Abstract
Objective:
Because the no-reflow phenomenon in patients with ST- segment elevation myocardial infarction can lead to poor outcomes and early identification of patients at high risk may alter the clinical outcome, we aimed to study possible differences in the predictive utility among hematological parameters for early identification of patients at high risk of the no-reflow phenomenon during the primary percutaneous coronary intervention.
Methods:
A total of 612 patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention were enrolled. The patients were divided into 2 groups: no-reflow and normal reflow. Hematological parameters were measured on admission. Sensitivity, specificity, positive and negative predictive values, and receiver–operating characteristic areas under the curve were determined to evaluate the predictive values of these parameters.
Results:
The patients in the no-reflow group had a significantly higher neutrophil count, neutrophil–lymphocyte ratio, platelet–lymphocyte ratio, and mean platelet volume-to-lymphocyte ratio when compared to the normal reflow patients. We identified mean platelet volume-to-lymphocyte ratio to have a moderate predictive value and high specificity (66.8%) for the no-reflow phenomenon. Neutrophil–lymphocyte ratio provided the largest area under the curve for predicting no reflow. Regarding the predictive utility for no reflow, the comparison showed no statically significant differences among evaluated hematological parameters.
Conclusion:
For the prediction of no reflow, mean platelet volume-to-lymphocyte ratio yielded moderate performance. No hematological parameter on admission had persuasive superior capacities to predict no-reflow in patients receiving the primary percutaneous coronary intervention.
Keywords: hematological parameters, no-reflow phenomenon, ST-segment elevation myocardial infarction
Introduction
Angiographic no reflow, a reduced coronary antegrade flow (thrombolysis in myocardial infarction [TIMI] flow grade ≤2) without mechanical obstruction after recanalization, is known to be associated with short- and long-term morbidity and mortality in patients who underwent primary percutaneous coronary intervention (PPCI) for acute ST-segment elevation myocardial infarction (STEMI),1,2,3 and early identification of patients at high risk regarding no reflow is very important for the prevention and treatment of this condition. Previous studies have suggested various hematological parameters, such as the platelet-to-lymphocyte ratio (PLR),4,5 mean platelet volume-to-lymphocyte ratio (MPVLR),6 and neutrophil-to-lymphocyte ratio (NLR) to be inflammatory and thrombotic markers in patients with coronary atherosclerotic disease and may predict poor cardiovascular outcomes7 and no reflow in patients with acute STEMI undergoing mechanical reperfusion.8 However, the literature reports on the predictive value of these parameters vary, and data on the comparison of the utility of such parameters are rare or not available. So in this study, we aim to investigate the association between no-reflow and NLR, PLR, MPVLR, and other hematological parameters in patients with STEMI undergoing PPCI and compare the utility of these parameters for early detection of patients at high risk of no-reflow phenomenon.
Patients and Methods
Study Population
Patients diagnosed with STEMI undergoing PPCI between September 2012 and October 2016 in our hospital were enrolled in this retrospective observational study. ST-segment elevation myocardial infarction was defined as typical chest pain >30 minutes with ST-segment elevation of >1 mm in at least 2 consecutive leads on the electrocardiogram or new-onset left bundle branch block and more than 2-fold increase in serum cardiac markers. Exclusion criteria included cardiogenic shock on admission, thrombolytic drugs in the previous 24 hours, active infections, systemic inflammatory disease history, clinical evidence of autoimmune disease or hematological proliferative disorders, known malignancy, liver disease as well as patients with renal failure. Demographic data (age and gender), history of diseases (diabetes mellitus, hypertension, and hyperlipidemia), and smoking status were collected in all patients. The study protocol was approved by the ethics committee of our institution, and written informed consent was obtained from all patients.
Coronary Angiography and PCI Procedure
Pharmacological treatment of all enrolled patients before PPCI included aspirin (300 mg loading dose), clopidogrel (600 mg loading dose), and an intravenous bolus of unfractionated heparin at a dose of 70 U/kg of body weight. The PPCI was performed using the standard radial or femoral approach with a 6F or 7F guiding catheter. The stent was deployed in all patients. The use of balloon predilatation or postdilatation, the type of stents (bare-metal or drug-eluting), and the use of thrombus aspiration were left to the operator’s decision. The glycoprotein IIb/IIIa receptor inhibitor tirofiban was given by judgment of the operator and initiated during PCI procedure with 10 μg/kg intracoronary bolus followed by 0.15 μg/kg/min intravenous infusion. Technically, successful stent implantation was defined as the residual stenosis <10% in the culprit lesion after the procedure as visually assessed by angiography, without occlusion of a significant side branch, flow-limiting dissection, distal embolization, or angiographic thrombus.9 The TIMI flow grades were evaluated by consensus of 2 experienced interventional cardiologists who did not have knowledge of the clinical and laboratory data using the quantitative cardiovascular angiographic software. No reflow after PPCI was defined as TIMI flow grade ≤2 after vessel recanalization despite the absence of angiographic stenosis, spasm, dissection, or thrombosis. Normal reflow was defined as postintervention TIMI grade 3 flow.10 Study participants were divided into 2 groups according to TIMI flow grades after PPCI. Multivessel disease was defined as >50% diameter stenosis of 2 or more major epicardial coronary arteries. To evaluate the intracoronary thrombus burden, we performed TIMI thrombus scale11 in all patients after antegrade flow achievement through guidewire crossing or small balloon dilatation (final TIMI thrombus grade). In TIMI thrombus grade 0, no cine-angiographic characteristics of thrombus are present; in TIMI thrombus grade 1, possible thrombus is present with angiographic characteristics such as decreased contrast density, haziness, irregular lesion contour, or a smooth convex “meniscus” at the site of total occlusion suggestive but not diagnostic of thrombus; in TIMI thrombus grade 2, there is definite thrombus, with the largest dimensions ≤1/2 the vessel diameter; in TIMI thrombus grade 3, there is definite thrombus with the largest linear dimension >1/2 but <2 vessel diameters; in TIMI thrombus grade 4, there is definite thrombus, with the largest dimension ≥2 vessel diameters; and in TIMI thrombus grade 5, there is total occlusion. Low-thrombus burden group was defined as a thrombus grade of 0 to 2, and high-thrombus burden was defined as a thrombus grade of at least 3.
Angiographic and procedural data (eg, time from symptom onset to PPCI, stent parameters) were collected for each participant.
Laboratory Analysis and Echocardiography
In all patients, venous blood samples were drawn into standard EDTA-containing tubes on admission in the emergency department before the administration of aspirin and clopidogrel. The hematological parameters such as neutrophil, platelet, and lymphocyte count were measured by an automated blood cell counter (XS-1000i; Sysmex Co, Kobe, Japan). Creatinine, high-sensitivity C-reactive protein (hs-CRP), and cardiac enzymes on admission were also measured in all patients determined by the standard methods. Echocardiography investigation was routinely performed on admission before PPCI, using GE ViVidE7 ultrasound machine (GE Healthcare, Piscataway, New Jersey) with a 3.5-MHz transducer. Left ventricular ejection fraction (LVEF) was measured by Simpson method in the 2-dimensional echocardiographic apical 4-chamber view.
Statistical Analysis
Continuous variables with a normal distribution (eg, age, LVEF, peak cardiac troponin I [cTnI] level, and creatinine level) were expressed as a mean (SD), and the differences between groups were tested by independent samples t test. Continuous variables with nonnormal distribution (eg, hs-CRP and hematological parameters) were expressed as medians and interquartile ranges, and the differences between the groups were analyzed with Mann-Whitney U tests. Categorical variables were summarized as percentages and compared to the χ2 test. A 2-sided P value <.05 was considered significant. Multivariate logistic regression analysis was used to identify independent predictors for the development of the no-reflow phenomenon. Receiver–operating characteristic (ROC) curves of the NLR, PLR, MPVLR, lymphocyte-to-monocyte ratio (LMR), lymphocyte count, and neutrophil count were drawn to compute the area under the curve (AUC). The Youden-index method was applied to set an optimal cutoff value for optimal differentiation. The DeLong test was used to compare ROC-AUCs of different parameters. Statistical analysis was performed using the SPSS 22.0 Statistical Package Program for Windows (SPSS Inc, Chicago, Illinois).
Results
Our study included 612 patients (435 men; mean age: 62 [14] years) with STEMI who had undergone PPCI within 12 hours from symptom onset. In the current study, the incidence of the angiographic no-reflow phenomenon was 16.0% (n = 97) after PPCI. Patients with TIMI flow grades 0 to 2 formed the no-reflow group (n = 97; 67 men; mean age: 63 [16] years), and patients with TIMI 3 flow grade formed the normal reflow group (n = 515; 368 men; mean age: 62 [13] years), respectively. Baseline demographic and biochemical characteristics of the patients in no-reflow and normal reflow groups are shown in Table 1.
Table 1.
Baseline Demographic and Biochemical Characteristics of Patients in Groups.
| Variables | Normal-reflow (n = 515) | No-reflow (n = 97) | P |
|---|---|---|---|
| Age, years | 62 (13) | 63 (16) | .477 |
| Male sex | 368 (71.5%) | 29 (69.1%) | .324 |
| Diabetes mellitus | 164 (31.9%) | 20 (30.3%) | 1.000 |
| Hypertension | 306 (59.5%) | 63 (65.2%) | .426 |
| Hyperlipidemia | 346 (67.3%) | 45.5 (46.9%) | .335 |
| Active smokers | 237 (46.2%) | 32 (33.3%) | .213 |
| Prior MI | 17 (3.5%) | 3 (3.0%) | 1.000 |
| LVEF, % | 53.6 (9.6) | 52.4 (8.2) | .425 |
| Creatinine, µmol/L | 78.0 (24.7) | 81.3 (25.3) | .411 |
| hs-CRP level, mg/L | 4.08 (1.51-11.50) | 5.16 (1.86-13.47) | .654 |
| Peak cTnI, ng/mLa | 35.9 (11.3-90.5) | 59.7 (16.6-119.7) | .344 |
Abbreviations: cTnI, cardiac troponin I; hs-CRP, high sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
aData are presented as the median value (25th, 75th percentiles).
There was no statistically significant difference between the 2 groups in the presence of hypertension, hyperlipidemia, diabetes mellitus, and previous myocardial infarction. Age, sex distribution, and serum creatinine admission LVEF levels were similar between the groups. Median peak cTnI levels were not significantly different between the no-reflow group and the normal reflow group (59.7 ng/mL vs 35.9 ng/mL; P = .344). There was no significant difference in median hs-CRP levels between the no-reflow group and the normal reflow group (5.16 mg/L vs 4.08 mg/L; P = .654).
The comparison of admission hematological parameters between 2 groups of patients is presented in Table 2. White blood cell, platelet count, mean platelet volume (MPV), hemoglobin, hematocrit, and monocyte count were similar in both the groups. The patients in the no-reflow group had a significantly higher median neutrophil count (7.10 × 109/L vs 6.30 × 109/L; P = .031), NLR (4.45 vs 3.00; P = .004), PLR (131.20 vs 102.50; P = .006), MPVLR (6.71 vs 5.14; P = .027), but lower median lymphocyte count (1.50 × 109/L vs 2.00 × 109/L; P = .010) and LMR (3.25 vs 4.00; P = .008) when compared to the normal reflow patients.
Table 2.
Hematological Parameters of the Study Population.a
| Variable | Normal-reflow (n = 515) | No-reflow (n = 97) | P |
|---|---|---|---|
| White blood cell count, ×109/L | 9.10 (7.50-11.28) | 9.81 (7.81-11.47) | .221 |
| Neutrophil count, ×109/L | 6.30 (4.55-8.05) | 7.10 (5.28-9.43) | .031 |
| Hemoglobin, g/dL | 148.00 (135.50-156.50) | 142.00 (132.50-155.00) | .082 |
| Platelet count, ×109/L | 206.00 (175.50-248.50) | 216.50 (165.50-277.00) | .519 |
| Hematocrit, % | 43.10 (40.25-45.50) | 41.40 (38.45-44.70) | .065 |
| Mean platelet volume, fL | 10.20 (9.70-10.90) | 10.50 (9.80-11.10) | .303 |
| Lymphocyte count, ×109/L | 2.00 (1.40-2.85) | 1.50 (1.00-2.50) | .010 |
| Monocyte count, ×109/L | 0.50 (0.40-0.70) | 0.52 (0.40-0.70) | .748 |
| Neutrophil/lymphocyte ratio | 3.00 (1.70-5.25) | 4.45 (2.50-8.65) | .004 |
| Platelet/lymphocyte ratio | 102.50 (71.70-151.55) | 131.20 (82.65-213.37) | .006 |
| Mean platelet volume-to-lymphocyte ratio | 5.14 (3.51-7.46) | 6.71 (4.01-9.93) | .027 |
| Lymphocyte-to-monocyte ratio | 4.00 (3.00-5.00) | 3.25 (2.38-4.57) | .008 |
aData are presented as median (interquartile range).
The angiographic and procedural characteristics of the patient population are presented in Table 3. No significant differences in the time from pain to intervention, infarct-related coronary artery, multivessel disease, and other angiographic or procedural characteristics were observed between the groups. However, the high-thrombus burden was more frequently observed in the no-reflow group compared to the normal reflow group (58.8% vs 29.2%; P = .000).
Table 3.
Angiographic and Procedural Characteristics of Study Population According to TIMI Flow.
| Variable | Normal-reflow (n = 515) | No-reflow (n = 97) | P |
|---|---|---|---|
| Time from symptom onset to PPCI | .227 | ||
| <3 hours | 145 (28.3%) | 22 (22.7%) | |
| 3-6 hours | 198 (38.4%) | 31 (31.8%) | |
| 6-12 hours | 171 (33.3%) | 44 (45.5%) | |
| Infarct-related coronary artery | .748 | ||
| Left main | 2 (0.4%) | 0 (0.0%) | |
| Left anterior descending | 274 (53.2%) | 50 (51.5%) | |
| Left circumflex | 57 (11.0%) | 15 (15.1%) | |
| Right coronary artery | 182 (35.4%) | 32 (33.4%) | |
| Multi-vessel disease | 435 (84.4%) | 69 (71.2%) | .225 |
| Proximal lesion | 291 (56.6%) | 46 (47.0%) | .365 |
| Preintervention TIMI flow grade | .548 | ||
| 0 | 470 (91.3%) | 82 (84.5%) | |
| 1 | 24 (4.6%) | 9 (9.3%) | |
| 2 | 15 (2.9%) | 5 (5.2%) | |
| 3 | 6 (1.2%) | 1 (1.0%) | |
| Thrombus burden | .000 | ||
| Low thrombus burden | 365 (70.8%) | 40 (41.2%) | |
| High thrombus burden | 150 (29.2%) | 57 (58.8%) | |
| Number of used stent, n | 1.59 (0.70) | 1.41 (0.94) | .149 |
| Stent length, mm | 36.18 (18.21) | 37.75 (20.53) | .618 |
| Stent diameter, mm | 3.08 (0.46) | 3.16 (0.44) | .310 |
| Use of thrombus aspiration | 116 (22.5%) | 44 (45.5%) | .000 |
| Tirofiban use | 284 (55.2%) | 81 (83.3%) | .000 |
Abbreviations: PPCI, primary percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
The multivariate logistic regression models revealed that neutrophil count (odds ratio [OR] = 1.122, 95% confidence interval [CI]: 1.007-1.250; P = .037), lymphocyte count (OR = 0.701, 95% CI: 0.509-0.965; P = .029), NLR (OR = 1.010, 95% CI: 1.020-1.188; P = .014), PLR (OR = 1.005, 95% CI: 1.001-1.009; P = .013), MPVLR (OR = 1.076, 95% CI: 1.001-1.157; P = .045), LMR (OR = 0.812, 95% CI: 0.682-0.966; P = .019), and thrombus burden (OR = 3.526, 95% CI: 1.858-6.695; P = .000) were independent factors for predicting no-reflow phenomenon in patients undergoing PPCI after adjustment for age, gender, history of hypertension, diabetes mellitus, hyperlipidemia, active smoking, preintervention TIMI flow grade, and hs-CRP level, as shown in Table 4.
Table 4.
Odd ratios (ORs) of Prognostic Factors for Predicting No-Reflow.a,b
| Factors | Model 1 | Model 2 | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Preintervention TIMI flow grade | 1.371 (0.847-2.217) | .199 | ||
| Thrombus burden | 3.454 (1.847-6.460) | .000 | 3.526 (1.858-6.695) | .000 |
| hs-CRP | 0.997 (0.988-1.006) | .526 | ||
| Neutrophil count | 1.112 (1.010-1.224) | .030 | 1.122 (1.007-1.250) | .037 |
| Lymphocyte count | 0.696 (0.512-0.947) | .021 | 0.701 (0.509-0.965) | .029 |
| NLR | 1.120 (1.044-1.201) | .002 | 1.101 (1.020-1.188) | .014 |
| PLR | 1.006 (1.002-1.010) | .003 | 1.005 (1.001-1.009) | .013 |
| MPVLR | 1.080 (1.010-1.155) | .025 | 1.076 (1.001-1.157) | .045 |
| LMR | 0.793 (0.665-0.945) | .010 | 0.812 (0.682-0.966) | .019 |
Abbreviations: CI, confidence interval; hs-CRP, high sensitivity C-reactive protein; LMR, lymphocyte-to-monocyte ratio; MPVLR, mean platelet volume-to-lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; TIMI, thrombolysis in myocardial infarction.
aModel 1: unadjusted model.
bModel 2: adjusted for age, gender, history of hypertension, diabetes mellitus, hyperlipedemia, active smoking, preintervention TIMI flow grade and hs-CRP.
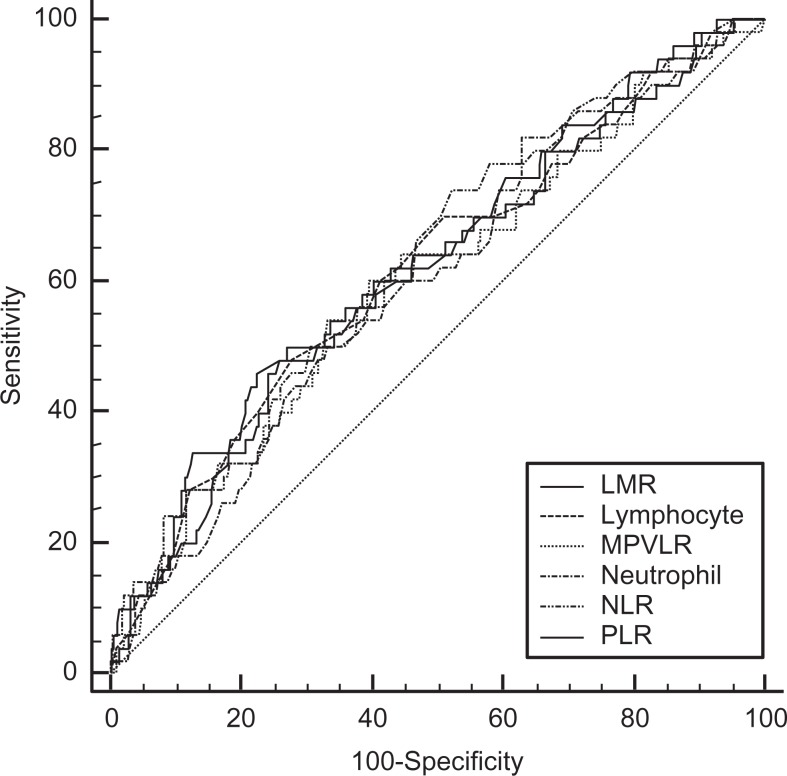
Information on the cutoff values and capacity of the individual hematological parameters to predict no-reflow phenomenon in patients with STEMI is provided in Table 5. Neutrophil-to-lymphocyte ratio provided largest AUC for predicting no reflow (AUC-ROC: 0.628, 95% CI: 0.569-0.685; P = .028). Using 2.8 as a cutoff value, the assessed sensitivity was 74.0%, with 47.6% specificity, 89.3% negative predictive value, and 23.7% positive predictive value. No statistically significant difference in ROC-AUC was found among evaluated parameters (Figure 1).
Table 5.
Predictive Capacities of Hematological Parameters for No-Reflow.
| Parameters | ROC-AUC (95% CI) | P Value | Cutoff Value | Sensitivity | Specificity | Negative Predictive Value | Positive Predictive Value |
|---|---|---|---|---|---|---|---|
| Neutrophil count, ×109/L | 0.597 (0.537-0.655) | .027 | 5.1 | 82.0 | 37.1 | 90.4 | 22.3 |
| Lymphocyte count, ×109/L | 0.616 (0.556-0.673) | .010 | 1.4 | 48.0 | 72.1 | 86.3 | 27.4 |
| NLR | 0.628 (0.569-0.685) | .028 | 2.8 | 74.0 | 47.6 | 89.3 | 23.7 |
| PLR | 0.624 (0.565-0.681) | .005 | 142.3 | 48.0 | 74.2 | 86.7 | 29.0 |
| MPVLR | 0.600 (0.540-0.658) | .028 | 6.69 | 54.0 | 66.8 | 86.2 | 27.5 |
| LMR | 0.620 (0.560-0.677) | .008 | 2.97 | 46.0 | 77.7 | 86.1 | 32.5 |
Abbreviations: CI, confidence interval; LMR, lymphocyte-to-monocyte ratio; MPVLR, mean platelet volume-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC-AUC, receiver operating characteristic-area under the curve.
Figure 1.
ROC-curves of various parameters for predicting no-reflow. For comparison between assessed hematologic parameters, the DeLong test was applied, no statically significant differences were found among evaluated hematologic parameters. LMR indicates lymphocyte-to-monocyte ratio; MPVLR, mean platelet volume-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic.
Discussion
In the present study, we demonstrated the predicting value of MPVLR for the no-reflow phenomenon in patients with STEMI for the first time. However, we found no significant differences in the hematological parameters on admission in the predictive power for the no-reflow phenomenon.
Despite successful recanalization of the infarct-related artery for patients with acute STEMI, myocardial tissue perfusion does not completely restore in up to 12% to 39% of patients.2,12 This effect is known as the no-reflow phenomenon, which may markedly reduce the benefits of recanalization of the ischemic-related artery and is associated with short-term and long-term adverse clinical outcomes.13,3 In the present study, 66 (16.0%) patients developed angiographic no reflow. The limited access to performant diagnostic tools or biomarkers restricts the therapeutic window in PPCI practice for patients with STEMI. Early risk stratification to detect patients at high risk of no reflow before PPCI may be beneficial from perspective awareness of this condition, which might lead to the use of certain prophylactic interventional techniques14 or intracoronary medications to ameliorate the development of no reflow.15 Although the pathophysiology of the no-reflow phenomenon has not been fully understood, previous studies have suggested important roles for inflammation16 and excessive thrombotic activity,15 which may eventually lead to distal microvascular obstruction and endothelial dysfunction in the process of no-reflow phenomenon. The complete blood count is inexpensive and routinely available on admission before PPCI and includes several inflammatory parameters. Previous studies, including ours, have reported that higher neutrophil count,17 lower lymphocyte count,18 MPV,19 NLR,7,8 PLR,4,5 and MPVLR6 seem to have association with the pathogenesis of no-reflow phenomenon and prognostic value in STEMI. To the best of our knowledge, this is the first study to determine the comparative value of these parameters in predicting the no-reflow phenomenon for patients with STEMI treated with PPCI. Our study showed that for the prediction of no reflow in patients with STEMI who underwent PPCI, no hematological parameters such as neutrophil count, lymphocyte count, MPV, NLR, PLR, or MPVLR displayed persuasive discriminatory capacities. In the present study, neutrophil count and NLR had moderately high sensitivities (82.0% and 74.0%, respectively) and negative predictive value (90.4%, 89.3%, respectively) in the current study, although the specificities and positive predictive value were relatively low.
Neutrophil infiltration was found in ruptured plaques in patients with acute coronary syndromes.20 Accumulation of neutrophils in capillaries probably contributed to microvascular obstruction and no-reflow development.21 Neutrophil-to-lymphocyte ratio, an index that reflects high neutrophil levels and relatively low lymphocyte count, has been determined to be a good indicator of inflammatory conditions.22 In the present study, the predictive value examined using ROC curve analysis revealed that, although without significant difference, NLR >2.8 (AUC = 0.628) had the higher discriminative ability for predicting the no reflow than neutrophil count >5.1 × 109/L (AUC = 0.597). One possible reason may be that NLR represents a combination of 2 important and opposite immune pathways, where neutrophils represent nonspecific systemic inflammation initiating the first line of defense, whereas lymphocytes represent the regulatory or protective component of the immune system physiological stress response. This may explain that NLR performs better than absolute neutrophil count alone in our study. Moreover, NLR has better stability compared to the neutrophil count, which may be altered by various physiological, pathological, and physical factors.23 The finding of no statistically significant result for the predictive value of neutrophil count and NLR in the current study may due to the retrospective nature and small sample size.
Furthermore, we identified PLR and MPVLR to have a moderate predictive value (AUC = 0.624 and 0.600, respectively) and high specificity (74.2%, 66.8%, respectively) for no-flow phenomenon. Higher platelet count and MPV may reflect underlying inflammation, platelet activation, and higher propensity to form thrombi.24,25 Kurtul et al revealed that PLR is a strong and independent predictor of no reflow after PPCI.26 The area under the ROC curve for PLR in that previous study was 0.78, and a PLR above 126 predicted no reflow with a sensitivity of 73% and specificity of 71%. A possible explanation for the different pattern of results between Kurtul et al and our study may be the relatively low platelet count in our study (237.0 × 109/L in normal reflow group and 254.0 × 109/L in no-reflow group in Kurtul et al vs 206.0 × 109/L in normal reflow group and 216.5 × 109/L in no-reflow group in our study). Our study included more patients with symptom onset >6 hours (35.5% in our study vs 8.5% in Kurtul et al) which may result in the consumption of platelets in the infarct region or thrombi formation and the reverse changes in MPV.27 These inconsistent results indicate that the utility of hematological parameters, such as PLR and MPVLR, may vary with the timing of STEMI onset. For the first time in our study, we demonstrated the predicting value of MPVLR for the no-reflow phenomenon in patients with STEMI. Many studies have shown a negative correlation between platelet count and MPV.27,28 So it is not surprising that PLR and MPVLR displayed same predictive value for no reflow in the present study.
Monocytes and lymphocytes are both key immune cells in the inflammatory response. Previous study revealed that the area under the ROC curve for the LMR for the no-reflow phenomenon was 0.835. The cutoff value of the LMR (2.292) was associated with 76.3% sensitivity and 72.5% specificity, respectively.29 However, there was relatively lower predictive capacity of LMR in our study with AUC = 0.620, 46.0% sensitivity, and 77.7% specificity, respectively. The discordance between these 2 studies may be partially due to the different sample sizes and clinical characteristics.
Study Limitations
This study investigated the comparative value of admission hematological parameters in predicting the no-reflow phenomenon for patients with STEMI treated with PPCI. However, this is a retrospective study, including a relatively small number of patients, and it was conducted at a single institution. These factors may have hindered our ability to identify differences among studied parameters, and the power of the analysis of our study might be restricted. We did not assess the effect of reference luminal diameter in the present study, which may be correlated with no reflow during PPCI and may influence the evaluation of the predictive value of the studied hematological parameters. Additional large-scale, prospective studies will be needed to further evaluate the comparative abilities of various hematological parameters to predict the no-reflow phenomenon.
Conclusion
We found that MPVLR and other previously studied hematological parameters, including neutrophil count, NLR, PLR, and LMR, showed a moderate diagnostic performance regarding the prediction of no reflow. The diagnostic abilities of these hematological parameters performed similarly, and no parameter had persuasive superior diagnostic capacities to identify the no-reflow phenomenon in patients with STEMI receiving PPCI.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Ito H. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2006;3(9):499–506. [DOI] [PubMed] [Google Scholar]
- 2. Morishima I, Sone T, Okumura K, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36(4):1202–1209. [DOI] [PubMed] [Google Scholar]
- 3. Brosh D, Assali AR, Mager A, et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol. 2007;99(4):442–445. [DOI] [PubMed] [Google Scholar]
- 4. Azab B, Shah N, Akerman M, McGinn JT., Jr Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012;34(3):326–334. [DOI] [PubMed] [Google Scholar]
- 5. Gürsoy OM, Karakoyun S, Kalçik M, et al. Usefulness of novel hematologic inflammatory parameters to predict prosthetic mitral valve. Am J Cardiol. 2014;113(5):860–864. [DOI] [PubMed] [Google Scholar]
- 6. Hudzik B, Szkodziński J, Lekston A, Gierlotka M, Poloński L, Gąsior M. Mean platelet volume-to-lymphocyte ratio: a novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complications. 2016;30(6):1097–1102. [DOI] [PubMed] [Google Scholar]
- 7. Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110(5):621–627. [DOI] [PubMed] [Google Scholar]
- 8. Kurtul A, Murat SN, Yarlioglues M, et al. Increased neutrophil-to-lymphocyte ratio predicts persistent coronary no-flow after wire insertion in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Clinics (Sao Paulo). 2015;70(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. 2011;124(23):e574–e651. [DOI] [PubMed] [Google Scholar]
- 10. Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54(4):281–292. [DOI] [PubMed] [Google Scholar]
- 11. Sianos G, Papafaklis MI, Serruys PW. Angiographic thrombus burden classification in patients with ST-segment elevation myocardial infarction treated with percutaneous coronary intervention. J Invasive Cardiol. 2010;22(10 suppl B):6B–14B. [PubMed] [Google Scholar]
- 12. Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast-enhanced magnetic resonance. J Am Coll Cardiol. 2005;46(7):1229–1235. [DOI] [PubMed] [Google Scholar]
- 13. Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55(21):2383–2389. [DOI] [PubMed] [Google Scholar]
- 14. Mancini JG, Filion KB, Windle SB, Habib B, Eisenberg MJ. Meta-analysis of the long-term effect of routine aspiration thrombectomy in patients undergoing primary percutaneous coronary intervention. Am J Cardiol. 2016;118(1):23–31. [DOI] [PubMed] [Google Scholar]
- 15. Rezkalla SH, Dharmashankar KC, Abdalrahman IB, Kloner RA. No-reflow phenomenon following percutaneous coronary intervention for acute myocardial infarction: incidence, outcome, and effect of pharmacologic therapy. J Interv Cardiol. 2010;23(5):429–436. [DOI] [PubMed] [Google Scholar]
- 16. Niccoli G, Lanza GA, Spaziani C, et al. Baseline systemic inflammatory status and no-reflow phenomenon after percutaneous coronary angioplasty for acute myocardial infarction. Int J Cardiol. 2007;117(3):306–311. [DOI] [PubMed] [Google Scholar]
- 17. Wang Z, Ren L, Lei L, Ye H, Peng J. The relationship between neutrophil count on admission and angiographic no-reflow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Acta Cardiol. 2016;71(2):241–246. [DOI] [PubMed] [Google Scholar]
- 18. Núñez J, Núñez E, Bodí V, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis. 2010;21(1):1–7. [DOI] [PubMed] [Google Scholar]
- 19. Celik T, Kaya MG, Akpek M, et al. Predictive value of admission platelet volume indices for in-hospital major adverse cardiovascular events in acute ST-segment elevation myocardial infarction. Angiology. 2015;66(2):155–162. [DOI] [PubMed] [Google Scholar]
- 20. Buffon A, Biasucci LM, Liuzzo G, D’Onofrio G, Crea F, Maseri A. Widespread coronary inflammation in unstable angina. N Engl J Med. 2002;347(1):5–12. [DOI] [PubMed] [Google Scholar]
- 21. Kloner RA, Rude RE, Carlson N, Maroko PR, DeBoer LW, Braunwald E. Ultrastructural evidence of microvascular damage and myocardial cell injury after coronary artery occlusion: which comes first? Circulation. 1980;62(5):945–952. [DOI] [PubMed] [Google Scholar]
- 22. Kaya MG. Inflammation and coronary artery disease: as a new biomarker neutrophil/lymphocyte ratio. Turk Kardiyol Dern Ars. 2013;41(3):191–192. [DOI] [PubMed] [Google Scholar]
- 23. Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154(5):995–1002. [DOI] [PubMed] [Google Scholar]
- 24. Martin JF, Shaw T, Heggie J, Penington DG. Measurement of the density of human platelets and its relationship to volume. Br J Haematol. 1983;54(3):337–352. [DOI] [PubMed] [Google Scholar]
- 25. Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9(3):177–190. [DOI] [PubMed] [Google Scholar]
- 26. Kurtul A, Yarlioglues M, Murat SN, et al. Usefulness of the platelet-to-lymphocyte ratio in predicting angiographic reflow after primary percutaneous coronary intervention in patients with acute ST-segment elevation myocardial infarction. Am J Cardiol. 2014;114(3):342–347. [DOI] [PubMed] [Google Scholar]
- 27. Glud T, Schmidt EB, Kristensen SD, Arnfred T. Platelet number and volume during myocardial infarction in relation to infarct size. Acta Med Scand. 1986;220(5):401–405. [DOI] [PubMed] [Google Scholar]
- 28. Yilmaz MB, Cihan G, Guray Y, et al. Role of mean platelet volume in triaging acute coronary syndromes. J Thromb Thrombolysis. 2008;26(1):49–54. [DOI] [PubMed] [Google Scholar]
- 29. Kurtul A, Yarlioglues M, Celik IE, et al. Association of lymphocyte-to-monocyte ratio with the no-reflow phenomenon in patients who underwent a primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis. 2015;26(8):706–712. [DOI] [PubMed] [Google Scholar]