Abstract
Progression of coronary artery calcification (CAC) was significantly associated with all-cause mortality, and high coronary artery calcium score (CACS) portends a particularly high risk of cardiovascular events. But how often one should rescan is still an unanswered question. Preliminary screening by testing circulating biomarker may be an alternative before repeat computed tomography (CT) scan. The aim of this study was to investigate the value of big endothelin-1 (bigET-1), the precursor of endothelin-1 (ET-1), in predicting the severity of CAC. A total of 428 consecutively patients who performed coronary computed tomography angiography (CCTA) due to chest pain in Fuwai Hospital were included in the study. The clinical characteristics, CACS, and laboratory data were collected, and plasma bigET-1 was detected by enzyme-linked immunosorbent assay (ELISA). The bigET-1 was positively correlated with the CACS (r = .232, P < .001), and the prevalence of CACS >400 increased significantly in the highest bigET-1 tertile than the lowest tertile. Multivariate analysis showed that bigET-1was the independent predictor of the presence of CACS >400 (odds ratio [OR] = 1.721, 95% confidence interval [CI], 1.002-2.956, P = .049). The receiver operating characteristic (ROC) curve analysis showed that the optimal cutoff value of bigET-1 for predicting CACS >400 was 0.38 pmol/L, with a sensitivity of 59% and specificity of 68% (area under curve [AUC] = 0.65, 95% CI, 0.58-0.72, P < .001). The present study demonstrated that the circulating bigET-1 was valuable in the assessment of the severity of CAC.
Keywords: atherosclerosis, big endothelin-1, coronary artery calcium score
Introduction
The coronary artery calcium score (CACS) is a direct marker of atherosclerosis and provides an assessment of the burden of coronary atherosclerosis.1 Numerous studies have revealed the prognostic value of CACS with data from population and clinical cohorts.2–7 Society of Cardiovascular Computed Tomography (SCCT) coronary artery calcification (CAC) expert consensus recommends that it is appropriate to perform CACS in the context of shared decision-making for asymptomatic individuals without clinical arteriosclerotic cardiovascular disease (ASCVD) in the 5% to 20% 10-year ASCVD risk group and selectively in the <5% ASCVD group.8
Moreover, progression of CAC was significantly associated with all-cause mortality, and a higher CAC burden carries a greater risk for future coronary heart disease (CHD) events.9 There is a direct proportional relationship between CACS and clinical events, including cardiac or all-cause mortality, myocardial infarction, unstable angina hospitalization, late revascularization >3 months, stroke, and transient ischemic attacks stratified by the CACS categories 0, 1 to 99, 100 to 399, and >400.8 A CACS >400 is a CHD equivalent with 10-year event rates exceeding 20% in asymptomatic patients according to Framingham risk score.10 Repetitive quantification of CAC may be necessary to measure the progression of CAC. But how often one should rescan is still an unanswered question. The test of computed tomography (CT) CAC screening is far from low cost in China. Therefore, preliminary screening by testing circulating biomarker may be an alternative before repeat CT scan.
Endothelin-1 (ET-1) is a vasoactive peptide and has a correlation with the development of atherosclerosis via its actions on all cells of the vasculature. The expression of ET-1 and its receptors is upregulated in experimental models of atherosclerosis and in human atherosclerotic lesions.11–13 As both CACS and ET-1 are associated with atherogenesis, the relationship between the severity of CAC and ET-1 is worth exploring, which remains unclear now. Being a peptide with high biologic activity, ET-1 is rapidly cleared and may never reach the circulation. The bigET-1, the precursor of ET-1, circulates in higher concentration and opens an analytic window.14 Therefore, the aim of this study was to investigate the value of bigET-1 in assessment of the severity of CAC.
Methods
Study Design and Population
We evaluated consecutive patients who performed coronary computed tomography angiography (CCTA) due to stable typical or atypical chest pain from May 2016 to July 2017 in Fuwai Hospital (National Center for Cardiovascular Diseases, Beijing, China) and collected their complete clinical and laboratory data. Exclusion criteria were as follows: (1) patients with acute coronary syndrome and without lipids and bigET-1 measurements available and (2) other diseases, such as congenital heart disease, New York Heart Association (NYHA) functional class III or IV heart failure, stage C or D of the progression of valvular heart disease according to the guideline of 2014 American Heart Association/American College of Cardiology (AHA/ACC) for the management of patients with valvular heart disease,15 hematological disease, cancer, and severe renal or liver disease. The study protocol complied with the Declaration of Helsinki and was approved by the hospital ethics review board. Written informed consent was obtained from all the participants.
Data Acquisition
Scans were performed using a 64-row spiral CT scanner (Light Speed VCT, GE Healthcare, Milwaukee, Wisconsin, USA). Patients were given 25 to 50 mg of metoprolol (Selokeen, AstraZeneca, Zoetermeer, the Netherlands) orally 1 hour before scanning according to the heart rates. Contrast medium was injected at different speed during the different stage. The main scanning parameters were as follows: 64 detectors; individual detector width, 0.625 mm; gantry rotation time, 350 ms; tube voltage, 120 kV; electrocardiographically modulated tube current; pitch, 0.16 to 0.22; table feed per rotation, 400 mm; and field of view, 200 to 250 mm.
Image Analysis
The scans were retrospectively analyzed on the workstation (Deep Blue, ADW4.3, GE Healthcare, Milwaukee, Wisconsin, USA). Calcium was defined as the presence of at least 3 contiguous pixels with a density >130 Hounsfield Units (HU). The total was quantified according to the scoring algorithm proposed by Agatston et al16 and predefined calcium score categories (0, 1-100, 101-400, and > 400) were used.17
Cardiovascular Risk Factors Assessment
Main cardiovascular risk factors such as diabetes mellitus, hypertension, dyslipidemia, cigarette smoking, alcohol consummation, and family history of coronary artery disease (CAD) were assessed. Diabetes mellitus was defined as fasting glucose of ≥126 mg/dL, nonfasting glucose of ≥200 mg/dL, or receiving hypoglycemic therapy (insulin, oral hypoglycemic therapy, or dietary advice). Hypertension was defined as a previously established diagnosis, systolic blood pressure ≥140 mm Hg and diastolic blood pressure ≥90 mm Hg, or taking antihypertensive medication. Dyslipidemia was defined according to the medical history or by the current use of lipid-lowering drugs. Smoking status and alcohol consumption were ascertained by the medical history. Family history of CAD was considered as a history of CAD, myocardial infarction, coronary revascularization, or sudden cardiac death before 55 years of age for the father or 65 years of age for the mother.
Laboratory Measurements
Venous blood samples were obtained at baseline before undergoing CCTA from each participant. The plasma bigET-1 was detected using a commercial sandwich enzyme immunoassay (BI-20082H, Biomedica, Wien, Austria). The levels of serum total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), creatinine (CREA), blood urea nitrogen (BUN), and uric acid (URIC) were measured using an automatic biochemistry analyzer (Olympus Diagnostics, California, USA) and conventional clinical analytical methods.
Statistical Analysis
Statistical analysis was performed with the statistical package SPSS 21.0 (SPSS Inc, Chicago, Illinois, USA). A two-sided P value of less than .05 was considered to be statistically significant. Normally distributed variables were expressed as mean + standard deviation (SD) and non-normally distributed variables are presented as median (interquartile range), which were analyzed by independent-samples t test, one-way ANOVA, or the Mann-Whitney U test (as appropriate). Categorical variables were expressed as percentages and were assessed by χ2 or Fisher exact tests (as appropriate). The correlation between two continuous variables was assessed with Pearson correlation test or Spearman correlation test (as appropriate). Multivariate logistic regression analysis was performed to identify the independent risk factors of the presence of CACS >400. The risk factors were prespecified on the basis of univariate P values of <.05.
Results
The study population consisted of 428 patients undergoing CCTA (average age: 58.86 ± 10.57 years; 61.4% were males). Baseline clinical and laboratory data of the 4 groups are shown in Table 1. The participants were divided into 4 groups based on predefined calcium score categories (0, 1-100, 101-400, and >400). The majority of the study population (n = 303, 70.8%) were found to have CAC, and the remaining 125 (29.2%) participants had no CAC. Among the 303 patients, there were 154 patients with CACS 1 to 100, 74 patients with CACS 101 to 400, and 75 patients with CACS >400.
Table 1.
Baseline Characteristics of the Study Population According to the CACS Categories.
| Variables | CACS = 0 (n = 125) | CACS 1 to 100 (n = 154) | CACS 101 to 400 (n = 74) | CACS >400 (n = 75) | P Value |
|---|---|---|---|---|---|
| Age (years) | 52.0 ± 9.9 | 58.9 ± 9.4a | 62.3 ± 8.6b | 66.8 ± 8.5c | <.001 |
| BMI (kg/m2) | 25.5 ± 3.3 | 26.4 ± 4.0a | 25.4 ± 3.5 | 24.9 ± 3.6 | .024 |
| Male, n (%) | 69 (55.2%) | 97 (63.0%) | 48 (64.9%) | 49 (65.3%) | .383 |
| Hypertension, n (%) | 56 (44.8%) | 110 (71.4%) | 54 (73.0%)b | 56 (74.7%)c | <.001 |
| Diabetes, n (%) | 21 (16.8%) | 29 (18.8%) | 19 (25.7%) | 23 (30.7%)c | .079 |
| Dyslipidemia, n (%) | 88 (70.4%) | 125 (81.2%) | 63 (85.1%) | 62 (82.7%) | .040 |
| Smoking, n (%) | 36 (28.8%) | 45 (29.2%) | 18 (24.3%) | 21 (28.0% | .855 |
| Alcohol consumption, n (%) | 35 (28.0%) | 49 (31.8%) | 26 (35.1%) | 21 (28.0%) | .691 |
| History of CAD, n (%) | 14 (11.2%) | 18 (11.7%) | 4 (5.4%) | 7 (9.3%) | .485 |
| Biochemical parameters | |||||
| TC (mmol/L) | 4.8 ± 1.0 | 4.7 ± 1.5 | 4.4 ± 0.9b | 4.1 ± 1.0c | <.001 |
| TG (mmol/L) | 1.6 (1.2, 2.3) | 1.6 (1.1, 2.1) | 1.4 (1.0, 2.1) | 1.4 (1.0, 1.9)c | <.001 |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | .498 |
| LDL-C (mmol/L) | 3.0 ± 0.8 | 2.9 ± 1.0 | 2.6 ± 0.8b | 2.4 ± 0.8c | <.001 |
| CREA (umol/L) | 68.6 ± 15.3 | 74.8 ± 19.2a | 73.6 ± 14.1b | 79.1 ± 17.9c | <.001 |
| BUN (mmol/L) | 5.5 ± 1.3 | 6.0 ± 2.0a | 6.0 ± 1.7b | 6.6 ± 1.8c | <.001 |
| URIC (umol/L) | 325.7 ± 90.7 | 349.3 ± 95.2a | 351.1 ± 85.7 | 347.2 ± 109.2 | .141 |
| BigET-1 (pmol/L) | 0.27 (0.20, 0.39) | 0.30 (0.22, 0.46) | 0.35 (0.23, 0.60)b | 0.46 (0.27, 0.73)c | <.001 |
| Medications, n (%) | |||||
| Statins | 74 (59.2%) | 107 (69.5%) | 60 (81.1%)b | 58 (77.3%)c | .004 |
| Aspirin | 84 (67.2%) | 121 (78.6%)a | 61 (82.4%)b | 60 (80.0%)c | .041 |
| Calcium antagonists | 23 (18.4%) | 68 (44.2%)a | 34 (45.9%)b | 36 (48.0%)c | <.001 |
| ARB/ACEI | 46 (36.8%) | 65 (42.2%) | 35 (47.3%) | 41 (54.7%)c | .085 |
| β-blockers | 61 (48.8%) | 80 (51.9%) | 35 (47.3%) | 53 (70.7%)c | .010 |
Abbreviations: CACS, coronary artery calcium score; BMI, body mass index; CAD, coronary artery disease; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CREA, creatinine; BUN, blood urea nitrogen; URIC, uric acid; BigET-1, big endothelin-1; ARB, angiotensin II receptor blockers; ACEI, angiotensin converting enzyme inhibitors.
a P < .05 for CACS 1 to 100 vs CACS = 0.
b P < .05 for CACS 101 to 400 vs CACS = 0.
c P < .05 for CACS >400 vs CACS = 0.
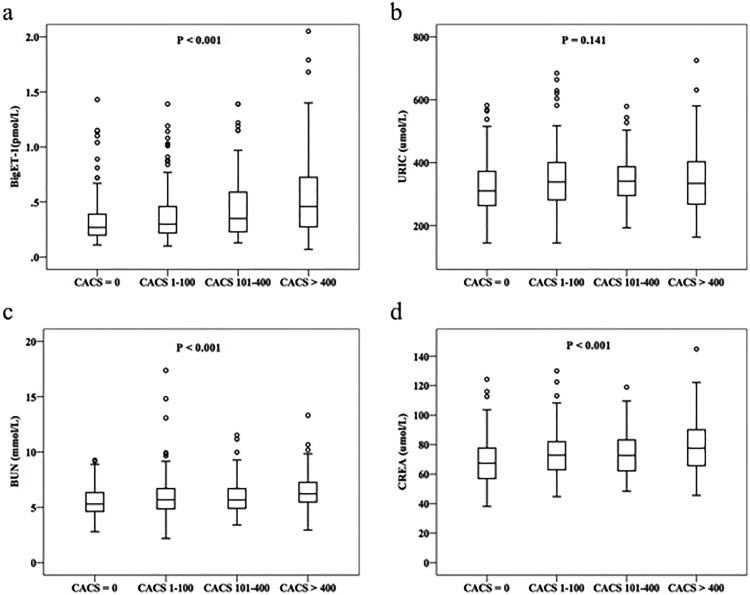
Patients with CACS >400 were older and had a higher percentage of hypertension and diabetes mellitus. But the levels of TC, TG, and LDL-C were lower in patients with CACS >400. Meanwhile, the levels of CREA, BUN, and bigET-1 showed a progressive increase among the 4 groups (Figure 1).
Figure 1.
The levels of bigET-1 and other biomarkers according to the CACS. CACS indicates coronary artery calcium score; BigET-1, big endothelin-1; URIC, uric acid; BUN, blood urea nitrogen; CREA, creatinine.
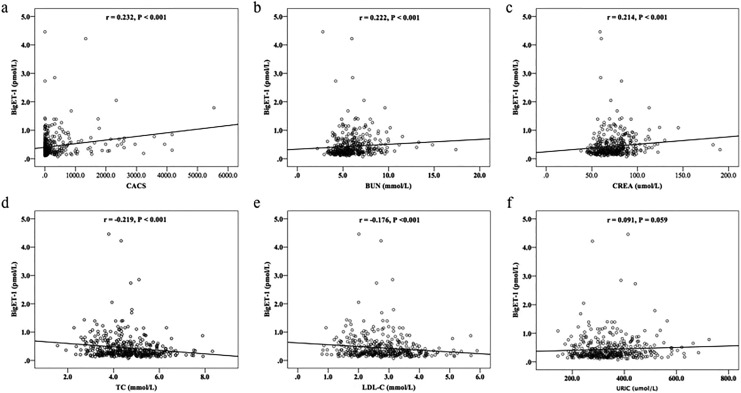
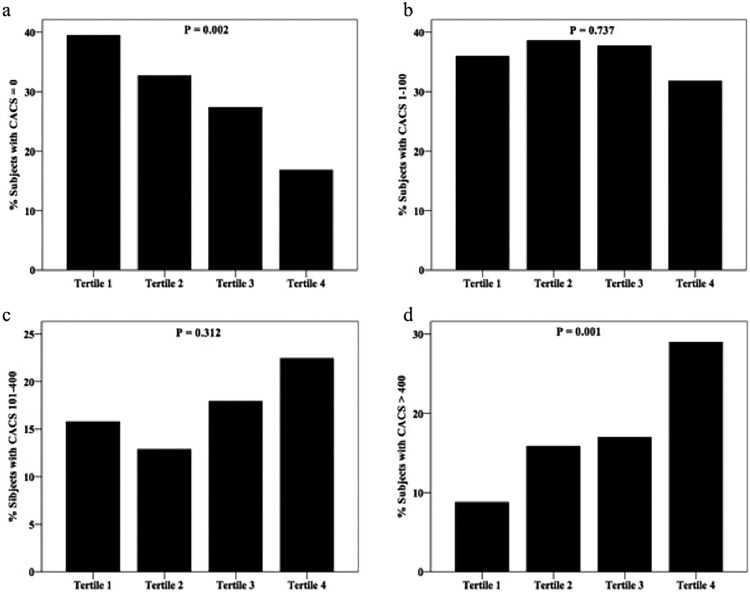
To explore the association of bigET-1 level with the CACS, Spearman correlation analysis was performed in the study. As shown in Figure 2a, the bigET-1 was positively correlated with the CACS (r = .232, P < .001). We additionally evaluated the relation of bigET-1 to other risk factors. The data showed that bigET-1 level was positively correlated with BUN and CREA (r = .222, P < .001, Figure 2b; r = .214, P < .001, Figure 2c) but inversely correlated with TC and LDL-C (r = −.219, P < .001, Figure 2d; r = −.176, P < .001, Figure 2e) and not correlated with URIC (r = .091, P = .059, Figure 2f). To further investigate the relationship between the bigET-1 and the severity of CAC burden, we classified the participants into 4 groups according to bigET-1 tertiles. As shown in Table 2, patients in tertile 3 and tertile 4 were older (P < .05). Further analysis showed that the prevalence of CACS >400 increased significantly from 8.8% in tertile 1 to 29.0% in tertile 4 groups (P < .001) and participants with CACS = 0 were more likely to be in tertile 1 and tertile 2 groups (P < .05). However, there was no significant difference in the proportion of patients with CACS 1 to 100 or CACS 101 to 400 among the 4 groups (P > .05; Figure 3).
Figure 2.
The correlation of bigET-1 with CACS, BUN, CREA, TC, LDL-C and URIC. BigET-1 indicates big endothelin-1; CACS, coronary artery calcium score; BUN, blood urea nitrogen; CREA, creatinine; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; URIC, uric acid.
Table 2.
Clinical and Demographic Characteristics According to bigET-1 Tertiles.
| Variables | Tertile 1 (<0.22) (n = 114) | Tertile 2 (0.22-0.31) (n = 101) | Tertile 3 (0.31-0.52) (n = 106) | Tertile 4 (>0.52) (n = 107) | P Value |
|---|---|---|---|---|---|
| Age (years) | 55.5 ± 8.7 | 57.6 ± 11.5 | 61.1 ± 10.3a | 61.4 ± 10.7b | <.001 |
| BMI (kg/m2) | 25.8 ± 3.6 | 25.6 ± 3.5 | 25.8 ± 3.4 | 25.4 ± 4.2 | .763 |
| Male, n (%) | 66 (57.9%) | 55 (54.5%) | 67 (63.2%) | 75 (70.1%) | .102 |
| Biochemical parameters | |||||
| TC (mmol/L) | 4.8 ± 1.1 | 4.8 ± 0.9 | 4.7 ± 1.6 | 4.2 ± 1.0b | .001 |
| TG (mmol/L) | 1.6 (1.2, 2.2) | 1.6 (1.3, 2.2) | 1.4 (1.0, 2.1) | 1.3 (0.9, 2.1) | .053 |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3b | .011 |
| LDL-C (mmol/L) | 2.9 ± 0.9 | 2.9 ± 0.8 | 2.8 ± 0.9 | 2.5 ± 0.8b | .005 |
| CACS, n (%) | |||||
| CAC = 0 | 45 (39.5%) | 33 (32.7%) | 29 (27.4%)a | 18 (16.8%)b | .002 |
| CACS 1 to 100 | 41 (36.0%) | 39 (38.6%) | 40 (37.7%) | 34 (31.8%) | .737 |
| CACS 101 to 400 | 18 (15.8%) | 13 (12.9%) | 19 (17.9%) | 24 (22.4%) | .312 |
| CACS >400 | 10 (8.8%) | 16 (15.8%) | 18 (17.0%) | 31 (29.0%)b | .001 |
Abbreviations: BigET-1, big endothelin-1; CACS, coronary artery calcium score; BMI, body mass index; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
a P < .05 for tertile 3 vs tertile 1.
b P < .05 for tertile 4 vs tertile 1.
Figure 3.
Percentage of patients with CACS = 0, CACS 1 to 100, CACS 101 to 400 and CACS >400 according to bigET-1 tertiles. BigET-1 indicates big endothelin-1; CACS, coronary artery calcium score.
Multivariable binary logistic regression analysis was performed to identify the predictors of the presence of CAC. Variables with a P < .05 in univariate analysis were included in the model of multivariate analysis including age, hypertension, diabetes mellitus, calcium antagonists, β-blockers, LDL-C, TC, BUN, CREA, and bigET-1. As shown in Table 3, multivariate analysis showed that bigET-1 was the independent predictor of the presence of CACS >400 (odds ratio [OR] = 1.721, 95% CI, 1.002-2.956, P = .049). Furthermore, the highest bigET-1 tertile was also independently associated with the presence of CACS >100.
Table 3.
Regression Analysis to Assess the Severity of CACS According to the bigET-1 and bigET-1 Tertiles.a
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Presence of CACS >0 | |||
| BigET-1 | 1.008 | 0.528-1.925 | .981 |
| BigET-1 tertiles | |||
| Tertile 1 | 1 | ||
| Tertile 2 | 1.195 | 0.620-2.303 | .596 |
| Tertile 3 | 1.060 | 0.485-1.833 | .863 |
| Tertile 4 | 1.692 | 0.814-3.515 | .159 |
| Presence of CACS >100 | |||
| BigET-1 | 1.580 | 0.890-2.803 | .118 |
| BigET-1 tertiles | |||
| Tertile 1 | 1 | ||
| Tertile 2 | 0.961 | 0.489-1.888 | .909 |
| Tertile 3 | 0.944 | 0.488-1.825 | .863 |
| Tertile 4 | 1.966 | 1.026-3.766 | .042 |
| Presence of CACS >400 | |||
| BigET-1 | 1.721 | 1.002-2.956 | .049 |
| BigET-1 tertiles | |||
| Tertile 1 | 1 | ||
| Tertile 2 | 1.605 | 0.639-4.035 | .314 |
| Tertile 3 | 1.067 | 0.428-2.658 | .890 |
| Tertile 4 | 2.435 | 1.013-5.853 | .047 |
Abbreviations: CACS, coronary artery calcium score; BigET-1, big endothelin-1; OR, odds ratio; CI, confidence interval; LDL-C, low-density lipoprotein cholesterol; TC, total cholesterol; CREA, creatinine; BUN, blood urea nitrogen.
aAdjusted for age, hypertension, diabetes mellitus, calcium antagonists, β-blockers, LDL-C, TC, BUN, CREA, and BigET-1.
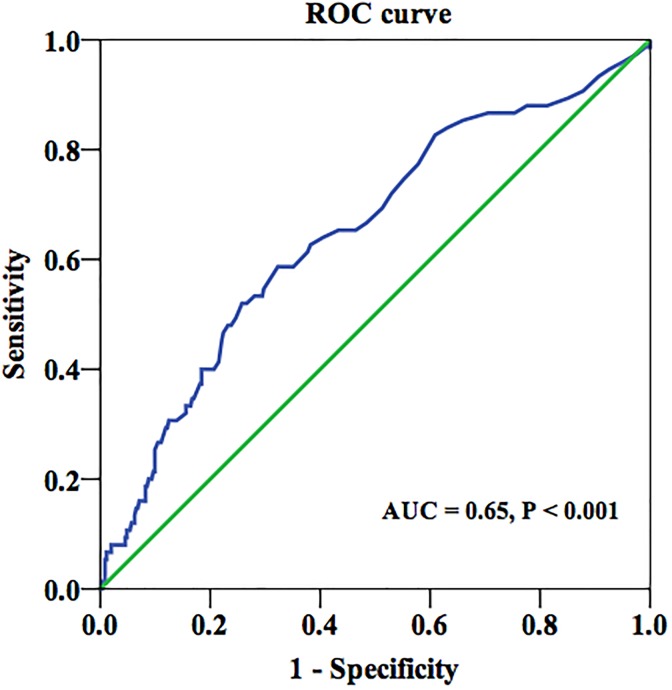
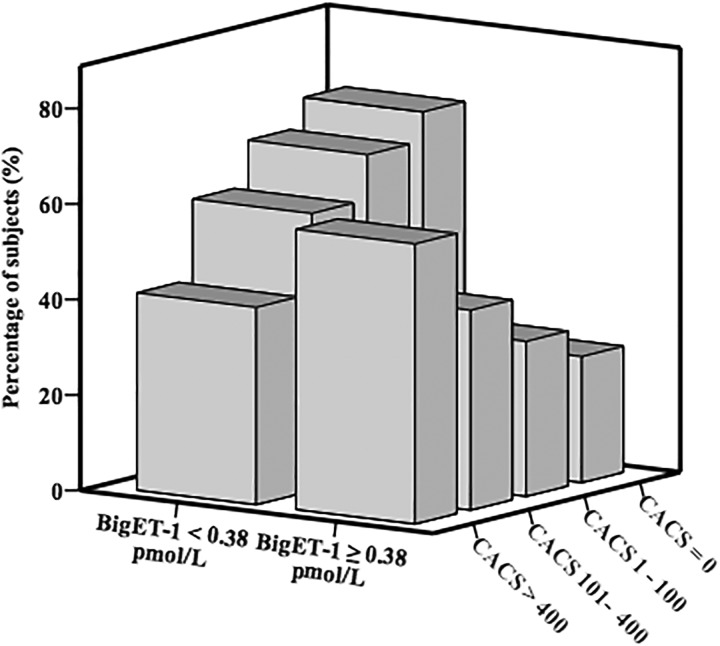
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the utility of bigET-1 as predictor of the presence of CACS >400 (Figure 4). The optimal cutoff value of big ET-1 for predicting CACS >400 was 0.38 pmol/L, with a sensitivity of 59% and specificity of 68% ( area under curve [AUC] = 0.65, 95% CI, 0.58-0.72, P < .001). Using this cutoff value, we additionally divided the participants into the 2 groups (high bigET-1 group ≥0.38 pmol/L and low bigET-1 group <0.38 pmol/L). As shown in Figure 5, the high bigET-1 group tended to have more severe CAC burden and low percentage of CACS = 0 compared to the low bigET-1 group.
Figure 4.
The ROC curve of bigET-1 in predicting CACS >400. ROC indicates receiver operating characteristic; BigET-1, big endothelin-1; AUC, area under the curve.
Figure 5.
Percentage of patients with CACS = 0, CACS 1 to 100, CACS 101 to 400 and CACS >400 according to the level of bigET-1. CACS indicates coronary artery calcium score; BigET-1, big endothelin-1.
Discussion
In the present study, we investigated for the first time the value of bigET-1 in assessment of the severity of CAC. The main findings of this study are as follows: (1) the bigET-1 was positively correlated with the CACS and the prevalence of CACS >400 increased significantly in the highest bigET-1 tertile than the lowest tertile; (2) bigET-1 was the independent predictor of the presence of CACS >400 and the highest bigET-1 tertile was also independently associated with the presence of CACS >100; (3) the ROC curve analysis showed that the optimal cutoff value of big ET-1 for predicting CACS >400 was 0.38 pmol/L, with a sensitivity of 59% and specificity of 68%.
The CACS is a powerful predictor of incident CHD and provides predictive information beyond that provided by standard cardiovascular risk factors. In the Multi-Ethnic Study of Atherosclerosis (MESA), in comparison with participants with 0 CACS, the adjusted risk of cardiovascular event was increased by a factor of 7.7 for those with CACS between 101 and 300 and by a factor of 9.7 for those with CACS >300.2 LaMonte et al18 reported that hazard ratios for major CHD events were 8.7 among men and 6.3 among women among participants with CACS of 400 or more. High CACS appears to portend a particularly high risk of cardiovascular event.8 Notably, asymptomatic individuals with no traditional risk factors and CACS ≥400 had a substantially higher mortality rate than those with ≥3 traditional risk factors but no CAC.19 In the present study, we found the bigET-1 was an independent predictor of the presence of CACS >400. Moreover, the highest bigET-1 tertile was independently associated with the presence of CACS >100. These results suggest the value of bigET-1 as a circulating biomarker in predicting severe CAC and progression of CAC. Recently, Qing et al20 found that plasma bigET-1 was associated with the presence of CAC in patients with chest pain. But they did not report the correlation between the level of bigET-1 and various CACS. Our study provided further information on the association of bigET-1 and CAC. In addition, we noticed that contrary to Qing’s study, we did not find bigET-1 was an independent predictor of the presence of CAC. The difference between the studies suggests that a large cohort study is needed.
An important question regarding CACS relates to how often one should follow up with repeat CT scan. On the one hand, studies indicate that significant progression of CACS is associated with increased risk of adverse cardiovascular events.21–22 On the other hand, a large proportion of the patients with baseline CACS = 0 did not develop any evidence of CAC on follow-up scans within the first 5 years.23 Circulating biomarker provides a possibility to avoid unnecessary CT CAC scan before CACS thresholds of high risk like CAC >400 are reached. In fact, some studies have investigated the value of biomarkers in predicting CAC presence and progression. In a recent prospective study, the association between CAC in asymptomatic participants and 15 biochemical markers was investigated.24 The results showed that only LDL and TC were associated with CAC incidence and phosphate with CAC progression, whereas 12 other biomarkers related to inflammation, kidney function, and myocardial necrosis had little value. A variety of mechanisms have been proposed for vascular calcification.25 Endothelial dysfunction is recognized to be a key early determinant in the progression of atherosclerosis and vascular calcification.26 The ET-1, released from endothelial cells, has an essential physiologic role in cardiovascular homeostasis and contributes to endothelial dysfunction.27 We found that there was a positive correlation between bigET-1 and CACS, which suggests that bigET-1as a biomarker related to endothelial dysfunction should be on the biomarkers list of CAC evaluation.
The ET-1, the first member of the endothelin peptide family identified in 1988, is the most abundant isoform in human cardiovascular system.26 Beyond its function as a vasoactive peptide, many studies propose ET-1 plays a crucial role in the process of atherosclerosis. The ET-1 in both peripheral blood and coronary circulation are increased in patients with atherosclerosis and coronary endothelial dysfunction.12,28 Long-term endothelin receptor antagonism improves endothelial function and attenuates plaque progression in patients with early atherosclerosis.29–30 But few studies focus on the role of ET-1 in coronary calcification involved in atherosclerosis. Increasing evidence suggest that ET-1 may mediate vascular calcification in chronic kidney disease. The mechanisms include increasing phosphate transport, promoting transformation of vascular smooth muscle cells into a bone-producing phenotype, breaking the balance between procalcification and anticalcification factors.31 A recent study demonstrates that endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease.32 Therefore, it is reasonable to assume that the endothelin system may be pivotal in the process of CAC. Our findings of the positive correlation between bigET-1and CACS in this study provide new clue for the research on mechanism of ET-1 in CAC.
There are several limitations in this study. First, the study was a cross-sectional study, which did not permit the determination of causality. Second, the size of the study was relatively small. Third, because the patients admitted had symptoms of chest pain, the findings may not applicable for general population. Whether such associations are present in populations should be studied in the future. Fourth, owing to the patients without the examination of peripheral artery diseases or carotid stenosis, it is unknown whether those factors affect the level of bigET-1. Finally, serial measurements of bigET-1 and CACS would be useful to further explore the dynamic correlation between them.
Conclusion
The bigET-1 levels are significantly and independently associated with the severity of CAC, and the bigET-1 tertile is independently associated with the presence of CACS >400. These findings imply that the circulating bigET-1 is valuable in the assessment of the severity of CAC.
Footnotes
Author Contribution: Fang Wang and Tiewei Li have contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the National Natural Science Foundation of China (81371887 and 81470484).
References
- 1. Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31(1):126–133. [DOI] [PubMed] [Google Scholar]
- 2. Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. [DOI] [PubMed] [Google Scholar]
- 3. Paixao AR, Ayers CR, El Sabbagh A, et al. Coronary artery calcium improves risk classification in younger populations. JACC Cardiovasc Imaging. 2015;8(11):1285–1293. [DOI] [PubMed] [Google Scholar]
- 4. Baber U, Mehran R, Sartori S, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol. 2015;65(11):1065–1074. [DOI] [PubMed] [Google Scholar]
- 5. Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med. 2015;163(1):14–21. [DOI] [PubMed] [Google Scholar]
- 6. Yeboah J, Young R, McClelland RL, et al. Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. J Am Coll Cardiol. 2016;67(2):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClelland RL, Jorgensen NW, Budoff M, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (multi-ethnic study of atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the society of cardiovascular computed tomography. J Cardiovasc Comput Tomogr. 2017;11(2):157–168. [DOI] [PubMed] [Google Scholar]
- 9. Budoff MJ, Hokanson JE, Nasir K, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging. 2010;3(12):1229–1236. [DOI] [PubMed] [Google Scholar]
- 10. Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8(5):579–596. [DOI] [PubMed] [Google Scholar]
- 11. Lerman A, Webster MW, Chesebro JH, et al. Circulating and tissue endothelin immunoreactivity in hypercholesterolemic pigs. Circulation. 1993;88(6):2923–2928. [DOI] [PubMed] [Google Scholar]
- 12. Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325(14):997–1001. [DOI] [PubMed] [Google Scholar]
- 13. Iwasa S, Fan J, Shimokama T, Nagata M, Watanabe T. Increased immunoreactivity of endothelin-1 and endothelin B receptor in human atherosclerotic lesions. a possible role in atherogenesis. Atherosclerosis. 1999;146(1):93–100. [DOI] [PubMed] [Google Scholar]
- 14. Van Beneden R, Gurne O, Selvais PL, et al. Superiority of big endothelin-1 and endothelin-1 over natriuretic peptides in predicting survival in severe congestive heart failure: a 7-year follow-up study. J Card Fail. 2004;10(6):490–495. [DOI] [PubMed] [Google Scholar]
- 15. Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;70(2):252–289. [DOI] [PubMed] [Google Scholar]
- 16. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 17. Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56(17):1397–1406. [DOI] [PubMed] [Google Scholar]
- 18. LaMonte MJ, FitzGerald SJ, Church TS, et al. Coronary artery calcium score and coronary heart disease events in a large cohort of asymptomatic men and women. Am J Epidemiol. 2005;162(5):421–429. [DOI] [PubMed] [Google Scholar]
- 19. Nasir K, Rubin J, Blaha MJ, et al. Interplay of coronary artery calcification and traditional risk factors for the prediction of all-cause mortality in asymptomatic individuals. Circ Cardiovasc Imaging. 2012;5(4):467–473. [DOI] [PubMed] [Google Scholar]
- 20. Qing P, Li XL, Zhang Y, et al. Association of big endothelin-1 with coronary artery calcification. PLoS One. 2015;10(11):e0142458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24(7):1272–1277. [DOI] [PubMed] [Google Scholar]
- 22. Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovasc Imaging. 2009;2(6):692–700. [DOI] [PubMed] [Google Scholar]
- 23. Gopal A, Nasir K, Liu ST, Flores FR, Chen L, Budoff MJ. Coronary calcium progression rates with a zero initial score by electron beam tomography. Int J Cardiol. 2007;117(2):227–231. [DOI] [PubMed] [Google Scholar]
- 24. Diederichsen SZ, Gronhoj MH, Mickley H, et al. CT-detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc Imaging. 2017;10(8):858–866. [DOI] [PubMed] [Google Scholar]
- 25. Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34(4):715–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davenport AP, Hyndman KA, Dhaun N, et al. Endothelin. Pharmacol Rev. 2016;68(2):357–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Amiri F, Virdis A, Neves MF, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110(15):2233–2240. [DOI] [PubMed] [Google Scholar]
- 28. Lerman A, Holmes DR, Jr, Bell MR, et al. Endothelin in coronary endothelial dysfunction and early atherosclerosis in humans. Circulation. 1995;92(9):2426–2431. [DOI] [PubMed] [Google Scholar]
- 29. Reriani M, Raichlin E, Prasad A, et al. Long-term administration of endothelin receptor antagonist improves coronary endothelial function in patients with early atherosclerosis. Circulation. 2010;122(10):958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon MH, Reriani M, Mario G, et al. Long-term endothelin receptor antagonism attenuates coronary plaque progression in patients with early atherosclerosis. Int J Cardiol. 2013;168(2):1316–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rabkin SW. Endothelin but not angiotensin ii may mediate hypertension-induced coronary vascular calcification in chronic kidney disease. Int J Nephrol. 2011;2011:516237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lariviere R, Gauthier-Bastien A, Ung RV, et al. Endothelin type A receptor blockade reduces vascular calcification and inflammation in rats with chronic kidney disease. J Hypertens. 2017;35(2):376–384. [DOI] [PubMed] [Google Scholar]