scholar]
- 45. Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17(2):R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Astermark J, Donfield SM, DiMichele DM, et al. A randomized comparison of bypassing agents in haemophilia complicated by an inhibitor: the FEIBA NovoSeven Comarative (FENOC) Study. Blood. 2007;109(2):546–551. [DOI] [PubMed] [Google Scholar]
- 47. Shima M, Matsumoto T, Ogiwara K. New assays for monitoring haemophilia treatment. Haemophilia. 2008;4(suppl 3):83–92. [DOI] [PubMed] [Google Scholar]
- 48. Tran HT, Sorensen B, Bjornsen S, et al. Monitoring bypassing agent therapy - a prospective crossover study comparing thromboelastometry and thrombin generation assay. Haemophilia. 2015;21(2):275–283. [DOI] [PubMed] [Google Scholar]
- 49. Furukawa S, Nogami K, Ogiwara K, et al. Systematic monitoring of haemostatic management in haemophilia A patients with inhibitor in the perioperative period using rotational thromboelastometry. J Thromb Haemost. 2015;13(7):1279–1284. [DOI] [PubMed] [Google Scholar]
- 50. Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174(4):503–514. [DOI] [PubMed] [Google Scholar]
- 51. Yada K, Nogami K, Shida Y, et al. Enhanced global haemostatic potentials with a bispecific antibody to factor IXa and X (ACE910) in the whole blood by rotation thromboelastometry (ROTEM). Blood. 2015;126:3503. [Google Scholar]
- 52. Spiezia L, Meneghetti L, Dalla Valle F, et al. Potential role of thrombelastography in the monitoring of acquired factor VIII inhibitor hemophilia A: report on a 78-year-old woman with life-threatening bleedings. Clin Appl Thromb Hemost 2009;15(4):470–476. [DOI] [PubMed] [Google Scholar]
- 53. Chong BH. Diagnosis, treatment and pathophisiology of autoimmune thrombocytopenias. Crit Rev Oncol Hematol. 1995;20(1):271–296. [DOI] [PubMed] [Google Scholar]
- 54. Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura. Blood. 2005;106(7):2244–2251. [DOI] [PubMed] [Google Scholar]
- 55. Wang ZY, Shen ZX. Megakaryocytes and platelet in immune thrombocytopenic purpura. Baillieres Clin Haematol. 1997;10(1):89–107. [DOI] [PubMed] [Google Scholar]
- 56. Gunduz E, Akay OM, Bal C, et al. Can thrombelastography be a new tool to assess bleeding risk in patients with idiopathic thrombocytopenic purpura? Platelets. 2011;22(7):516–520. [DOI] [PubMed] [Google Scholar]
- 57. Larsen OH, Stentoft J, Radia D, et al. Combination of recombinant factor VIIa and fibrinogen corrects clot formation in primary immune thrombocytopenia at very low platelet counts. Br J Haematol. 2013;160(2):228–236. [DOI] [PubMed] [Google Scholar]
- 58. Lang T, Johanning K, Metzler H, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Analg. 2009;108(3):751–758. [DOI] [PubMed] [Google Scholar]
- 59. Kakkar VV. Prevention of venous thromboembolism. Clin Haematol. 1981;10(2):543–582. [PubMed] [Google Scholar]
- 60. Hincker A, Feit J, Sladen RN, et al. Rotational thromboelastography predictes thromboembolic complications after major non-cardiac surgery. Crit Care 2014;18(5):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nijziel MR, van Oerle R, Hillen HFP, et al. From Trousseau to angiogenesis: the link between the hemostatic system and cancer. Neth J Med. 2006;64(11):403–410. [PubMed] [Google Scholar]
- 62. Cohen E, Caprini JP, Zuckerman L, Vagher P, Robinson B. Evaluation of three methods used to identify accelerated coagulability. Thromb Res. 1997;10(4):587–604. [DOI] [PubMed] [Google Scholar]
- 63. Arcelus JI, Traverso CI, Caprini JA. Thromboelastography for the assessment of hypercoagulabilityduring general surgery. Semin Thromb Hemost. 1995;21(suppl 4):21–26. [DOI] [PubMed] [Google Scholar]
- 64. Akay OM, Ustuner Z, Canturk Z, et al. Laboratory investigation of hypercoagulability in cancer patients using rotation thromboelastography. Med Oncol. 2009;26(3):358–364. [DOI] [PubMed] [Google Scholar]
- 65. Na HS, Shin HJ, Kang SB, et al. Effects of magnesium sulphate on coagulation after laparoscopic colorectal cancer surgery, measured by rotational thromboelastometry (ROTEM). Anaesthesia. 2014;69(12):1314–1321. [DOI] [PubMed] [Google Scholar]
- 66. Davies NA, Harrison NK, Sabra A, et al. Application of ROTEM to assess hypercoagulability in patients with lung cancer. Thromb Res. 2015;135(6):1075–1080. [DOI] [PubMed] [Google Scholar]
- 67. Thorson CM, Van Haren RM, Ryan ML, et al. Pre-existing hypercoagulability in patients undergoing potentially curative cancer resection. Surgery. 2014;155(1):134–144. [DOI] [PubMed] [Google Scholar]
- 68. Thorson CM, Van Haren RM, Ryan ML, et al. Persistence of hypercoagulable state after resection of intra-abdominal malignancies. J Am Coll Surg. 2013;26(4):580–589. [DOI] [PubMed] [Google Scholar]
- 69. Blasi A, Molina V, Sanchez-Cabus S, et al. Prediction of thromboembolic complications after liver resection for cholangiocarcinoma: is there a place for thromboelastometry? Blood Coagul Fibrinolysis. 2018;29(1):61–66. [DOI] [PubMed] [Google Scholar]
- 70. Leiba M, Seligsohn U, Sidi Y, et al. Thrombophilic factors are not the leading cause of thrombosis in Behçet’s disease. Ann Rheum Dis. 2004;63(11):1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yasar Bilge N, Akay OM, Kaşifoğlu T, et al. The role of hemostatic mechanisms in the development of thrombosis in Behcet’s disease: an analysis by modified rotation thromboelastogram (ROTEM). Clin Rheumatol. 2013;32(12):1815–1818. [DOI] [PubMed] [Google Scholar]
- 72. Karadogan I, Ozdogan M, Undar L. Single automated donor plateletpheresis increases the plasma level of proinflammatory cytokine tumor necrosis factor-alpha which does not associate with endothelial release markers von Willebrand factor and fibronectin. Transfus Sci. 2000;23(3):171–175. [DOI] [PubMed] [Google Scholar]
- 73. Kobayashi I, Hamaoka S, Ozawa H, et al. Hypercoagulable state induced by thrombocytapheresis. J Clin Apheresis. 1993;8(3):147–152. [DOI] [PubMed] [Google Scholar]
- 74. Ziemer S, Roeseler E, Monike A, et al. Therapeutic plasmapheresis and haemostatic system. Folia Haematol Int Mag Klin Morphol Blutforcsh. 1998;115(4):563–568. [PubMed] [Google Scholar]
- 75. Blasi U, Jung F, Mrowietz C, et al. Determination of platelet aggregation of donor blood before and after thrombocytapheresis. Beitr Infusionsther Transfusionmed. 1994;32:339–340. [PubMed] [Google Scholar]
- 76. Boehlen F, Michel M, Reber G, et al. Analysis of platelet donors function before and after thrombapheresis using the platelet function analyzer PFA-100. Thromb Res. 2001;102(1):49–52. [DOI] [PubMed] [Google Scholar]
- 77. Akay OM, Karagulle M, Kus G, et al. Thrombelastographic evaluation of the influence of 2-RBC apheresis on donor’s coagulation system. Transfus Apher Sci. 2013;48(3):387–390. [DOI] [PubMed] [Google Scholar]